Abstract
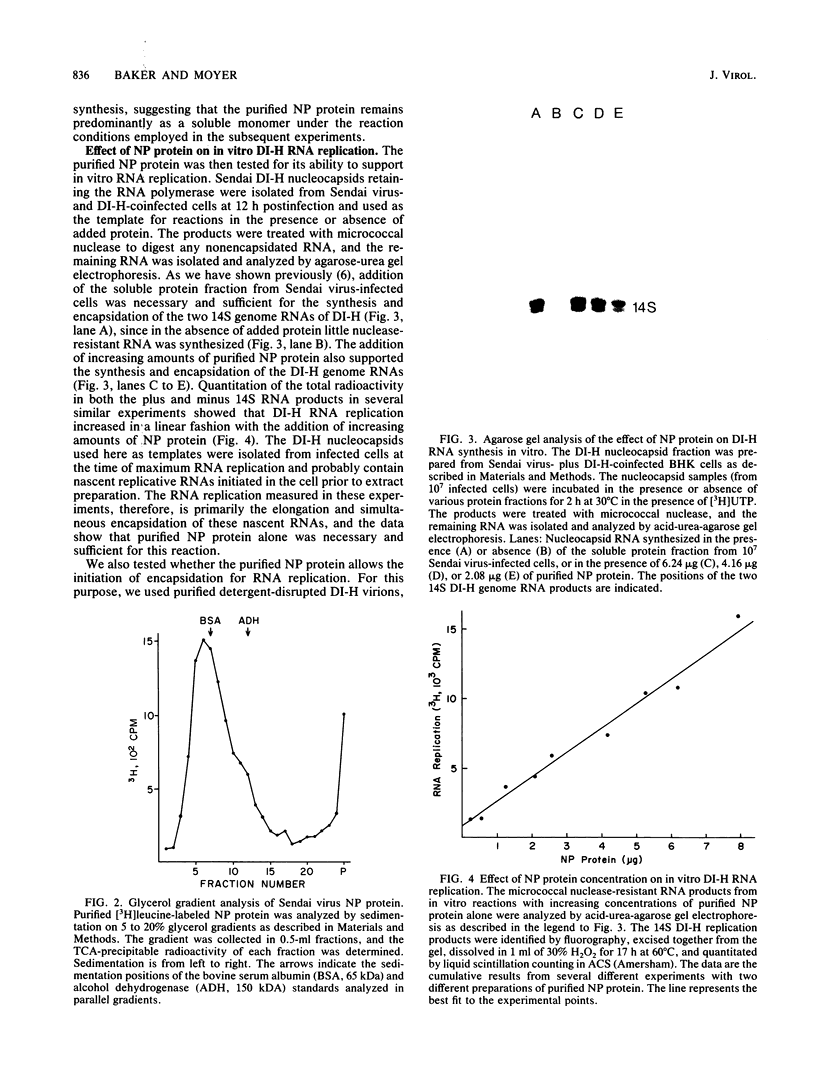
The ability of the Sendai virus major nucleocapsid protein, NP, to support the in vitro synthesis and encapsidation of viral genome RNA during Sendai virus RNA replication was studied. NP protein was purified from viral nucleocapsids isolated from Sendai virus-infected BHK cells and shown to be a soluble monomer under the reaction conditions used for RNA synthesis. The purified NP protein alone was necessary and sufficient for in vitro genome RNA synthesis and encapsidation from preinitiated intracellular Sendai virus defective interfering particle (DI-H) nucleocapsid templates. The amount of DI-H RNA replication increased linearly with the addition of increasing amounts of NP protein. With purified detergent-disrupted DI-H virions as the template, however, there was no genome RNA synthesis in either the absence or presence of the NP protein. Furthermore, addition of the soluble protein fraction of uninfected cells alone or in the presence of purified NP protein also did not support DI-H genome RNA synthesis from purified DI-H. Another viral component in addition to the NP protein appears to be required for the initiation of encapsidation, since the soluble protein fraction of infected but not uninfected cells did support DI-H genome replication from purified DI-H.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Rose K., Kolakofsky D. Preparation and analysis of the nucleocapsid proteins of vesicular stomatitis virus and sendai virus, and analysis of the sendai virus leader-NP gene region. J Gen Virol. 1984 Apr;65(Pt 4):769–779. doi: 10.1099/0022-1317-65-4-769. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlsen S. R., Peluso R. W., Moyer S. A. In vitro replication of Sendai virus wild-type and defective interfering particle genome RNAs. J Virol. 1985 May;54(2):493–500. doi: 10.1128/jvi.54.2.493-500.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Arnheiter H., Wertz G. W. Vesicular stomatitis virus N and NS proteins form multiple complexes. J Virol. 1986 Sep;59(3):751–754. doi: 10.1128/jvi.59.3.751-754.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande K. L., Portner A. Monoclonal antibodies to the P protein of Sendai virus define its structure and role in transcription. Virology. 1985 Jan 15;140(1):125–134. doi: 10.1016/0042-6822(85)90451-9. [DOI] [PubMed] [Google Scholar]
- Deshpande K. L., Portner A. Structural and functional analysis of Sendai virus nucleocapsid protein NP with monoclonal antibodies. Virology. 1984 Nov;139(1):32–42. doi: 10.1016/0042-6822(84)90327-1. [DOI] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Glazier K., Raghow R., Kingsbury D. W. Regulation of Sendai virus transcription: evidence for a single promoter in vivo. J Virol. 1977 Mar;21(3):863–871. doi: 10.1128/jvi.21.3.863-871.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Determination by peptide mapping of the unique polypeptides in Sendai virions and infected cells. Virology. 1978 Feb;84(2):469–478. doi: 10.1016/0042-6822(78)90263-5. [DOI] [PubMed] [Google Scholar]
- Leppert M., Rittenhouse L., Perrault J., Summers D. F., Kolakofsky D. Plus and minus strand leader RNAs in negative strand virus-infected cells. Cell. 1979 Nov;18(3):735–747. doi: 10.1016/0092-8674(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re G. G., Gupta K. C., Kingsbury D. W. Genomic and copy-back 3' termini in Sendai virus defective interfering RNA species. J Virol. 1983 Feb;45(2):659–664. doi: 10.1128/jvi.45.2.659-664.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Iwasaki K., Shibuta H. Determination of the complete nucleotide sequence of the Sendai virus genome RNA and the predicted amino acid sequences of the F, HN and L proteins. Nucleic Acids Res. 1986 Feb 25;14(4):1545–1563. doi: 10.1093/nar/14.4.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]