Abstract
Epithelio–mesenchymal interactions during kidney organogenesis are disrupted in integrin α8β1-deficient mice. However, the known ligands for integrin α8β1—fibronectin, vitronectin, and tenascin-C—are not appropriately localized to mediate all α8β1 functions in the kidney. Using a method of general utility for determining the distribution of unknown integrin ligands in situ and biochemical characterization of these ligands, we identified osteopontin (OPN) as a ligand for α8β1. We have coexpressed the extracellular domains of the mouse α8 and β1 integrin subunits as a soluble heterodimer with one subunit fused to alkaline phosphatase (AP) and have used the α8β1-AP chimera as a histochemical reagent on sections of mouse embryos. Ligand localization with α8β1-AP in developing bone and kidney was observed to be overlapping with the distribution of OPN. In “far Western” blots of mouse embryonic protein extracts, bands were detected with sizes corresponding to fibronectin, vitronectin, and unknown proteins, one of which was identical to the size of OPN. In a solid-phase binding assay we demonstrated that purified OPN binds specifically to α8β1-AP. Cell adhesion assays using K562 cells expressing α8β1 were used to confirm this result. Together with a recent report that anti-OPN antibodies disrupt kidney morphogenesis, our results suggest that interactions between OPN and integrin α8β1 may help regulate kidney development and other morphogenetic processes.
INTRODUCTION
Integrins are a family of transmembrane glycoproteins that serve as receptors for a wide variety of ligands, including extracellular matrix (ECM)1 constituents, Ig and cadherin class cell adhesion molecules, and cell surface–associated and soluble members of the disintegrin family (Sonnenberg, 1993; Wolfsberg et al., 1995). Integrins are noncovalently linked heterodimers with α and β subunits, which play an important role in development, wound healing, and immune responses (Hynes, 1992). We have previously shown that the integrin α8 subunit is expressed as a heterodimer with the integrin β1 subunit (Bossy et al., 1991). Affinity chromatography with detergent-solubilized integrins and cell adhesion assays have been used to identify fibronectin (FN), vitronectin (VN), and tenascin-C (TN-C) as ligands for the integrin α8β1 (Müller et al., 1995; Schnapp et al., 1995; Varnum-Finney et al., 1995).
Recently, we have shown that the integrin α8β1 mediates essential steps in metanephric kidney development, both ingrowth of the ureteric bud and formation of epithelial tubular structures from metanephric mesenchyme (Müller et al., 1997). Although this integrin is strongly expressed in metanephric mesenchyme, none of the known α8β1 ligands appears to have an appropriate expression pattern to account alone for the requirement for this integrin during kidney morphogenesis. FN is expressed in the metanephric mesenchyme before invasion by the ureteric bud and is down-regulated upon induction (Ekblom, 1981; Aufderheide et al., 1987). VN is not expressed in the embryonic kidney (Seiffert et al., 1995), and TN-C is expressed too late (Aufderheide et al., 1987) to account for the phenotype in α8β1-deficient animals. Consistent with these observations, mice with targeted mutations in the VN and TN-C genes develop without kidney abnormalities (Saga et al., 1992; Zheng et al., 1995). Mice lacking FN die too early to analyze kidney development, but antibodies to FN do not perturb kidney development in organ cultures (Klein et al., 1988; Sariola et al., 1988; George et al., 1993). Antibodies to TN-C also have failed to perturb kidney development (Talts et al., 1997).
Motivated in part by these observations, we developed a strategy to identify and characterize ligands that are coexpressed in vivo with their cognate integrin receptor(s) and that may mediate essential developmental functions. Previous strategies for detection of integrin ligands based on in vitro assays are limited to use of purified proteins as candidate ligands. In addition, some receptor–ligand interactions may not occur in vivo, because the ligand and receptor are not codistributed. To search for α8β1 ligand(s) in vivo, we chose a combination of biochemical and histochemical approaches using a recombinant soluble integrin heterodimer expressed as a fusion protein with alkaline phosphatase (AP). Although AP chimeras have been used to identify the ligands for receptor tyrosine kinases (Flanagan and Leder, 1990; Cheng and Flanagan, 1994), they have not been previously used to identify ligands of heterodimeric receptor molecules such as integrins. Using the α8β1-AP chimera as a histochemical reagent, we observed codistribution of α8β1 ligand(s) and osteopontin (OPN) in areas of bone formation and within the developing kidney. Using the α8β1-AP chimera as a probe in far Western blots of tissue extracts and in solid-phase binding assays with purified proteins, we were able to confirm that OPN is an additional α8β1 ligand. Based on the recent finding that anti-OPN antibodies and RGD peptides disrupt kidney morphogenesis (Rogers et al., 1997), it seems likely that interactions between OPN and integrin α8β1 may contribute to the regulation of epithelio–mesenchymal interactions during kidney development.
MATERIALS AND METHODS
Materials
Human plasma FN, the 120-kDa fragment of FN (FN120), bovine plasma VN, and the peptides GRGDSP and GRGESP were purchased from Life Technologies (Gaithersburg, MD). A glutathione S-transferase-human OPN fusion protein (GST-OPN) (Hu et al., 1995) was a gift from Dr. J.W. Smith (Burnham Institute, La Jolla, CA). Purified human urine OPN (Shiraga et al., 1992) was a gift from Dr. J.R. Hoyer (Children’s Hospital of Philadelphia, Philadelphia, PA). Recombinant mouse entactin/nidogen was a gift from Dr. R. Timpl (Max-Planck-Institut für Biochemie, Martinsried, Germany). GST–entactin fragment fusion proteins were gifts from Dr. A. E. Chung (University of Pittsburgh, Pittsburgh, PA).
Antibodies
Anti-mouse integrin α8 subunit antibody was prepared by immunizing rabbits with affinity-purified mouse integrin α8t monomer (Figure 1B, lane 2) expressed in COS cells. The antibody reacted specifically with integrin α8 in Western blot, immunoprecipitation, and immunohistochemistry of mouse tissue sections. The other antibodies were as follows: anti-rat OPN peptide (residues 291–306) antiserum OST-1 (a gift from Dr. B.B. Mukherjee, McGill University, Montreal, Quebec, Canada); anti-OPN antiserum pp69 (Nemir et al., 1989); anti-GST-mouse OPN antibody (Stern et al., 1995); anti-OPN monoclonal antibody (mAb) MPIIIB10 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, and Johns Hopkins University, Baltimore, MD); anti-rat FN antiserum (Yang et al., 1993); anti-human FN antibody (Organon Teknika, Durham, NC); anti-mouse VN antibody (Seiffert, 1996); anti-rat integrin β1 mAb HA2/11 (Mendrick and Kelly, 1993); anti-mouse integrin β1 mAb 9EG7 (Lenter et al., 1993); anti-human integrin αvβ5 mAb P1F6 (Life Technologies); anti-human integrin α5 mAb B1E5 (Hall et al., 1990); anti-human integrin β1 mAb AIIB2 (Hall et al., 1990); and anti-human placental AP mAb MIA1801 (Medix Biotech, San Carlos, CA). For some experiments, IgG fractions were prepared from antisera using protein A-Sepharose (Pharmacia LKB Biotechnology, Piscataway, NJ).
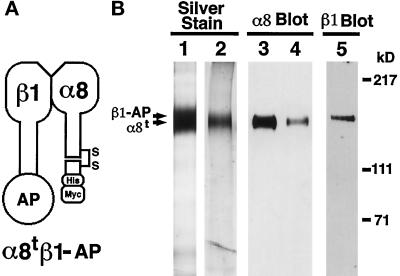
Figure 1.
Purification of soluble truncated integrin α8β1 heterodimer and α8 monomer. (A) Schematic representation of the soluble α8tβ1-AP heterodimer. Mature integrin α8 and β1 subunits consist of extracellular, transmembrane, and cytoplasmic domains. The truncated α8 (α8t) consists of the entire extracellular domain of mouse α8 cDNA with c-terminal (His)6 and c-myc epitope tags. The β1-AP chimera (β1-AP) consists of the entire extracellular domain of β1 fused in frame to human placental AP. α8tβ1-AP is a heterodimer of α8t and β1-AP. (B) The purified α8tβ1-AP heterodimer (lanes 1, 3, and 5) and α8t monomer (lanes 2 and 4) were separated by electrophoresis in 6% polyacrylamide gels in nonreducing conditions. The gels were either silver stained (lanes 1 and 2) or transferred to nitrocellulose membranes and probed with anti-α8 antiserum (lanes 3 and 4) or anti-β1 mAb 9EG7 (lane 5). In lane 1, both α8t and β1-AP bands are within the overexposed band shown. The purified α8t monomer (lane 2) was used as an antigen to prepare the rabbit polyclonal anti-mouse α8 antibody.
Preparation of Expression Vectors for Expression of Modified Extracellular Domains of α8 and β1 Subunits
Cloning of murine integrin α8 cDNA and the constructs to express truncated soluble heterodimers are described elsewhere (Denda et al., 1998). In brief, using mRNA purified from NIH 3T3 cells, cDNA was synthesized, and clones encoding α8 cDNA were amplified using primers based on the sequences of the human and chick integrin α8 subunits. The sequence data are available from GenBank under accession number AF041409.
To express a secreted protein, a synthetic DNA fragment encoding a chick α8 signal peptide was fused to the N-terminal portion of the mature mouse α8 subunit. A clone encoding a truncated mouse α8 (α8t) was generated by PCR with a gene-specific 5′ primer and a 3′ primer encoding the C terminus of the deduced extracellular domain of mouse α8 plus a (His)6-myc tag and a stop codon (VIWATPNVSHHHHHHGEQKLISEEDL-stop). The truncated mouse β1 (β1t) was generated by introducing a stop codon after the end of extracellular domain (amino acid sequence number 728) by PCR using the mouse β1 cDNA clone ST1 as a template. These were subcloned into pCR3 (Invitrogen, San Diego, CA). The mouse β1 extracellular domain-AP chimera (β1-AP) was generated by isolating a modified pCR3-β1t in which the stop codon at the end of the extracellular domain of β1t was eliminated by PCR and was then ligated to an SnaBI (filled in with Klenow)–XhoI fragment containing the AP from APtag-1 (Flanagan and Leder, 1990). All PCR-generated clones were sequenced to ensure that they did not contain mutations.
Expression and Purification of Soluble Truncated Integrin Heterodimers
COS-7 cells were transiently cotransfected using LipofectAMINE Reagent (Life Technologies) with the two plasmids encoding extracellular domains of the α and β subunits plus indicated C-terminal tags (α8t and β1-AP) to express α8tβ1-AP (Figure 1A). The conditioned medium was collected every 2–4 d for 1 wk. After addition of MgCl2 (at a final concentration of 1 mM), phenylmethylsulfonyl fluoride (PMSF, 1 mM), and sodium azide (0.02%), the conditioned medium was filtered and concentrated 10- to 20-fold using a YM100 membrane (Amicon, Beverly, MA). After addition of Tris-Cl (pH 8.0; 20 mM), imidazole (10 mM), and PMSF (0.5 mM), the solution was incubated with Ni-NTA beads (Qiagen, Chatsworth, CA), which were transferred into an empty column and washed with 20 mM Tris-Cl, pH 7.5, 300 mM NaCl, 1 mM MgCl2, 0.02% sodium azide, 20 mM imidazole, 0.5 mM PMSF. After washing, bound proteins were eluted with 20 mM Tris-Cl (pH 7.5), 50 mM NaCl, 1 mM MgCl2, 0.02% sodium azide, 100 mM imidazole, 0.5 mM PMSF. To remove imidazole, α8t monomer, and nonspecifically bound contaminants of low molecular weight, the buffer of the eluate was exchanged by repeating concentration and dilution with the elution buffer without imidazole, using a Centricon 100 or a Centriplus 100 filter apparatus (Amicon, Beverly, MA). The truncated integrin α8 monomer (α8t) was purified as above, except that a YM30 membrane (Amicon) was used to concentrate the medium from cells expressing only α8t. Protein concentrations were determined by both Coomassie Plus protein assay reagent (Pierce, Rockford, IL) and silver staining of proteins after fractionation by SDS-PAGE. The purified heterodimers retained activity for at least 5 mo when stored at either 4 or −80°C.
Histochemistry
C57Bl/6 mouse embryos were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned at 7 μm, stained with antibodies, and counterstained as described (Jones et al., 1994). Staining with α8tβ1-AP was performed as described (Cheng and Flanagan, 1994) with some modifications. Sections were blocked with 1% BSA, 25 mM Tris-Cl (pH 7.5), 100 mM NaCl; incubated for 4–12 h at room temperature with 7 μg/ml α8tβ1-AP in 20 mM Tris-Cl (pH 7.5), 50 mM NaCl, 1 mM MnCl2, 0.05% BSA, 0.02% sodium azide; washed with the same buffer; fixed with 60% acetone, 3% formaldehyde, 20 mM HEPES (pH 7.0); washed with 20 mM HEPES (pH 7.0), 150 mM NaCl; heated at 65°C for 1 h in this buffer; rinsed in AP buffer (100 mM Tris-Cl [pH 9.5], 100 mM NaCl, 5 mM MgCl2); and incubated at room temperature with AP substrate solution (0.33 mg/ml nitroblue tetrazolium and 0.17 mg/ml 5-bromo-4-chloro-3-indoyl-phosphate in AP buffer). Sections were counterstained with methyl green (Zymed, South San Francisco, CA) and mounted with GVA-mount (Zymed).
Far Western Blotting
Protein extracts were obtained by homogenizing mouse embryos and kidneys in ice-cold extraction buffer (50 mM Tris-Cl [pH 7.5], 50 mM octylglucoside, 20 mM NaCl, 1 mM MgCl2, 1 mM sodium vanadate, 1 mM sodium molybdate, 1 mM PMSF, 10 μg/ml leupeptin, 3 μg/ml pepstatin A) followed by centrifugation to remove the debris. Protein concentrations were determined by Coomassie assays (Pierce). Far Western blotting was performed as described (Hildebrand et al., 1995) with some modifications. Proteins were separated by electrophoresis on SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and renatured. Membranes were blocked at 4°C with 10% BSA, 20 mM HEPES (pH 7.5), 75 mM KCl, 0.1 mM EDTA, 2.5 mM MgCl2, 0.05% NP40 for 1 h and then 1% BSA, 25 mM Tris-Cl (pH 7.5), 50 mM NaCl, 0.05% NP-40 for 1 h. Membranes were washed in 0.1% BSA, 25 mM Tris-Cl (pH 7.5), 50 mM NaCl, 1 mM MnCl2, 0.05% NP-40, incubated with 0.3 μg/ml α8tβ1-AP in the same solution for 2 h at room temperature, washed with this solution, rinsed in AP buffer containing 0.5 mM MnCl2, and incubated with AP-substrate solution containing 0.5 mM MnCl2. Where indicated, extracts were depleted of FN by passing the extracts three times through an FN antibody column, or extracts were immunoprecipitated with OPN antiserum (pp69; Nemir et al., 1989) and protein A beads before far Western analysis.
Solid-Phase Binding Assays, Cell Adhesion Assays, and Cell-spreading Assays
Ninety-six-well plates (Maxisorp, Nunc, Rochester, NY) were coated with substrate proteins in Tris-buffered saline (TBS; 25 mM Tris-Cl [pH 7.5], 100 mM NaCl) at 4°C overnight, blocked with 1% BSA in TBS, and washed with TBS-Mn (1 mM MnCl2 in TBS). As background control and positive control, wells were coated with 1% BSA and 10 μg/ml FN120, respectively. α8tβ1-AP (5 μg/ml in TBS-Mn) was then added to each well and incubated at room temperature for 2 h. After washing with TBS-Mn, 100 μl AP substrate (1 M diethanolamine [pH 9.8], 0.5 mM MgCl2, 12 mM p-nitrophenyl phosphate) was added and incubated at room temperature. Integrin binding was quantified by measuring absorbance at 405 nm.
The KA8 cells (α8-transfected K562 cells), K562 cells, and cell adhesion and cell-spreading assays have been described previously (Müller et al., 1995).
RESULTS
Soluble Recombinant Integrin α8β1 Heterodimers Bind FN In Vitro and In Situ
To generate soluble integrin heterodimers, we have designed truncated mouse α8 and mouse β1 cDNA constructs (Figure 1A). The truncated α8 (α8t) has (His)6 and c-myc epitope tags for the purpose of affinity purification and detection, respectively. Human placental AP was chosen for incorporation into the truncated β1-AP chimera (β1-AP) because it has well-characterized detection properties (Cheng and Flanagan, 1994). The two cDNA constructs (α8t and β1-AP) were coexpressed in COS cells, and the secreted integrin heterodimers (α8tβ1-AP) were purified from the conditioned medium (Figure 1B, lane 1).
The secreted α8tβ1-AP chimera shows similar ligand-binding properties to integrin α8β1 expressed on the cell surface, because purified α8tβ1-AP interacts specifically with ECM ligands in a solid-phase binding assay; α8tβ1-AP bound to the known ligands (FN and VN) in an RGD-dependent manner (see Figure 5A, discussed later in this article); it did not bind to several other ECM molecules, such as laminin-1, collagen I, and collagen IV, in agreement with earlier observations that these molecules do not appear to be ligands for cell surface–expressed α8β1 (Müller et al., 1995, Denda et al., 1998). Binding to each of these substrate-bound ligands was completely blocked by the inhibitory anti-β1 mAb HA2/11 (Denda et al., 1998), and binding was observed in the presence of Mn2+ but not detectably in the absence of Mn2+. α8β1 expressed on the cell surface of K562 cells exhibits the same requirement for Mn2+ (Müller et al., 1995).
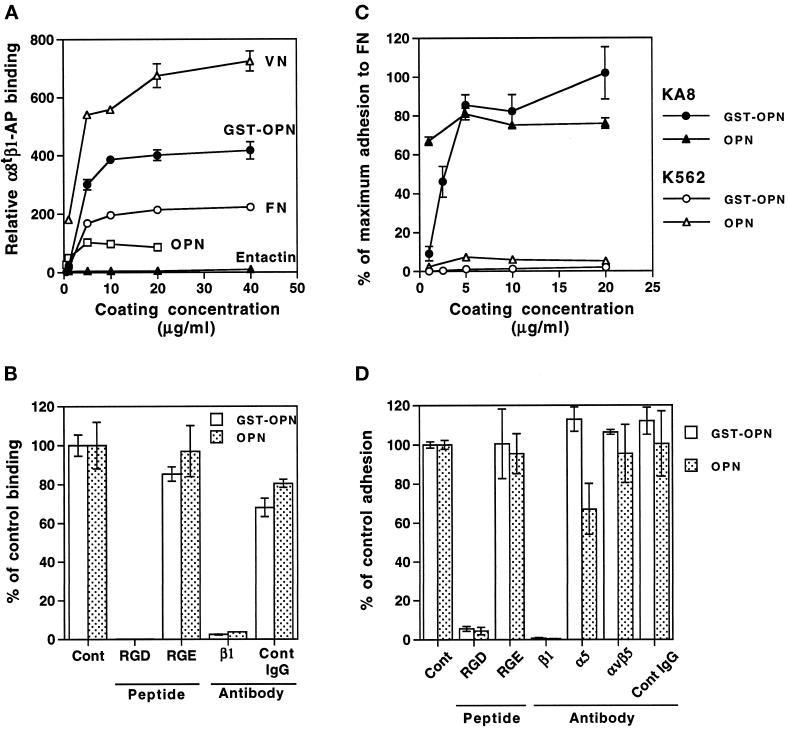
Figure 5.
Binding of α8tβ1-AP and cells expressing α8β1 to known ligands and to purified native OPN and recombinant GST-OPN. (A and B) Solid-phase binding assays were performed with α8tβ1-AP in the presence of 1 mM MnCl2. (A) α8tβ1-AP binding to entactin, VN, FN, native OPN, and GST-OPN. Plates were coated with indicated concentrations of purified proteins. The absorbance values were normalized using the absorbance value for binding to substrata coated with 10 μg/ml FN120. Results demonstrate that α8tβ1-AP binds to OPN and GST-OPN as well as to FN and VN. (B) Effects of peptides and an inhibitory anti-β1 antibody on α8tβ1-AP binding to native OPN and GST-OPN. Wells coated with 5 μg/ml OPN or 10 μg/ml GST-OPN were incubated with α8tβ1-AP with additions indicated: (Cont) no addition; (RGD) 0.1 μg/ml GRGDSP peptide; (RGE) 0.1 μg/ml GRGESP peptide; (β1) 100 μg/ml anti-integrin β1 mAb HA2/11; and (Cont IgG) 100 μg/ml control IgG. The absorbance was quantified as the percentage of control binding (no addition). (C) Cell adhesion to native OPN and GST-OPN. Cell adhesion assays were carried out with KA8 and K562 cells on plates coated with indicated concentrations of OPN and GST-OPN in the presence of 1 mM MnCl2. The absorbance was quantified as the percentage of maximum adhesion to FN coated at 5 μg/ml. Note that only KA8 cells adhered to OPN and GST-OPN. (D) Effects of peptides and anti-integrin mAbs on KA8 cell adhesion to OPN and GST-OPN. KA8 cell adhesion assays were carried out on OPN and GST-OPN (coated at 5 μg/ml) in the presence of 1 mM MnCl2 with additions as indicated: (Cont) no addition; (RGD) 10 μg/ml GRGDSP peptide; (RGE) 10 μg/ml GRGESP peptide; (β1) anti-β1 mAb AIIB2; (α5) anti-α5 mAb B1E5; (αvβ5) anti-αvβ5 mAb P1F6; and (Cont IgG) 100 μg/ml control IgG. Adhesion with no addition (Cont) was set at 100%. Note that KA8 cell adhesion to OPN and GST-OPN was RGD sensitive and was blocked by the anti-β1 mAb. Data represent mean ± SD of triplicate wells.
Purified soluble heterodimers also bound to an FN affinity column and could be used to localize FN secreted into the ECM by cultured fibroblasts, as shown by double immunofluorescence with α8tβ1-AP and anti-FN antibodies. Both reagents stained FN fibrils on the surface of fibroblasts (our unpublished results). The α8tβ1-AP binding was specific, because it was completely inhibited by an RGDS-containing peptide and by the anti-integrin β1 mAb HA2/11 (our unpublished results).
Ligand Detection with α8tβ1-AP in Tissue Sections: Colocalization of an α8β1 Ligand and OPN
Because the α8tβ1-AP chimera recognized the known ligands of this integrin, we extended the use of α8tβ1-AP to histochemistry on tissue sections to localize ligands in situ (Figures 2 and 3). On sections of embryonic day (E) 13.5 and E16.5 mouse embryos, the α8tβ1-AP bound to restricted sites, including developing rib bones (Figure 2) and kidneys (Figure 3).
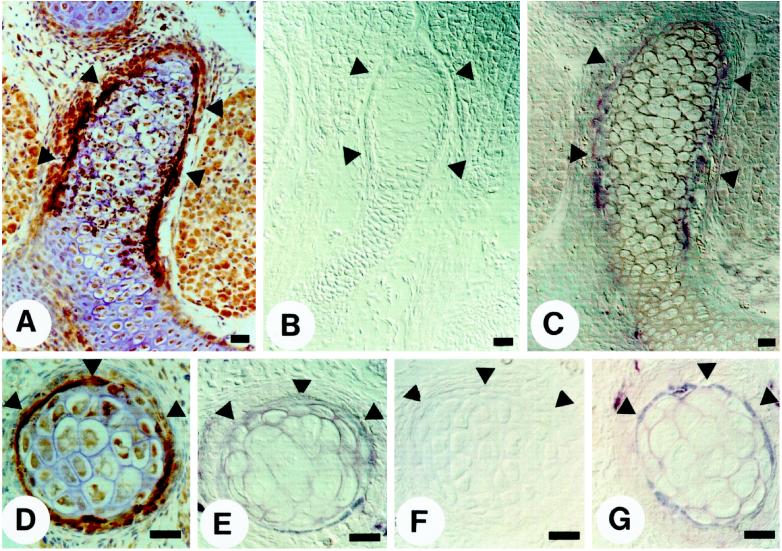
Figure 2.
Colocalization of α8tβ1-AP binding sites and OPN in the regions of bone morphogenesis. Paraffin sections of E16.5 (A–C) and E13.5 (D–G) mouse embryos were incubated with indicated reagents: (A) anti-OPN peptide antiserum; (B) control conditioned medium containing nonfused, secreted AP; (C and E) α8tβ1-AP; (D) anti-GST-mouse OPN IgG; (F) α8tβ1-AP in the presence of 50 μg/ml GRGDSP peptide; and (G) α8tβ1-AP in the presence of 50 μg/ml GRGESP peptide. Arrowheads indicate the bony collar. Bar, 10 μm.
Figure 3.
Colocalization of α8tβ1-AP binding sites, OPN, and the integrin α8 subunit in the developing kidney. Paraffin sections (A and C) and frozen sections (B) of an E16.5 kidney were incubated with α8tβ1-AP (A), anti-OPN peptide antiserum (B), and affinity-purified anti-mouse integrin α8 subunit IgG (C). The panels show a cross-section through the ureter epithelium, and the arrows point to the border between the epithelium and surrounding mesenchyme. Note that the section is at a level below the tips of the ureter; thererfore, no condensing mesenchymal cells are visible. Bar, 50 μm.
Within the developing bone, α8tβ1-AP binding (staining) was confined to the bony collar (Figure 2, C and E, arrowheads), and the binding was specific, because it was inhibited by the β1 mAb HA2/11 (our unpublished results). Binding was also inhibited by an RGDS-containing peptide (Figure 2F), whereas a control RGES peptide had no effect (Figure 2G), indicating that the binding pattern of α8tβ1-AP reflects the distribution of RGD-containing ligand(s). The α8tβ1-AP binding pattern (Figure 2, C and E) was similar to the previously reported immunohistochemical localization of OPN, an ECM protein with an RGD cell attachment site (Mark et al., 1988; Gorski et al., 1990; Chen et al., 1991; Stern et al., 1995). We confirmed this by staining the adjacent sections with anti-OPN antibodies (Figure 2, A and D). The anti-OPN staining pattern overlapped with α8tβ1-AP staining, although the α8tβ1-AP staining was more restricted and concentrated at cell–cell junctions.
We have previously shown that the integrin α8 subunit is expressed in the metanephric mesenchyme with high levels in the condensing mesenchymal cells surrounding the tips of the branching ureter and in the mesenchymal cells bordering on the growing and maturing ureter epithelium (Müller et al., 1997). The α8tβ1-AP chimera stained the surface of the ureter epithelium in a pattern complementary to staining with antibodies against the integrin α8 subunit, as described (Figure 3, A and C; Müller et al., 1997). We have previously demonstrated also that the α8tβ1-AP binding is specific, because it can be inhibited by an RGDS-containing peptide but not by a control RGES peptide (Müller et al., 1997). We now demonstrate by immunohistochemistry that OPN is also localized to the ureter epithelium in a pattern that overlaps with that of α8tβ1-AP staining (Figure 3, compare A and B). The staining for OPN was confined to the growing and maturing ureteric epithelium, and no staining was apparent in the surrounding mesenchymal cells. The same OPN expression pattern was observed with three different antibodies against OPN (Figure 3B and our unpublished results). This is in agreement with recent analysis of OPN expression by in situ hybridization (Rogers et al., 1997). Our data suggest that α8tβ1-AP may recognize OPN in situ, and OPN is appropriately localized to mediate interactions with integrin α8β1 in vivo in the kidney.
Ligand Detection by Far Western Blot Analysis
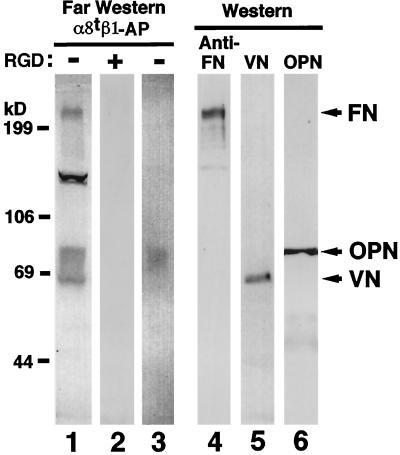
To characterize the biochemical nature of α8β1 ligands in mouse embryos, extracts from E13.5 mouse embryos were analyzed by far Western blot using α8tβ1-AP as a probe. The α8tβ1-AP bound to proteins of approximate molecular masses of 240, 130, and 65–85 kDa (Figure 4, lane 1). Bands with the same molecular masses were recognized in an extract from E16.5 kidney (Müller et al., 1997). The binding to each of these proteins was completely inhibited by RGDS (Figure 4, lane 2) but not by RGES peptides (our unpublished results). The sizes of some of the proteins recognized by α8tβ1-AP appeared the same as those of FN, VN, and OPN, as detected by Western blotting (Figure 4, lanes 4–6). Indeed, the 240-kDa band (Figure 4, lane 1) was identified as FN, because it could be depleted from extracts by FN–antibody affinity chromatography (our unpublished results). Proteins in the range of 65 kDa (Figure 4, lane 1) were purified from tissue extracts over an α8tβ1-AP affinity column and identified by microsequencing as VN (our unpublished results). We were not able to purify the proteins of ∼130 and 65–85 kDa (Figure 4, lane 1) with an α8tβ1-AP column in sufficient quantities for microsequencing or blotting with OPN antibodies. In addition, the OPN antibodies that were available immunoprecipitated OPN inefficiently; so we could not immunodeplete OPN from extracts. However, using anti-OPN immunoprecipitates from the E16.5 kidney extract, a band in the expected size range for OPN was detected in far Western blots (Figure 4, lane 3), indicating that OPN in the extract can be recognized by α8tβ1-AP and that OPN may be among the proteins of 65- to 85-kDa bands detected in the E13.5 embryo and E16.5 kidney extracts. The data prompted us to determine whether there are interactions between purified OPN and integrin α8β1. The data presented below clearly show that OPN is a novel ligand for integrin α8β1, and the 80-kDa protein observed in far Western blots may correspond to OPN.
Figure 4.
RGD-dependent binding of α8tβ1-AP to proteins including OPN in mouse embryo extract. Fifty micrograms of protein per lane of the extract from E13.5 embryo (lanes 1, 2, and 4–6) and immunoprecipitates from E16.5 kidney extract with OPN antiserum pp69 (lane 3) were separated electrophoretically on a 7.5% polyacrylamide gel in reducing conditions and analyzed by far Western (lanes 1–3) and Western (lanes 4–6) blotting. For far Western blotting of the E13.5 extract, membrane strips were incubated with α8tβ1-AP in the absence (lane 1) or presence (lane 2) of 50 μg/ml GRGDSP peptide. For Western blotting, the membrane strips were probed with anti-FN IgG (lane 4), anti-VN IgG (lane 5), or anti-OPN mAb MPIIIB10 (lane 6). The integrin-AP chimera bound to protein bands of 240, 130, and 65–85 kDa in the extract (lane 1) and ∼80 kDa in the anti-OPN immunoprecipitates (lane 3). This 80-kDa band comigrates with the band detected by OPN Western blot (lane 6).
OPN is a Novel Ligand for Integrin α8β1
The colocalization and far Western studies described above suggest that OPN is an additional ligand for integrin α8β1. To pursue this possibility, we analyzed interactions of α8tβ1-AP with purified recombinant GST-OPN and native human OPN in a solid-phase binding assay (Figure 5, A and B). The α8tβ1-AP bound to GST-OPN and native OPN (Figure 5A) and the binding was specific, as it was inhibited by an RGDS-containing peptide and a function blocking mAb against the β1 subunit (Figure 5B). Binding of α8tβ1-AP to purified, bacterially expressed GST-OPN was stronger than binding to purified human OPN; this suggests that the α8tβ1-AP binding site within the purified human OPN may have been partially masked, for example, by posttranslational modification, or that the preparation may have only been partially active (see DISCUSSION).
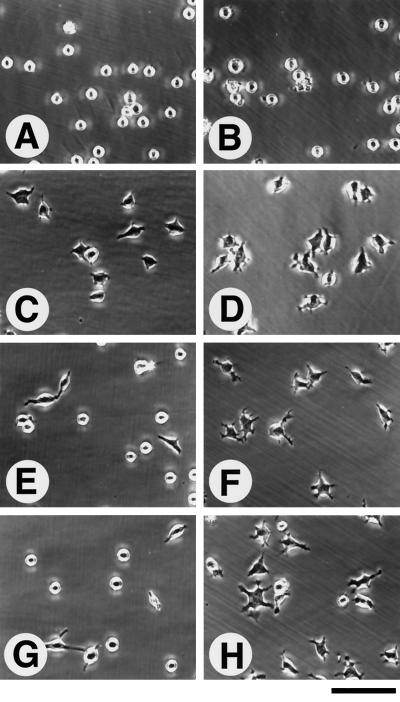
To confirm that OPN binds to integrin α8β1, we performed cell adhesion assays with K562 and KA8 cells on GST-OPN and purified OPN. KA8 cells were obtained by transfecting K562 cells with an α8 cDNA expression vector (Müller et al., 1995). KA8 cells express integrin α8β1 and α5β1, whereas K562 cells express only α5β1 as major integrin receptors. KA8 and K562 cells also express low amounts of αv-containing integrins (e.g. Müller et al., 1995). Interaction of both cell lines with integrin ligands such as FN has been shown to be dependent on prior activation of the integrin by activating antibodies or by Mn2+ (Müller et al., 1995). KA8 cells but not K562 cells adhered strongly to OPN in the presence of Mn2+ (Figure 5C). Neither Mg2+ nor Ca2+ promoted KA8 cell adhesion to OPN in the absence of Mn2+ (our unpublished results). Adhesion of KA8 cells was inhibited by an RGDS-containing peptide and by a function-blocking anti-β1 mAb but not by other integrin antibodies tested (Figure 5D). In addition to adhesion, KA8 cells spread on GST-OPN and purified OPN as efficiently as on FN (Figure 6). The parent K562 cells did not spread on either substrate.
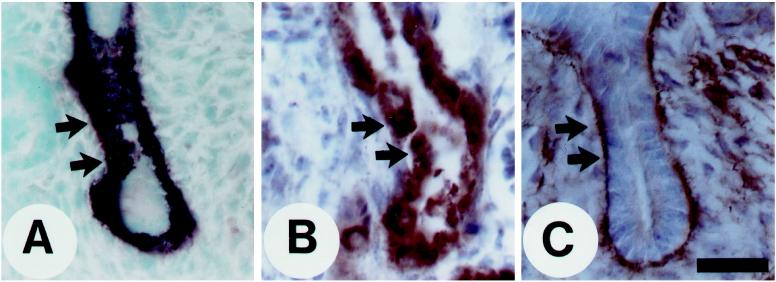
Figure 6.
Cell-spreading assay. In the presence of 1 mM MnCl2, K562 cells (A, C, E, and G) and KA8 cells (B, D, F, and H) were plated onto different substrata: (A and B) BSA; (C and D) FN (coated at 5 μg/ml); (E and F) purified native OPN (5 μg/ml); and (G and H) recombinant GST-OPN (5 μg/ml). Note that both cell types spread on FN, but only KA8 cells spread on native OPN and recombinant GST-OPN. Bar, 100 μm.
To exclude that integrin α8β1 interacts nonspecifically with purified proteins that contain an RGD motif, we tested interactions of α8tβ1-AP and KA8 cells with purified entactin and recombinant entactin fragments that contain an RGD site (Figure 5A and our unpublished results). We did not observe any interactions, further supporting that binding to OPN was specific. We thus conclude that OPN is a ligand for the integrin α8β1.
DISCUSSION
In the present study, we describe methodological approaches that should be of general usefulness for characterizing interactions between integrins and their ligands in situ and in vitro. With this method, we have identified OPN as a novel ligand for integrin α8β1. We demonstrate that the α8β1 integrin, OPN, and α8β1 ligands show partially overlapping expression patterns within the developing kidney. This raises the possibility that developmental defects in early stages of kidney morphogenesis observed in integrin α8β1-deficient mice (Müller et al., 1997) may be a consequence, at least in part, of disrupted interactions between α8β1 and OPN. In agreement, it has recently been shown that kidney morphogenesis is disrupted in organ cultures by antibodies to OPN and by RGD peptides (Rogers et al., 1997), which would be expected to block interactions of integrin α8β1 and OPN.
Our data demonstrating an interaction between the α8β1 integrin and OPN is in apparent disagreement with previous data of Schnapp et al. (1995), who reported that human kidney-derived 293 cells transfected with a human α8 cDNA (and therefore expressing the human α8β1 heterodimer) did not bind detectably to this ligand, although they did bind to other α8β1 ligands. As one possible explanation, the ligand-binding properties of several integrin receptors has been shown to vary, depending on cell type (reviewed by Hynes, 1992). It is also possible that differences in assay conditions or differences between the human α8β1 and the mouse and chick–human chimeric α8β1 used in the present article explain these apparent discrepancies. In addition, Schnapp et al. (1995) performed their assay in the presence of Mg2+ and Ca2+, although Ca2+ has been reported to inhibit the binding of αv integrins to OPN (Hu et al., 1995). Our data show convincingly that in some cells and after purification integrin α8β1 can interact with OPN.
Interactions of α8tβ1-AP with GST-OPN prepared by expression in bacteria were stronger than interactions with OPN purified from human urine. Surprisingly, no such difference was observed in cell adhesion assays. As one possible explanation, the binding site within native OPN but not GST-OPN may have been partially masked, preventing strong interactions between the purified integrin and native OPN. Cellular integrin α8β1 may in contrast exhibit cooperative interactions, strengthening cell binding to native OPN. Masking of integrin binding sites in native OPN may reflect conformational differences between native OPN and GST-OPN, posttranslational modifications of the former, or both. Native OPN is known to be highly phosphorylated, which could potentially modify the affinity of integrin binding sites (Shiraga et al., 1992). Nevertheless, the fact that both the purified integrin and the same integrin expressed on the surface of K562 cells bind to purified OPN and to GST-OPN argues that these interactions are specific; the inhibition of these interactions by an anti-integrin β1 mAb and an RGD-containing peptide indicates that the interactions are mediated by the integrin ligand binding site. The possible importance of posttranslational modifications of OPN for regulating integrin interactions in vivo is an interesting subject for future investigation.
Soluble integrin-AP chimera should be generally useful for detecting endogenous ligands in situ on the surface of isolated cells and in tissue sections. Integrin-AP chimeras are easy to prepare and appear to be more sensitive and specific probes of ligand receptor interactions than biotinylated or iodinated integrins (e.g., compare data in this article to data of DeFreitas et al., 1995). Fusion of the C terminus of the β1 subunit with AP does not appear to affect ligand–receptor interactions, because we have detected interactions of the chimera with the previously described ligands for this integrin. Binding of α8tβ1-AP requires integrin activation and is inhibited by an anti-β1 mAb or peptides containing the RGDS sequence. The specificity of binding observed in these assays appears to be identical to the specificity observed in cell adhesion assays using K562 cells modified by transfection to express the integrin α8β1 heterodimer. The integrin-AP chimera are also useful to identify and characterize novel integrin ligands. We show that integrin chimera can be used to map ligand distributions in situ and that potential ligands can be identified in far Western blots after biochemical fractionation. Such blots have the potential to reveal the repertoire of integrin ligands in different tissues. Combined with simple biochemical fractionation procedures, such blots can indicate whether potential ligands are associated with the ECM or cell membrane. As described in this article for FN, the identifies of candidate ligands in tissue extracts can be confirmed by immunodepletion of extracts before far Western blotting.
In our previous analysis of function of integrin α8β1 during murine development, we have shown that this integrin is strongly expressed in the metanephric mesenchyme but not the ureteric bud or epithelial tubules within the developing kidney (Müller et al., 1997). We further showed that it is critically important both for initial ingrowth of the ureteric bud and for subsequent formation of epithelial structures from the metanephric mesenchyme. We show here that histochemical staining of sections of embryonic mouse tissues with α8tβ1-AP reveals several interesting sites where ligands for integrin α8β1 are concentrated, including the surface of the ureteric epithelium within the developing kidney and areas where condensing mesenchyme is forming bone. The distribution of ligands in areas of bone and kidney formation has striking similarities to the previously described distribution of OPN, and integrin–ligand interactions were inhibited completely by RGD peptides; however, we were not able to demonstrate directly that the staining pattern obtained with α8tβ1-AP was due to OPN. This would require an antibody that inhibits effectively interactions of integrins with the RGD site in murine OPN. We do not have such an antibody available. Consequently, it remains to be determined whether binding of this integrin chimera to sections of developing bone and kidney is mediated primarily by OPN or reflects the presence also of additional ligands. For example, in bone matrix, there are several proteins that contain an RGD sequence such as type I collagen, thrombospondin, FN, bone sialoprotein, TN-C, VN, and OPN (Mackie and Tucker, 1992; Grzesik et al., 1994). The detection of additional bands in far Western blots using this integrin suggests that there are additional, uncharacterized ligands.
In the present study we show that purified OPN binds integrin α8β1 and that OPN protein is appropriately localized within the developing kidney to mediate essential functions of this integrin during kidney organogenesis. Rogers et al. (1997) have recently shown by in situ hybridization that the OPN gene is expressed within the developing ureter. We have shown that integrin α8β1 is expressed in the mesenchyme surrounding the ureter and that ureter development is defective in α8β1-deficient mice (Müller et al., 1997). Thus, OPN is expressed in cells that are defective in α8β1-deficient animals, suggesting that it may mediate α8β1 functions on these cells during development. In agreement, Rogers et al. (1997) demonstrate that block of OPN activity with anti-OPN antibodies leads to disruption of kidney tubulogenesis in organ culture. RGD peptides that inhibit ligand–receptor interactions of several integrins, including interactions of integrin α8β1 with OPN, lead to a similar block in kidney morphogenesis (Rogers et al., 1997). This raises the possibility that the effects of RGD peptide in these organ cultures are consequences of blocking interactions of integrin α8β1 with OPN. Surprisingly, mice carrying a targeted mutation in the OPN gene are viable, and a preliminary analysis suggests that kidney development progresses normally (Hogan, personal communication). This suggests that other integrin α8β1 ligands, such as FN, TN-C, VN, or yet to be identified proteins, may substitute for the absence of OPN in OPN knock-out mice and may also help mediate essential functions of α8β1 during normal kidney organogenesis.
OPN has been identified as a major protein constituent of bone- and cartilage-associated ECM, where it is expressed by osteoblasts, osteoclasts, and hypertrophic chondrocytes (Denhardt and Guo, 1993). Thus it may function in bone morphogenesis and remodeling. The importance in bone morphogenesis and remodeling of interactions of the integrin α8β1 with OPN remains uncertain. Analysis of mice lacking integrin α8β1 or OPN has failed to reveal any deficits in bone development (Müller, unpublished observations; Hogan, personal communication). There are several reports of β1 integrins with unidentified α subunits expressed by developing bone or cultured osteoblasts (Clover et al., 1992; Grano et al., 1994). By immunoprecipitation and immunofluorescence, expression of α8β1 has been detected in rat embryonic calvarial bone (Damsky, personal communication). Using paraffin sections, though, we have not seen prominent expression of this integrin at areas of primary bone formation in E13.5 and older mouse embryos. On balance, these data suggest that interactions of α8β1 with OPN will prove to modulate, but not be essential for, bone morphogenesis in some physiological circumstances.
In summary, findings in this article have identified a novel ligand for the integrin α8β1 and suggest strongly that integrin-AP chimeras will be generally applicable for characterizing interactions of integrins with known ligands and for identifying additional receptor–ligand interactions with important physiological or developmental roles. It will be important in the future to establish whether OPN indeed mediates essential functions of integrin α8β1 in the kidney, whether other known or unknown ligands are involved, and whether any of these ligands can substitute for lack of OPN in OPN-deficient animals.
ACKNOWLEDGMENTS
We thank Drs. J.W. Smith, J.R. Hoyer, R. Timpl, A.E. Chung, B.B. Mukherjee, R.A. Saavedra, R.O. Hynes, D. Seiffert, D.L. Mendrick, D. Vestweber, C.H. Damsky, S. Tominaga, and J.G. Flanagan for kindly providing reagents used in this study. We thank Dr. C.W. Turck for peptide microsequencing. We thank Drs. Amanda Littlewood and Dean Evans for help with analyzing bone sections. This work was supported by United States Public Health Service grant P01-16033 and by the Howard Hughes Medical Institute. L.F.R. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Abbreviations used: AP, alkaline phosphatase; E, embryonic day; ECM, extracellular matrix; FN, fibronectin; FN120, 120-kDa fragment of FN; GST, glutathione S-transferase; mAb, monoclonal antibody; OPN, osteopontin; PMSF, phenylmethylsulfonyl fluoride; TBS, Tris-buffered saline; TN-C, tenascin-C; VN, vitronectin.
REFERENCES
- Aufderheide E, Chiquet-Ehrismann R, Ekblom P. Epithelial-mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchyme. J Cell Biol. 1987;105:599–608. doi: 10.1083/jcb.105.1.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy B, Bossy-Wetzel E, Reichardt LF. Characterization of the integrin alpha 8 subunit: a new integrin beta 1-associated subunit, which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J. 1991;10:2375–2385. doi: 10.1002/j.1460-2075.1991.tb07776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang Q, McCulloch CAG, Sodek J. Immunohistochemical localization of bone sialoprotein in foetal procine bone tissues: comparisons with secreted phosphoprotein 1 (SPP-1, osteopontin) and SPARC (osteonectin) Histochem J. 1991;23:281–289. doi: 10.1007/BF01045047. [DOI] [PubMed] [Google Scholar]
- Cheng H-J, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Clover J, Dodds RA, Gowen M. Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 1992;103:267–271. doi: 10.1242/jcs.103.1.267. [DOI] [PubMed] [Google Scholar]
- DeFreitas MF, Yoshida CK, Frazier WA, Mendrick DL, Kypta RM, Reichardt LF. Identification of integrin alpha 3 beta 1 as a neuronal thrombospondin receptor mediating neurite outgrowth. Neuron. 1995;15:333–343. doi: 10.1016/0896-6273(95)90038-1. [DOI] [PubMed] [Google Scholar]
- Denda S, Müller U, Crossin KL, Erickson HP, Reichardt LF. Utilization of a soluble integrin-alkaline phophatase chimera to characterize integrin alpha 8 beta 1 receptor interactions with tenascin: murine alpha 8 beta 1 binds to the RGD site in tenascin-C fragments, but not to native tenascin-C. Biochemistry. 1998;37:5464–5474. doi: 10.1021/bi9727489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt DT, Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- Ekblom P. Formation of basement membranes in the embryonic kidney: an immunohistological study. J Cell Biol. 1981;91:1–10. doi: 10.1083/jcb.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JG, Leder P. The kit ligand: a cell surface molecule altered in steel mutant fibroblasts. Cell. 1990;63:185–194. doi: 10.1016/0092-8674(90)90299-t. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburm H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Gorski JP, Griffin D, Dudley G, Stanford C, Thomas R, Huang C, Lai E, Karr B, Solursh M. Bone acidic glycoprotein-75 is a major synthetic product of osteoblastic cells and localized as 75- and/or 50-kDa forms in mineralized phases of bone and growth plate and in serum. J Biol Chem. 1990;265:14956–14963. [PubMed] [Google Scholar]
- Grano M, Zigrino P, Colucci S, Zambonin G, Trusolino L, Serra M, Baldini N, Teti A, Marchisio PC, Zallone AZ. Adhesion properties and integrin expression of cultured human osteoclast-like cells. Exp Cell Res. 1994;212:209–218. doi: 10.1006/excr.1994.1136. [DOI] [PubMed] [Google Scholar]
- Grzesik WJ, Robey PG. Bone matrix RGD glycoproteins: immunolocalization and interaction with human primary osteoblastic bone cells in vitro. J Bone Miner Res. 1994;9:487–496. doi: 10.1002/jbmr.5650090408. [DOI] [PubMed] [Google Scholar]
- Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu DD, Hoyer JR, Smith JW. Ca2+ suppresses cell adhesion to osteopontin by attenuating binding affinity for integrin alpha v beta 3. J Biol Chem. 1995;270:9917–9925. doi: 10.1074/jbc.270.17.9917. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timple R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci USA. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie EJ, Tucker RP. Tenascin in bone morphogenesis: expression by osteoblasts and cell type-specific expression of splice variants. J Cell Sci. 1992;103:765–771. doi: 10.1242/jcs.103.3.765. [DOI] [PubMed] [Google Scholar]
- Mark MP, Buttler WT, Prince CW, Finkelman RD, Ruch J-V. Developmental expression of 44-kDa bone phosphoprotein (osteopontin) and bone γ-carboxyglutamic acid (Gla)-containing protein (osteocalcin) in calcifying tissues of rat. Differentiation. 1988;37:123–136. doi: 10.1111/j.1432-0436.1988.tb00804.x. [DOI] [PubMed] [Google Scholar]
- Mendrick DL, Kelly DM. Temporal expression of VLA-2 and modulation of its ligand specificity by rat glomerular epithelial cells in vitro. Lab Invest. 1993;69:690–702. [PubMed] [Google Scholar]
- Müller U, Bossy B, Venstrom K, Reichardt LF. Integrin alpha 8 beta 1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–448. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha 8 beta 1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–613. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemir M, DeVouge MW, Mukherjee BB. Normal rat kidney cells secrete both phosphorylated and nonphosphorylated forms of osteopontin showing different physiological properties. J Biol Chem. 1989;264:18202–18208. [PubMed] [Google Scholar]
- Rogers SN, Padanilam BJ, Hruska KA, Giachelli CM, Hammerman MR. Metanephric osteopontin regulates nephrogenesis in vitro. Am J Physiol. 1997;272:F469–F476. doi: 10.1152/ajprenal.1997.272.4.F469. [DOI] [PubMed] [Google Scholar]
- Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Sariola H, Aufderheide E, Bernhard H, Henke-Fahle S, Dippold W, Ekblom P. Antibodies to cell surface ganglioside Gd3 perturb inductive epithelial-mesenchymal interactions. Cell. 1988;54:235–245. doi: 10.1016/0092-8674(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J Biol Chem. 1995;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- Seiffert D. Detection of vitronectin in mineralized bone matrix. J Histochem Cytochem. 1996;44:275–280. doi: 10.1177/44.3.8648088. [DOI] [PubMed] [Google Scholar]
- Seiffert D, Iruela-Arispe ML, Sage EH, Loskutoff DJ. Distribution of vitronectin mRNA during murine development. Dev Dyn. 1995;203:71–79. doi: 10.1002/aja.1002030108. [DOI] [PubMed] [Google Scholar]
- Shiraga H, et al. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci USA. 1992;89:426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A. Integrins and their ligands. Curr Top Microbiol Immunol. 1993;184:7–35. doi: 10.1007/978-3-642-78253-4_2. [DOI] [PubMed] [Google Scholar]
- Stern DN, Glimcher MJ, Saavedra RA. Localization of osteopontin during mouse development. Ann NY Acad Sci. 1995;760:367–370. doi: 10.1111/j.1749-6632.1995.tb44659.x. [DOI] [PubMed] [Google Scholar]
- Talts JF, Eng H, Zhang H-Y, Faissner A, Ekblom P. Characterization of monoclonal antibodies against tenascin-C: no apparent effect on kidney development in vitro. Int J Dev Biol. 1997;41:39–48. [PubMed] [Google Scholar]
- Varnum-Finney B, Venstrom K, Müller U, Kypta R, Backus C, Chiquet M, Reichardt LF. The integrin receptor alpha 8 beta 1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfsberg TG, Primakoff P, Myles DG, White JM. ADAM, a novel family of membrane proteins containing a disintegrin and metalloprotease domain: multipotential functions in cell-cell and cell-matrix interactions. J Cell Biol. 1995;131:275–278. doi: 10.1083/jcb.131.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Zheng X, Saunders TL, Camper SA, Samuelson LC, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proc Natl Acad Sci USA. 1995;92:12426–12430. doi: 10.1073/pnas.92.26.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]