Abstract
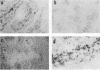
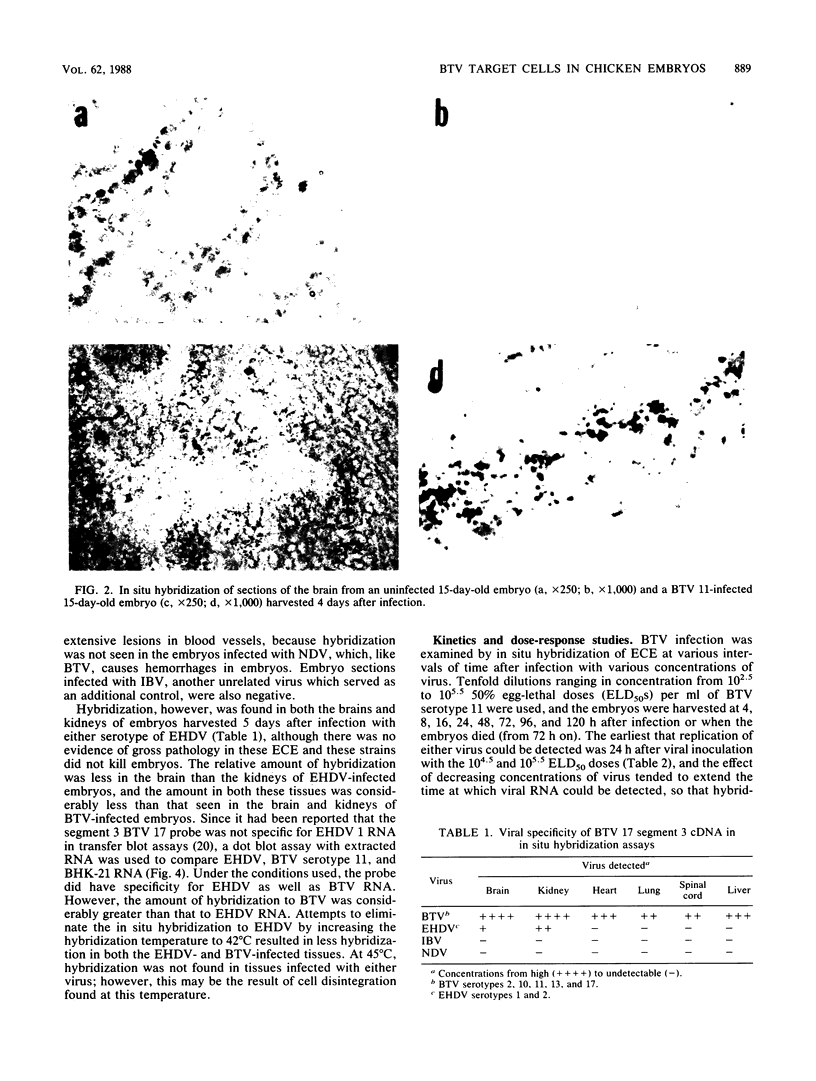
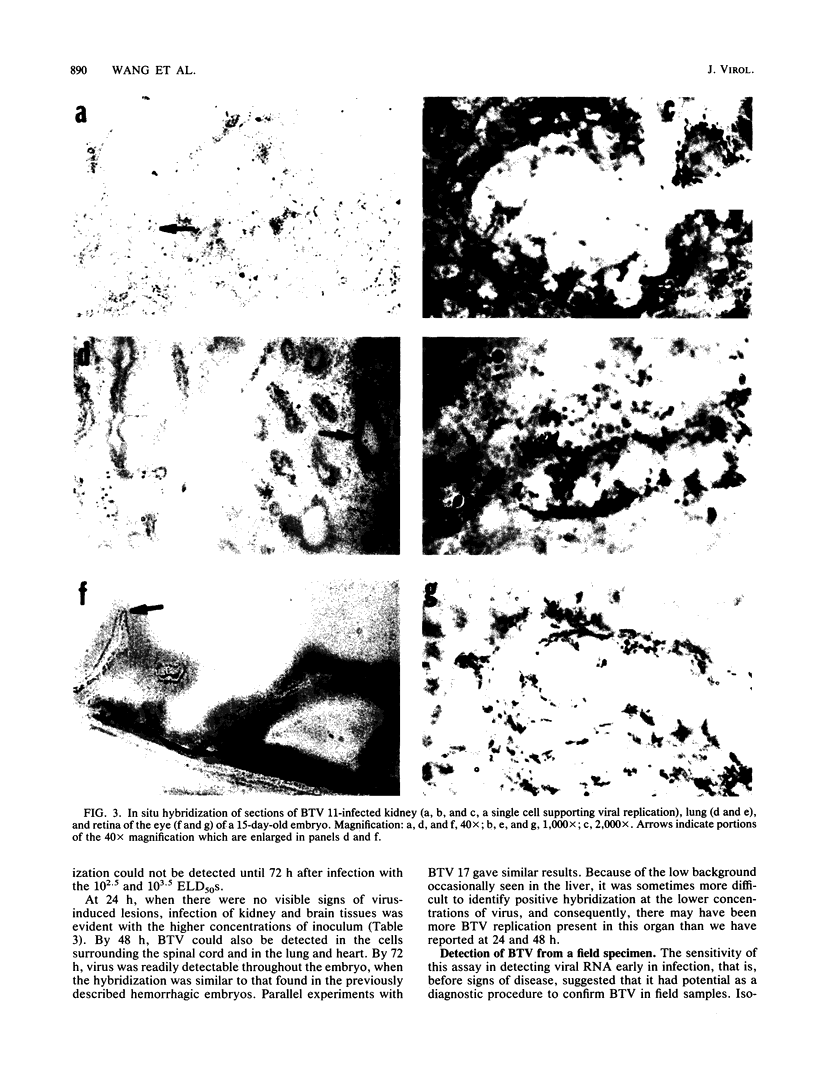
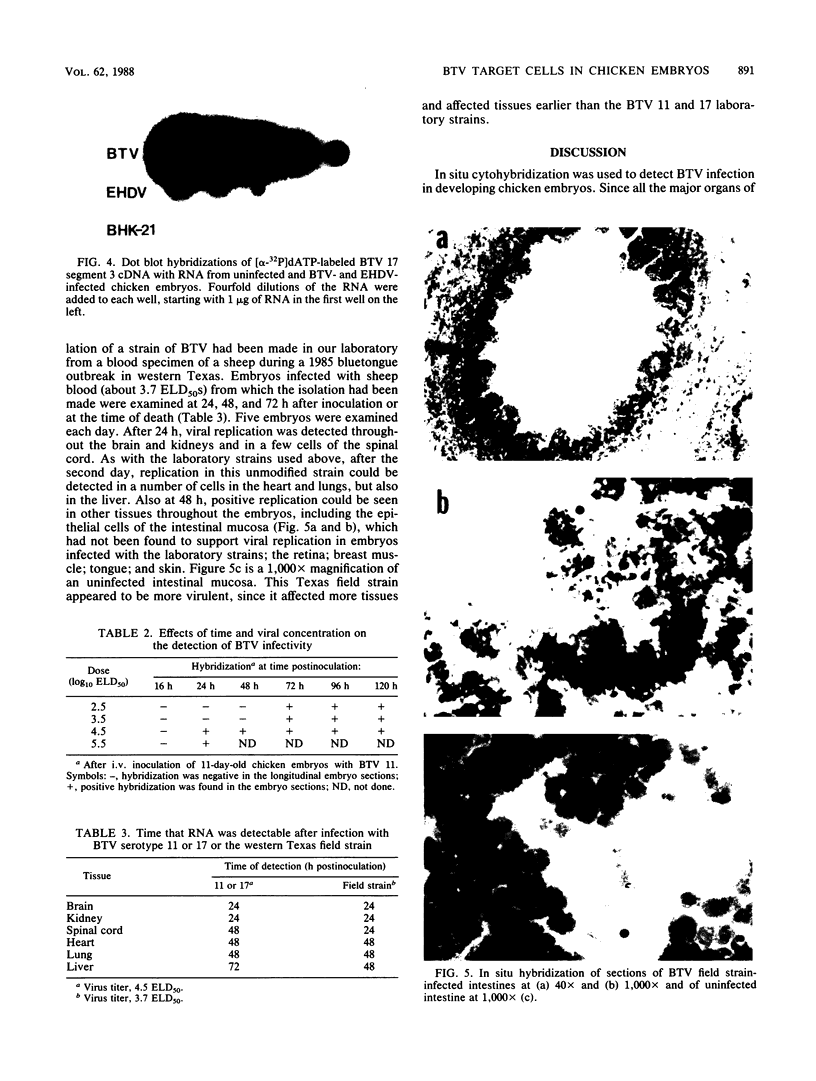
In situ cytohybridization was used to determine the tissue tropism and target cells for replication of bluetongue virus (BTV) in the developing chicken embryo. Hybridization with a biotinylated probe specific for segment 3 of BTV serotype 17 detected viral replication in embryos inoculated with U.S. serotypes 2, 10, 11, 13, and 17 or sheep blood containing a BTV field strain. At the final stages of infection, when the embryos were hemorrhagic, viral infection could consistently be detected in the brain, kidney, spinal cord, heart, lung, and liver, with the brain and kidney most severely affected. Other tissues, such as the retina, skin, tongue, and intestinal villi, also supported viral replication in some embryos. Greater concentrations of virus tended to be localized within epithelial cells, such as those lining the kidney tubules and tertiary bronchi of the lungs. Kinetics studies with BTV serotypes 11 and 17 and a field strain indicated that within 24 h after inoculation, viral replication occurred initially in the brain and kidney. By 48 h, viral replication was also detected in the lungs, heart, and spinal cord, with the liver being severely infected by 72 h. Low levels of hybridization could be detected in embryos infected with epizootic hemorrhagic disease virus, which is antigenically related to BTV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caskey C. T. Disease diagnosis by recombinant DNA methods. Science. 1987 Jun 5;236(4806):1223–1229. doi: 10.1126/science.3296189. [DOI] [PubMed] [Google Scholar]
- Collisson E. W., Barber T. L. Isolation and identification of bluetongue virus: a serotype new to the U.S. Prog Clin Biol Res. 1985;178:319–327. [PubMed] [Google Scholar]
- Dunn D. C., Blair C. D., Ward D. C., Beaty B. J. Detection of bovine herpesvirus-specific nucleic acids by in situ hybridization with biotinylated DNA probes. Am J Vet Res. 1986 Apr;47(4):740–746. [PubMed] [Google Scholar]
- Foster N. M., Leudke A. J. Direct assay for bluetongue virus by intravascular inoculation of embryonating chicken eggs. Am J Vet Res. 1968 Mar;29(3):749–753. [PubMed] [Google Scholar]
- Gould A. R. The complete nucleotide sequence of bluetongue virus serotype 1 RNA3 and a comparison with other geographic serotypes from Australia, South Africa and the United States of America, and with other orbivirus isolates. Virus Res. 1987 Apr;7(2):169–183. doi: 10.1016/0168-1702(87)90078-5. [DOI] [PubMed] [Google Scholar]
- Huismans H., Bremer C. W., Barber T. L. The nucleic acid and proteins of epizootic haemorrhagic disease virus. Onderstepoort J Vet Res. 1979 Jun;46(2):95–104. [PubMed] [Google Scholar]
- Jochim M. M., Jones S. C. Plaque neutralization of bluetongue virus and epizootic hemorrhagic disease virus in BHK21 cells. Am J Vet Res. 1976 Nov;37(11):1345–1347. [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedke A. J., Jochim M. M., Jones R. H. Bluetongue in cattle: effects of Culicoides variipennis-transmitted bluetongue virus on pregnant heifers and their calves. Am J Vet Res. 1977 Nov;38(11):1687–1695. [PubMed] [Google Scholar]
- MacLachlan N. J., Osburn B. I. Bluetongue virus-induced hydranencephaly in cattle. Vet Pathol. 1983 Sep;20(5):563–573. doi: 10.1177/030098588302000508. [DOI] [PubMed] [Google Scholar]
- Osburn B. I., Johnson R. T., Silverstein A. M., Prendergast R. A., Jochim M. M., Levy S. E. Experimental viral-induced congenital encephalopathies. II. The pathogenesis of bluetongue vaccine virus infection in fetal lambs. Lab Invest. 1971 Sep;25(3):206–210. [PubMed] [Google Scholar]
- Osburn B. I., Silverstein A. M., Prendergast R. A., Johnson R. T., Parshall C. J., Jr Experimental viral-induced congenital encephalopathies. I. Pathology of hydranencephaly and porencephaly caused by bluetongue vaccine virus. Lab Invest. 1971 Sep;25(3):197–205. [PubMed] [Google Scholar]
- Purdy M., Petre J., Roy P. Cloning of the bluetongue virus L3 gene. J Virol. 1984 Sep;51(3):754–759. doi: 10.1128/jvi.51.3.754-759.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Ritter G. D., Jr, Akashi H., Collisson E., Inaba Y. A genetic probe for identifying bluetongue virus infections in vivo and in vitro. J Gen Virol. 1985 Jul;66(Pt 7):1613–1619. doi: 10.1099/0022-1317-66-7-1613. [DOI] [PubMed] [Google Scholar]
- Thomas F. C., Trainer D. O. Bluetongue virus in white-tailed deer. Am J Vet Res. 1970 Feb;31(2):271–278. [PubMed] [Google Scholar]
- Verwoerd D. W. Purification and characterization of bluetongue virus. Virology. 1969 Jun;38(2):203–212. doi: 10.1016/0042-6822(69)90361-4. [DOI] [PubMed] [Google Scholar]