Abstract
This paper describes an electroactive substrate that displays two independent dynamic functions for controlling the adhesion of cells. The approach is based on self assembled monolayers on gold that are patterned into regions presenting the Arg-Gly-Asp peptide cell adhesion ligand. The patterned regions differ in the electrochemical properties of the linkers that tether the peptides to the monolayer. In this work, three distinct chemistries are employed, which provide for release of the ligand on application of a negative potential, release of the ligand on application of a positive potential, and no change in response to a potential, respectively. Cells were allowed to attach to a monolayer patterned into circular regions comprising the three chemistries. Treatment with electric potentials of 650 mV or −650 mV resulted in selective release of adherent cells only from regions that display the relevant electroactive groups. This example establishes the preparation of dynamic substrates with multiple functions and will be important to preparing model cultures derived from multiple cell types, with control over the temporal interactions of each cell population.
Introduction
The development of strategies for controlling the interface between materials and adherent cells remains an important challenge in materials science.1, 2 Surfaces that are tailored to influence the behaviors of cells are important in cell-based sensors, drug screening and in fundamental studies of cell migration.3–5 We have introduced an approach for creating dynamic substrates—based on self-assembled monolayers that present electroactive groups—wherein the activities of immobilized ligands can be switched on and off in response to applied potentials.5–12 These methods give real-time control over the molecular interactions that mediate the adhesion of cells and, together with related strategies using polymeric substrates, give unprecedented control over assembling and manipulating the positions of one or more cell types on a common substrate.13–15 This paper extends on previous work by demonstrating a monolayer that is patterned into distinct regions that present cell adhesive ligands that are tethered to the monolayer by way of different redox-active tethers. By using tethers that respond to positive or negative applied potentials, respectively, the adhesion of different populations of cells can be manipulated independently.[9] Further, we report the design and synthesis of alkanethiol reagents that incorporate these electroactive moieties and that are substituted with a maleimide group. These reagents permit a broad class of biologically-active ligands to be immobilized to a monolayer and thereby increase the scope of applications that can be addressed with dynamic substrates. The work that follows in this paper concerns the application of the patterned monolayers to control the adhesion of cells.
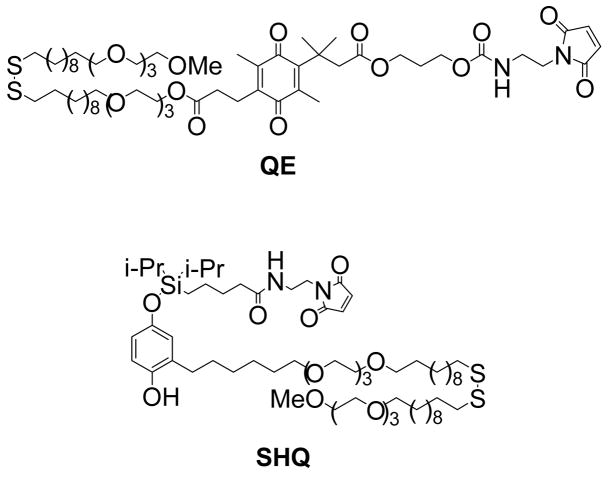
Our approach utilizes self-assembled monolayers (SAMs) on gold that incorporate alkanethiolates terminated in electroactive moieties that respond to electrical potentials by releasing attached ligands (Figure 1). We prepare monolayers from alkanethiolates that incorporate the electroactive groups and maleimide groups, which can be used to immobilize ligands. In this way, we introduce the ligands after the monolayer has been assembled.16 The tri(ethylene glycol) groups of the monolayer serve to prevent the non-specific attachment of cells to the monolayer.17 The demonstration in this paper is based on two electroactive groups that release the tethered ligands in response to either reductive or oxidative potentials (Chart 1). In the first case, an electroactive quinone ester (QE) undergoes reduction to give the corresponding hydroquinone, which then undergoes a cyclization reaction to give a lactone with release of the ligand (Figure 1A). In the second case, an O-silyl hydroquinone (SHQ) undergoes electrochemical oxidation to give a benzoquinone, with hydrolysis of the silyl ether and selective release of ligand (Figure 1B). In all cases, we used as the ligand the peptide Cys-Gly-Arg-Gly-Asp-Ser (CGRGDS). This peptide contains the RGD sequence that serves as a ligand for receptor-mediated adhesion of cells.18 We have shown that monolayers that present this ligand support the adhesion and spreading of mammalian cells.19, 20
Figure 1.
Molecular strategies used to prepare dynamic substrates that can release tethered ligands in response to applied potentials. (A) A monolayer presenting a maleimide tethered to an electroactive quinone ester reacts with a cysteine-terminated RGD peptide to immobilize the ligand. Upon electrochemical reduction of the quinone to the corresponding hydroquinone, a cyclization reaction ensues to give a lactone with release of the RGD ligand. (B) A monolayer presenting a maleimide group tethered to an electroactive O-silyl hydroquinone is used to immobilize a cysteine-terminated RGD peptide, and undergoes electrochemical oxidation to give a benzoquinone, with hydrolysis of the silyl ether and selective release of RGD ligands.
Chart 1.
Results and Discussion
Synthesis
The synthesis of electroactive quinone ester QE started with intermediate 1, which was converted to ester 2 by coupling with the hydroxyl-terminated disulfide 10.21 The t-butyldimethylsilyl (TBS) protecting group was removed under acidic conditions to reveal the alcohol, which was then coupled to the maleimide with a Mitsunobu reaction to give QE (Scheme 1). Separately, 4 was treated with trifluoroacetic acid (TFA) and a triethylsilane scavenger to deprotect the thiol. The resulting thiol was coupled with activated disulfide 9 to give disulfide 5. The hydroquinone was treated with a chlorosilane reagent to provide 6, which was then reacted with the aminoethylmaleimide to provide SHQ (Scheme 2). To prepare intermediate 9, the terminal alcohol of 7 was methylated by treatment with sodium hydride followed by iodomethane. The trityl group was removed to provide thiol 8, which was then activated with Aldrithiol affording 9. Intermediate 10 was accessed by treatment of 9 with a tri(ethylene glycol)-terminated alkanethiol (Scheme 3).
Scheme 1.
Synthesis of electroactive quinone ester QE
Scheme 2.
Synthesis of electroactive O-silyl hydroquinone SHQ
Scheme 3.
Synthesis of intermediates 9 and 10
Characterization of Electrochemical Reactions
We prepared a self-assembled monolayer from a mixture of a QE-terminated and a tri(ethylene glycol)-terminated alkanethiols in a ratio of 1:3. The mass spectrum of this monolayer showed two major peaks at m/z 1134.5 and m/z 1548.3 (Figure 2A, top).22 These peaks correspond to the mixed disulfide derived from a tri(ethylene glycol)-terminated and a QE-terminated alkanethiolate, and a symmetric disulfide derived from the QE-terminated alkanethiolate, respectively. After treatment with the CGRGDS peptide (1mg/ml in phosphate buffered saline (PBS) at pH 7.4) for 30 min, the original peaks were absent and gave rise to new peaks corresponding to the peptide-conjugated products at m/z 1357.1 and m/z 1706.4 (Figure 2A, middle). An identical monolayer was subjected to an electrical potential of −650 mV (vs. Ag/AgCl reference) for 4 min and analyzed by mass spectrometry, which gave two peaks corresponding to electrochemical reaction products at m/z 982.0 and m/z 1242.0 (Figure 2A, bottom).
Figure 2.
Characterization of the immobilization and electrochemical release of RGD ligands using MALDI-ToF MS. (A) A monolayer presenting QE groups gave two molecular ion peaks corresponding to disulfides as shown above the peaks (top). After treatment with a CGRGDS solution, the original peaks were absent and gave rise to new peaks corresponding to the peptide-conjugated products (middle). The identical monolayer was treated with an electrical potential of −650 mV for 4 min and analyzed by mass, which gave two peaks corresponding to electrochemical reaction products. (B) The mass spectrum of the monolayer presenting SHQ groups displayed peaks at m/z 1236.2 and m/z 1752.6 corresponding to the SHQ containing disulfides (top). Treatment of the monolayer with the CGRGDS peptide gave new peaks at m/z 1457.9 and m/z 1806.9 corresponding to the peptide-conjugated products (middle). After application of an electrical potential of 650 mV for 4 min and −550 mV for 30 seconds, the original peaks disappeared and gave rise to a new peak corresponding to the hydroquinone-terminated disulfide at m/z 899.9 (bottom).
In a separate experiment, we prepared a monolayer presenting SHQ groups and repeated the same line of experiments. The mass spectrum of the initial monolayer displayed peaks at m/z 1236.2 and m/z 1752.6 corresponding to the SHQ containing disulfides (Figure 2A, top). Treatment of the monolayer with the CGRGDS peptide gave new peaks at m/z 1457.9 and m/z 1806.9 which corresponded to the peptide-conjugated products (Figure 2B, middle). Upon application of an electrical potential of 650 mV for 4 min and −550 mV for 30 seconds, the original peaks were absent and gave rise to a new peak that represents the hydroquinone-terminated disulfide at m/z 899.9 (Figure 2B, bottom).
Preparation of Electroactive Substrates
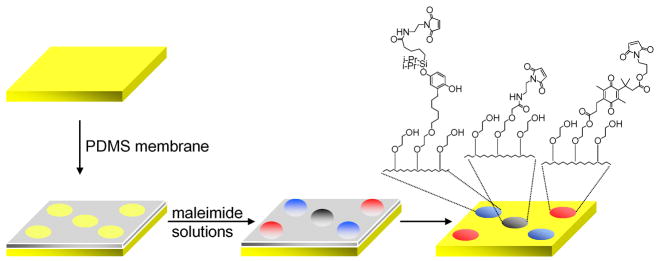
To demonstrate that these chemistries can be used to prepare a dynamic substrate that releases ligands—and therefore adherent cells—at two different potentials, we patterned a monolayer into several circular regions with the two electroactive alkanethiolates and a non-electroactive alkanethiolate, all of which present the RGD ligand for cell adhesion. We prepared a thin PDMS membrane with five holes (500 μm in diameter) and applied this structure to a gold-coated glass slide. Three different solutions of maleimide-containing disulfides (0.5 μL, mixed with tri(ethylene glycol)-terminated disulfide in a ratio of 1:200, total concentration of 0.2 mM in EtOH) were applied to the holes according to the scheme in Figure 3. After 5 minutes, the membrane was removed, and the monolayer was immersed in an ethanolic solution of tri(ethylene glycol)-terminated disulfide (1 mM) for 12 hr. The RGD peptide was then immobilized on this monolayer by treatment with a solution of CGRGDS 30 min. With this procedure, the initial assembly of the monolayers in the wells was not complete, but the final incubation in a solution containing the tri(ethylene glycol)-terminated disulfide resulted in complete monolayers. Further, we used disulfide reagents—and not the more common alkanethiols—because the latter are not compatible with the maleimide groups of the reagents.
Figure 3.
Preparation of an electroactive substrate combines two dynamic functions. A monolayer patterned into several regions was prepared as described in the text.
Selective Release of Adherent Cells
Swiss 3T3 fibroblast cells were harvested and allowed to attach to the patterned monolayer. An optical micrograph 2 hr after incubation showed that cells only adhered to the circular regions that present RGD ligands (Figure 4a). An electrical potential of 650 mV was applied to the monolayer and cells were efficiently released only from regions that present peptides tethered by way of the electroactive O-silyl hydroquinone groups (Figure 4b). In this experiment, the substrate was incubated for one hour after the electrochemical treatment and then the medium was exchanged prior to acquiring the image. A separate culture prepared in the same manner was subjected to an electrical potential of −650 mV and resulted in release of cells only from regions that present the electroactive quinone ester groups (Figure 4c). For this experiment, the cells were incubated for five minutes after application of the potential, after which the medium was exchanged and the image acquired. In both cases, the monolayers were not rinsed or otherwise treated to assist detachment of the cells. Subsequent application of an electrical potential of −650 mV to the monolayer of panel b or 650 mV to the monolayer of panel c caused additional release of cells from regions that present remaining electroactive groups (Figure 4d). In both cases, cells remained adherent to the monolayer only at the regions presenting the non-electroactive RGD ligands. This example illustrates that the electrochemical strategies can be used to release cells selectively and non-invasively.
Figure 4.
Demonstration of selective release of adherent cells under electrochemical control. An optical micrograph showed that Swiss 3T3 fibroblast cells adhered to the circular regions that presented RGD ligands (a). After treatment with an electrical potential of 650 mV, cells were efficiently released only from regions presenting electroactive O-silyl hydroquinone (b), while an electrical potential of −650 mV resulted in release of cells only from regions presenting electroactive quinone ester (c). Subsequent application of an electrical potential of −650 mV to the monolayer of panel b and of 650 mV to the monolayer of panel c caused additional release of cells from regions that present remaining electroactive groups (d). Note that the image shown in panel c was taken from a separate experiment.
This paper emphasizes the development and combination of surface chemistries that provide for orthogonal control of the presentation of two ligands on a monolayer. By utilizing electrochemically-active tethers that respond to either positive or negative potentials, it is possible to trigger the release of either ligand without manipulating the second ligand (even in cases where the second ligand is structurally identical to the first). We used relatively simple PDMS templates to pattern the assembly of monolayers in regions that are 500 μm in size. The PDMS templates can be made to have smaller regions, but at sizes below 200 μm it is difficult to manually apply solutions of alkanethiols to the wells. For the preparation of surfaces that are patterned with different monolayers at smaller scales, the use of dip pen nanolithography 23 or of microfluidic flow cells 24 represent effective approaches. Finally, the electroactive alkanethiol reagents described in this paper are substituted with maleimide groups and therefore provide a general route towards the immobilization of a broad range of ligands, provided that the ligands can be prepared with a primary thiol functional group.
Conclusion
The development of dynamic substrates that can modulate the ligand-receptor interactions with adherent cells has brought new opportunities for fundamental studies of cell behavior and for integrating cells with microfabricated devices. The protein matrix to which cells adhere not only provides a structural scaffold for organizing cells into tissue but also provides cells with several molecular signals that are required to direct or maintain regulatory functions. The development of dynamic substrates has now made it possible to mimic the signal modulation from the protein matrix and therefore to bring these complex problems under study.25, 26 In applications, it is clear that materials that can modulate their biological properties will be important in the development of scaffolds for tissue engineering and perhaps for the surfaces of medical devices.27–28 Indeed, with the acceleration of research in stem cell biology, these active surfaces may prove instrumental in directing cell maturation.30 Other applications include the development of assays that require control over cell migration and the patterning of multiple cell types to build functional models of multicellular organs.
Experimental
Preparation of SAMs
Titanium (5 nm) and then gold (15 nm) were evaporated onto glass coverslips (25 mm × 25 mm) using an electron beam evaporator (Thermionics VE-100) at a rate of 0.2 ~ 0.4 nm/sec and at a pressure of 9 × 10−7 Torr. A thin PDMS membrane with five holes (500 μm in diameter) was placed on the gold-coated coverslip. Solutions of maleimide-terminated disulfides (0.5 μL, mixed with tri(ethylene glycol)-terminated disulfide in a ratio of 1:200, total concentration of 0.2 mM in EtOH) were applied to the monolayer. After 5 minutes, the membrane was removed, and the monolayer was immersed in an ethanolic solution of tri(ethylene glycol)-terminated disulfide (1 mM) for 12 hr. The monolayer was rinsed with ethanol, dried under a stream of nitrogen, and treated with a solution of the CGRGDS peptide(1mg/ml, in PBS, at pH 7.4) for 30 min. The monolayer was rinsed with deionized water and ethanol, and dried under a stream of nitrogen.
MALDI-ToF analysis
Mass analysis was performed using a Voyager DE-PRO Biospectrometry mass spectrometer (Applied Biosystems, Framingham, MA). A 337 nm nitrogen laser was used as a desorption-ionization source, and all the spectra were acquired with 20 kV accelerating voltage using reflector mode in positive ions with a 2,4,6-trihydroxyacetophenone (1 μl, 5 mg/ml in acetonitrile) as a matrix.
Cell culture
A PDMS cylinder (inner diameter of 130 mm, outer diameter of 220 mm, and height of 150 mm) was placed on the monolayer. 25,000 ~ 50,000 Swiss Albino 3T3 cells were seeded in the PDMS cylinder and all cultures were maintained at 37 °C in a humidified 5 % CO2 atmosphere. Cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) containing 10 % fetal bovine serum and 1 % penicillin/streptomycin.
Electrochemistry
Electrochemistry was performed with a Bioanalytical Systems CV-50W potentiostat using PBS (10 mM phosphate and 150 mM NaCl, pH 7.4) or cell culture media as the electrolyte. A custom-designed electrochemical cell was used for measurement with the monolayer as the working electrode, a Pt wire as the counter electrode, and an Ag/AgCl reference electrode.
3-{2,5-Dimethyl-3,6-dioxo-4-[tri(ethylene glycol)undecane disulfide]-cyclohexa-1,4-dienyl}-3-methyl-butyric acid 3-(tert-butyl-dimethyl-silanyloxy)-propyl ester (2)
To a solution of 3-[4-(2-Carboxy-ethyl)-2,5-dimethyl-3,6-dioxo-cyclohexa-1,4-dienyl]-3-methyl-butyric acid 3-(tert-butyl-dimethyl-silanyloxy)-propyl ester (1) (62 mg, 0.13 mmol) dissolved in CH2Cl2, were added disulfide 10 (84 mg, 0.13 mmol), EDC (27 mg, 0.13 mmol), and DMAP (2 mg). The reaction mixture was stirred for 2 h, washed with 10 ml of water and 10 ml of brine, and then dried over MgSO4. After concentration, the residue was purified by column chromatography (hex/EtOAc, 1:1) to give 46 mg (31 %) of adduct as pale yellow oil. 1H NMR (500MHz, CDCl3) δ 0.03 (s, 6H), 0.87 (s, 9H), 1.0–1.3 (m, 28H), 1.41 (s, 6H), 1.57 (quint, J = 7.2 Hz, 2H), 1.66 (quint, J = 7.4 Hz, 2H), 1.75 (quint, J = 6.25 Hz, 2H), 1.99 (s, 3H), 2.12 (s, 3H), 2.48 (t, J = 8.0 Hz, 2H), 2.67 (t, J = 7.3 Hz, 4H), 2.73 (t, J = 7.6 Hz, 2H), 2.95 (s, 2H), 3.38 (s, 3H), 3.40–3.65 (m, 32H), 4.05 (t, J = 6.4 Hz, 2H), 4.23 (t, J = 4.9 Hz, 2H).
3-{2,5-Dimethyl-3,6-dioxo-4-[tri(ethylene glycol)undecane disulfide]-cyclohexa-1,4-dienyl}-3-methyl-butyric acid 3-propyl ester (3)
The conjugate 2 (46 mg, 0.04 mmol) was dissolved in 10 ml of AcOH : THF : H2O (3 : 1 : 1). The reaction mixture was stirred overnight. Evaporation of solvent gave 40 mg (97 %) product 3 as a pale yellow oil. 1H NMR (500MHz, CDCl3) δ 1.0–1.3 (m, 28H), 1.41 (s, 6H), 1.57 (quint, J = 7.2 Hz, 2H), 1.66 (quint, J = 7.4 Hz, 2H), 1.78 (quint, J = 6.25 Hz, 2H), 1.99 (s, 3H), 2.12 (s, 3H), 2.48 (t, J = 8.0 Hz, 2H), 2.67 (t, J = 7.3 Hz, 4H), 2.72 (t, J = 7.6 Hz, 2H), 2.97 (s, 2H), 3.37 (s, 3H), 3.40–3.65 (m, 32H), 4.12 (t, J = 6.4 Hz, 2H), 4.22 (t, J = 4.9 Hz, 2H).
QE
To a solution of alcohol 3 in THF (5 ml), were added DEAD (2 μl), triphenylphosphine (3.5 mg), and maleimide (2 mg). The reaction mixture was stirred for 1 hr, and reduced to give an oil. Silica gel chromatography (hex/EtOAc, 1:1) gave 4 mg (34 %) of the adduct as pale yellow oil. 1H NMR (500MHz, CDCl3) δ 1.0–1.3 (m, 28H), 1.41 (s, 6H), 1.57 (quint, J = 7.2 Hz, 2H), 1.66 (quint, J = 7.4 Hz, 2H), 1.87 (quint, J = 6.25 Hz, 2H), 1.99 (s, 3H), 2.12 (s, 3H), 2.48 (t, J = 8.0 Hz, 2H), 2.67 (t, J = 7.3 Hz, 4H), 2.72 (t, J = 7.6 Hz, 2H), 2.97 (s, 2H), 3.37 (s, 3H), 3.40–3.65 (m, 32H), 3.96 (t, J = 6.4 Hz, 2H), 4.22 (t, J = 4.9 Hz, 2H), 6.70 (s, 2H).
2-(6-(2-(2-(2-(11-(2-(11-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)undecyl)disulfanyl)undecyloxy) ethoxy)ethoxy)ethoxy)hexyl)benzene-1,4-diol (5)
To a solution of 2-(6-(2-(2-(2-(11-(tritylthio) undecyloxy) ethoxy) ethoxy) ethoxy) hexyl) benzene-1,4-diol (4) (167mg, 0.22 mmol) in 10 % TFA/methylene chloride (v/v, 5 ml), was added triethylsilane ( 50 μl) as a carbocation scavenger. The reaction mixture was stirred for 2 hrs and then concentrated to an oil. The residue was purified by silica gel chromatography (hex/EtOAc, 1:1) to give 105 mg (92 %) of 2-(6-(2-(2-(2-(11-mercaptoundecyloxy) ethoxy)ethoxy)ethoxy)hexyl)benzene-1,4-diol as a clear oil. 1H NMR (500MHz, CDCl3) δ 1–1.6 (br, 26H), 2.52 (t, J = 7.3 Hz, 2H), 2.58 (t, J = 7.5 Hz, 2H), 3.40–3.65 (m, 16H), 6.54 (m, 1H), 6.63 (m, 2H).
The resulting product (105 mg, 0.20 mmol) was dissolved in THF (5 ml), and added 92 mg (0.20 mmol) of 2-(2-(11-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)undecyl) disulfanyl)pyridine (9). The reaction mixture was stirred for 2 days. Silica el chromatography (hex/EtOAc, 1:1, then EtOAc only) to give 156 mg (90 %) of adduct as pale yellow oil. 1H NMR (400MHz, CDCl3) δ 1–1.6 (br, 44H), 2.52 (t, J = 7.3 Hz, 2H), 2.67 (t, J = 7.5 Hz, 4H), 3.37 (s, 3H), 3.40–3.65 (m, 30H), 6.54 (m, 1H), 6.63 (m, 2H).
1-hydroxy-4-diisopropylsiloxy pentanoic NHS ester-2-(6-(2-(2-(2-(11-(2-(11-(2-(2-(2-methoxy ethoxy)ethoxy)ethoxy)undecyl)disulfanyl)undecyloxy)ethoxy) ethoxy)ethoxy)hexyl)benzene (6)
To a solution of 5 (68 mg, 0.078 mmol) in THF (10 ml), was added DBU (23 μl, 0.155 mmol) followed by 5-(chlorodiisopropylsilyl) pentanoic NHS ester (60 mg, 0.11 mmol) at The reaction mixture was stirred 4 hrs and then diluted with EtOAc, washed with saturated NH4Cl, then brine, and dried over MgSO4. The organic layer was concentrated and purified by column chromatography with 2:1 ethyl acetate/hexane to give 55 mg (52 %) of 6 as a pale yellow oil. 1H NMR (400MHz, CDCl3) δ 0.76 (m, 2H), 0.88 (m, 2H), 1.04 (m, 12H), 1.1–1.6 (br, 48H), 2.54 (m, 4H), 2.66 (t, J = 7.5 Hz, 4H), 2.83 (brm, 4H), 3.37 (s, 3H), 3.40–3.65 (m, 30H), 6.53 (m, 1H), 6.60 (m, 2H).
SHQ
To a solution of NHS ester 6 (4 mg, 0.003 mmol) in THF (2 ml), was added DIEA (2.5 μl) followed by 1-(2-aminoethyl)-1H-pyrrole-2,5-dione (1.5 mg). The reaction mixture was stirred overnight at 50 °C and then diluted with EtOAc, washed with saturated NH4Cl, then brine, and dried over MgSO4. The organic layer was concentrated and purified by column chromatography with 2:1 ethyl acetate/hexane to give 2 mg (55 %) of adduct as a pale yellow oil. 1 H NMR (500MHz, CDCl3) δ 0.73 (m, 2H), 0.87 (m, 2H), 1.04 (m, 12H), 1.1–1.6 (br, 48H), 2.07 (t, J = 7.5 Hz, 2H), 2.54 (t, J = 7.5 Hz, 2H), 2.67 (t, J = 7.5 Hz, 4H), 3.38 (s, 3H), 3.40–3.65 (m, 34H), 6.53 (m, 1H), 6.60 (m, 2H), 6.71 (s, 2H).
11-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)undecane-1-thiol (8)
To a solution of a S-trityl tri(ethylene glycol)-terminated alkanethiol 7 (939 mg, 1.62 mmol) in THF (10 ml) was added NaH (162 mg) portionwise at 0 °C. The reaction mixture was stirred for 2 hrs at room temperature, followed by the addition of iodomethane (300 μl), and stirred for 30 min at room temperature. The reaction mixture was quenched with water, diluted with 30 ml of EtOAc, washed with saturated NH4Cl, then brine, and dried over MgSO4. The organic layer was concentrated and purified by column chromatography with 2:1 hexane/ethyl acetate to give 938 mg (98 %) of (11-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy) undecyl)(trityl)sulfane as a colorless oil. 1H NMR (400MHz, CDCl3) δ 1–1.6 (br, 18H), 2.10 (t, J = 7.3 Hz, 2H), 3.37 (s, 3H), 3.40–3.65 (m, 14H), 7.1–7.4 (m, 15H).
To a solution of the resulting product above (936mg, 1.58 mmol) in 10 % TFA/methylene chloride (v/v, 10 ml), was added triethylsilane (500 μl) as a carbocation scavenger. The reaction mixture was stirred for 2 hrs. The residue was purified by silicagel chromatography (hex/EtOAc, 2:1) to give 530 mg (96 %) of thiol as a clear oil. 1 H NMR (400MHz, CDCl3) δ 1–1.6 (br, 18H), 2.48 (q, J = 7.5 Hz, 2H), 3.37 (s, 3H), 3.40–3.65 (m, 14H).
2-(2-(11-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)undecyl)disulfanyl) pyridine (9)
To a solution of thiol 8 (258 mg, 0.736 mmol) in MeOH, was added aldrithiol (178 mg, 0.808 mmol) followed by DIEA (650 μl). The reaction mixture was stirred overnight and purified by column chromatography with 2:1 hexane/ethyl acetate to give 253 mg (78 %) of adduct as a yellow oil. 1 H NMR (500MHz, CDCl3) δ 1–1.6 (br, 18H), 2.73 (t, J = 7.5 Hz, 2H), 3.37 (s, 3H), 3.40–3.65 (m, 14H), 7.02 (m, 1H), 7.60 (m, 1H), 7.68 (m, 1H), 8.40 (m, 1H).
2-(2-(2-(11-(2-(11-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)undecyl)disulfanyl)undecyloxy) ethoxy)ethoxy)ethanol (10)
To a solution of 2-(2-(2-(11-mercapto undecyloxy) ethoxy)ethoxy)ethanol (270 mg, 0.80 mmol) in THF (10 ml), was added disulfide 9 (368 mg, 0.8 mmol). The reaction mixture was stirred 2 days, reduced a solvent, and purified by column chromatography with 1:1 hexane/ethyl acetate to give 422 mg (80 %) of adduct as a yellow oil. 1 H NMR (500MHz, CDCl3) δ 1–1.6 (br, 36H), 2.68 (t, J = 7.4 Hz, 4H), 3.37 (s, 3H), 3.40–3.65 (m, 28H).
Acknowledgments
This work was supported by the National Institutes of Health (NIH) and a MURI program from the Army Research Office.
References
- 1.Langer R, Tirrell DA. Nature. 2004;428:487. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DL, Craighead HG, Adams T, Mrksich M, Kapur R, Giuliano KA, Jung DR. Crit Rev Biotechnol. 2001;21:111. doi: 10.1080/20013891081700. [DOI] [PubMed] [Google Scholar]
- 3.Pancrazio JJ, Whelan JP, Borkholder DA, Ma W, Stenger DA. Ann Biomed Eng. 1999;27:697. doi: 10.1114/1.225. [DOI] [PubMed] [Google Scholar]
- 4.Manos P, Pancrazio JJ, Coulombe MG, Ma W, Stenger DA. Neurosci Lett. 1999;271:179. doi: 10.1016/s0304-3940(99)00520-0. [DOI] [PubMed] [Google Scholar]
- 5.Yousaf MN, Houseman BT, Mrksich M. Angew Chem Int Ed. 2001;40:1093. [PubMed] [Google Scholar]
- 6.Hodneland CD, Mrksich M. J Am Chem Soc. 2000;122:4235. [Google Scholar]
- 7.Yeo WS, Hodneland CD, Mrksich M. ChemBioChem. 2001;7/8:590. doi: 10.1002/1439-7633(20010803)2:7/8<590::AID-CBIC590>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 8.Yousaf MN, Houseman BT, Mrksich M. Proc Natl Acad Sci U S A. 2001;98:5992. doi: 10.1073/pnas.101112898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo WS, Yousaf MN, Mrksich M. J Am Chem Soc. 2003;125:14994. doi: 10.1021/ja038265b. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Ferrigno R, Mrksich M, Whitesides GM. J Am Chem Soc. 2003;125:2366. doi: 10.1021/ja029485c. [DOI] [PubMed] [Google Scholar]
- 11.Yeo WS, Mrksich M. Adv Mater. 2004;16:1352. [Google Scholar]
- 12.Dillmore WS, Yousaf MN, Mrksich M. Langmuir. 2004;20:7223. doi: 10.1021/la049826v. [DOI] [PubMed] [Google Scholar]
- 13.Collier TO, Anderson JM, Kikuchi A, Okano T. J Biomed Mater Res. 2002;59:136. doi: 10.1002/jbm.1225. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu T, Yamato M, Kikuchi A, Okano T. Biomaterials. 2003;24:2309. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 15.Nakanish J, Kikuchi Y, Takarada T, Nakayama H, Yamaguchi K, Maeda M. J Am Chem Soc. 2004;126:16314. doi: 10.1021/ja044684c. [DOI] [PubMed] [Google Scholar]
- 16.Houseman BT, Gawalt ES, Mrksich M. Langmuir. 2003;19:1522. [Google Scholar]
- 17.Mrksich M, Whitesides GM. American Chemical Society Symposium Series on Chemistry and Biological Applications of Polyethylene Glycol. 1997;680:361. [Google Scholar]
- 18.Ruoslahti E. Annu Rev Cell Dev Biol. 1996;12:697. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 19.Houseman BT, Mrksich M. J Org Chem. 1998;63:7552. doi: 10.1021/jo981113s. [DOI] [PubMed] [Google Scholar]
- 20.Houseman BT, Mrksich M. Biomaterials. 2001;22:943. doi: 10.1016/s0142-9612(00)00259-3. [DOI] [PubMed] [Google Scholar]
- 21.Zheng A, Shan D, Wang B. J Org Chem. 1999;64:156. doi: 10.1021/jo981528d. [DOI] [PubMed] [Google Scholar]
- 22.Sodium adducts of disulfides are the major species which are observed in MALDI spectra of SAMs of alkanethiolates: see Su J, Mrksich M. Angew Chem Int Ed. 2002;41:4715. doi: 10.1002/anie.200290026.Trevor JL, Lykke KR, Pellin MJ, Hanley L. Langmuir. 1998;14:1664.
- 23.Smith JC, Lee K-B, Wang Q, Finn MG, Johnson JE, Mrksich M, Mirkin CA. NanoLetters. 2003;3:883–886. [Google Scholar]
- 24.Su J, Bringer MR, Ismagilov RF, Mrksich M. J Am Chem Soc. 2005;127:7280. doi: 10.1021/ja051371o. [DOI] [PubMed] [Google Scholar]
- 25.Wong JY, Langer R, Ingber DE. Proc Natl Acad Sci U S A. 1994;91:3201. doi: 10.1073/pnas.91.8.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Proc Natl Acad Sci U S A. 1997;94:8948. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishida K, Yamato M, Hayashida Y, Watanabe K, Yamamoto K, Adachi E, Nagai S, Kikuchi A, Maeda N, Watanabe H, Okano T, Tano Y. New Eng J Med. 2004;351:1187. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 28.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Müller R, Hubbell JH. Nat Biotechnol. 2003;21:513. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 29.Hubbell JA. Curr Opin Biotechnol. 2003;14:551. doi: 10.1016/j.copbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Orner BP, Derda R, Lewis RL, Thomson JA, Kiessling LL. J Am Chem Soc. 2004;126:10808. doi: 10.1021/ja0474291. [DOI] [PubMed] [Google Scholar]