Olefins are fundamental building blocks for organic synthesis, and a multitude of methods exist for their preparation. Among various approaches, additions to alkynes offer the promise of convergence and stereoselectivity while leveraging the wealth of literature related to alkyne synthesis. Indeed, carbometalation of terminal alkynes represents a general method and has found widespread use in the synthesis of complex molecules. 1 In contrast, electronically unbiased internal alkynes react slowly and with poor regioselectivity. However, polar functionality near the alkyne has been found to affect both the rate and regioselectivity of carbometalation reactions (eq 1–2).2 For example, addition of Grignard reagents to propargylic alcohols introduces an organic fragment proximal to the alcohol (eq 1).2b,c The opposite regioselectivity has been observed in reactions of homopropargylic alcohols and ethers with vinyl titanium reagents,2d allyl magnesium bromide,2e,f butyl lithium2g and trimethyl aluminum2h (eq 2, n = 2; X = H or alkyl). Here we report an iron-catalyzed carbomagnesiation of propargylic and homopropargylic alcohols which generates tri- and tetra-substituted olefins with high regio- and stereoselectivity (eq 2; n = 1–2; X = H).
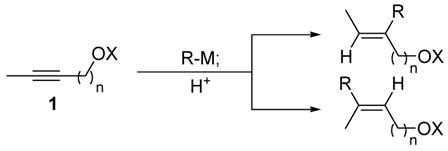 |
(1), (2) |
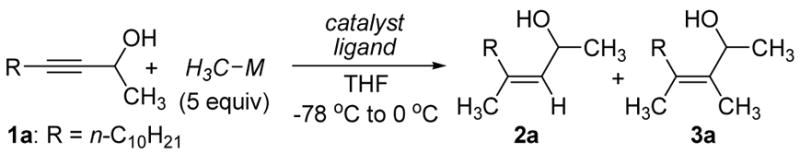
In the absence of catalysts CH3MgBr does not add to the internal propargylic alcohol 1a. Accordingly, we evaluated a variety of first-row transition metal salts for their ability to catalyze methylmagnesiation (Table 1). While Cu(I) salts did not effect addition,3 Co(II), Ni(II) and Fe(III) displayed promising catalytic activity (entries 2–4). In all cases regioselectivity and stereoselectivity was very high, but the desired trisubstituted olefin (2a) was contaminated with the dimethyl product 3a. The formation of this side product was largely suppressed when bis(diphenylphosphino)ethane (dppe, 1 equiv to Fe) was included in the reaction mixture (entry 6). Interestingly, methyllithium proved totally unreactive in the presence or absence of catalyst (entry 7).2g
Table 1.
Catalytic carbometalation of propargylic alcohol 1a.a
 | |||||
|---|---|---|---|---|---|
| entry | CH3M | catalystb | ligandb | conv. (%)c | 2a/3ad |
| 1 | CH3MgBr | CuBr | none | <5 | --- |
| 2 | CH3MgBr | Co(OAc)2 | none | 58 | 9/1 |
| 3 | CH3MgBr | Ni(acac)2 | none | 63 | 9/1 |
| 4 | CH3MgBr | Fe(acac)3 | none | 98 | 6/1 |
| 5e | CH3MgBr | Fe(ehx)3 | none | 98 | 5/1 |
| 6 | CH3MgBr | Fe(acac)3 | dppe | 97 | 21/1 |
| 7 | CH3Li | Fe(acac)3 | none | 0 | --- |
Reactions were carried out on 0.1 mmol scale under a N2 for 7 h.
20 mol%.
Determined by GC using an internal standard.
Determined by GC analysis of crude reaction mixture.
ehx = 2-ethyl hexanoate
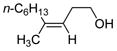
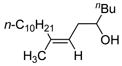
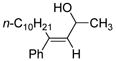
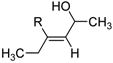
Table 2 presents the generality of the carbometalation. In the presence of Fe(acac)3 or Fe(ehx)3 (ehx = 2-ethyl hexanoate) propargylic alcohols react with methyl magnesium bromide to yield trisubstituted allylic alcohols as single regio- and stereoisomers. 4 Primary and secondary propargylic alcohols represent suitable substrates (entries 1–8), the latter reacting with complete conservation of optical purity (entry 7). Common oxygen protecting groups, olefins and tertiary nitrogens appear well-tolerated. Furthermore, primary and secondary homopropargylic alcohols provide the corresponding homoallylic alcohols on treatment with methyl Grignard reagent (entries 9–10, 12). In ongoing studies, we have found that the iron-catalyzed carbometalation can be extended to phenylation5 (entries 11–12) and ethylation (entries 13–14). In the latter experiments, no diethylation products (analogous to 3) were observed; instead, we obtained small amounts of the product arising from formal cis-hydrogenation (see below).6,7
Table 2.
Iron-catalyzed carbomagnesiation of propargylic and homopropargylic alcohols.a
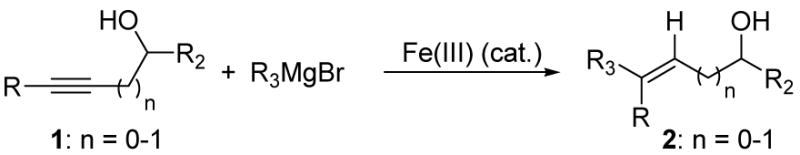 | |||
|---|---|---|---|
| entry | Conditions | Product | Yield (%)b |
| 1 | Fe(ehx)3 (0.20 equiv) dppe (0.20 equiv) |
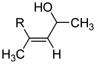
R = n-C10H21 |
75 |
| 2 | Fe(ehx)3 (0.20 equiv) dppe (0.20 equiv) | R = TBSO(CH2)4 | 80 |
| 3 | Fe(ehx)3 (0.20 equiv) dppe (0.20 equiv) | R = BnO(CH2)3 | 70 |
| 4 | Fe(acac)3 (0.20 equiv) dppe (0.20 equiv) |
|
78 |
| 5 | Fe(acac)3 (0.30 equiv) dppe (0.30 equiv) |
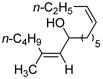
|
85 |
| 6 | Fe(acac)3 (0.15 equiv) |
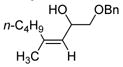
|
81 |
| 7 | Fe(acac)3 (0.30 equiv) |
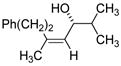
99% ee |
61 |
| 8 | Fe(ehx)3 (0.30 equiv) dppe (0.30 equiv) |
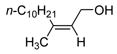
|
80 |
| 9c | Fe(acac)3 (0.20 equiv) |
|
75 |
| 10c | Fe(acac)3 (0.20 equiv) |
|
74 |
| 11 | Fe(acac)3 (0.50 equiv) CuBr (0.60 equiv) |
|
69 |
| 12c | Fe(acac)3 (0.40 equiv) |
|
63 |
| 13d | Fe(acac)3 (0.20 equiv) NMP (2.0 equiv) |
R=n-C10H21 |
70 |
| 14d | Fe(acac)3 (0.20 equiv) NMP (2.0 equiv) | R= TBSO(CH2)4 | 74 |
Reactions carried out in THF (0.1M in substrate) using 5.0 equivalents of RMgBr at 0 °C for 7h unless otherwise indicated.
Isolated yield.
In toluene at 23 °C.
NMP = N-methyl pyrrolidine.
Iron(III) salts are proposed to undergo ligand exchange and reduction with CH3MgBr to yield LnFeII(CH3)2 complexes at 0 °C.8b Lower oxidation states are available upon warming or in the presence of longer chain Grignard reagents.9 Accordingly, the oxidation sate of the catalytically active species here remains ambiguous.8a Regardless, alkoxide-directed carbometalation likely yields an intermediate (vinyl)Fe species (Scheme 1). In principle, direct coordination to the iron center could occur (4). Alternatively the interaction could be driven by association of iron with magnesium (5).10 The (vinyl)Fe(R) species can undergo metathesis with Grignard reagent to provide the carbometalated product and regenerate catalyst, or it can suffer reductive elimination to yield the geminally dialkylated product 3. β-Hydride elimination from an Fe(ethyl) intermediate could give rise to an Fe-H species 6. Subsequent hydrometalation could lead to the hydrogenated side products observed in ethylation reactions.
Scheme 1.
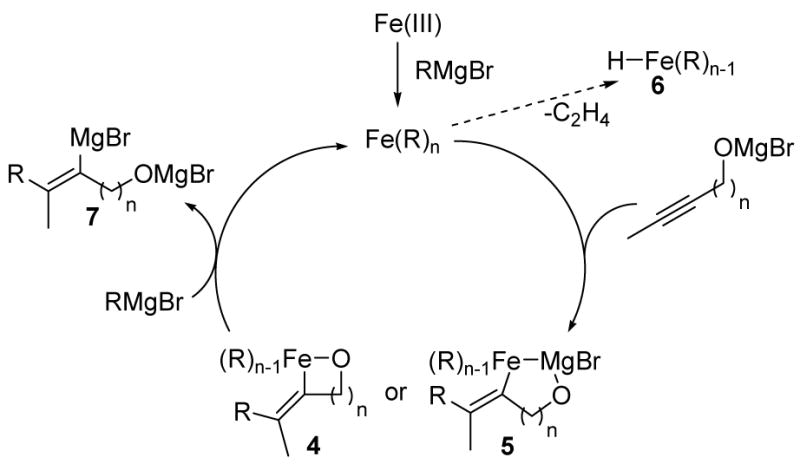
The presumptive vinyl Grignard intermediate 7 can be trapped with a variety of electrophiles to yield tetrasubstituted allylic alcohols. For example, deuteration, formylation, allylation and bromination proceeds under the conditions indicated in Scheme 2. Likewise, trapping with benzaldehyde provides the allylic alcohols 8 and 9 as single olefin isomers. Finally, trapping the vinyl iron or magnesium species with a pendant alkyne yields the cyclic diene 10 in good yield.
Scheme 2.
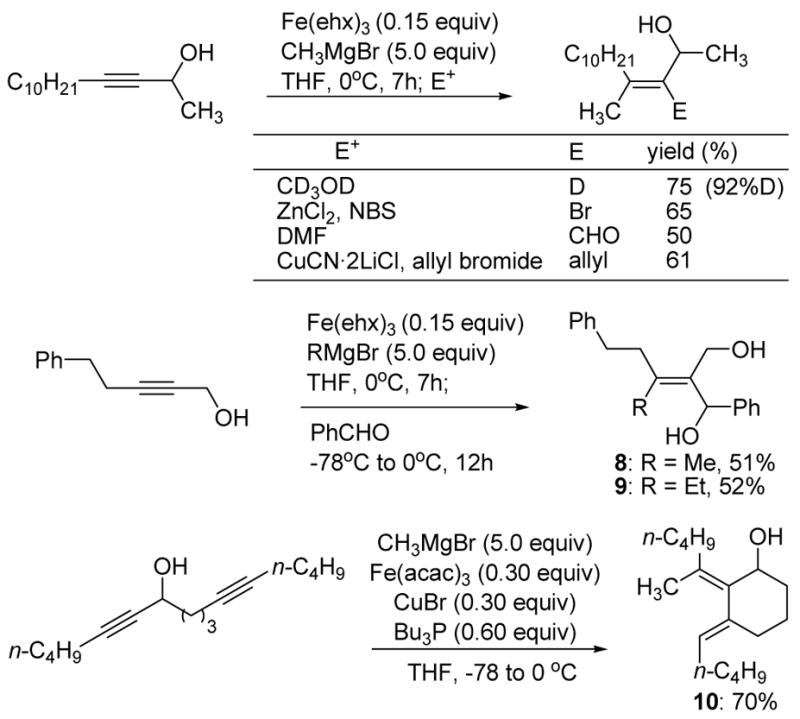
Synthesis of tetrasubstituted olefins.
Further studies of the iron-catalyzed carbomagnesiation may reveal intimate details of the reaction mechanism. In the meantime the method offers an efficient and stereoselective synthesis of tri- and tetrasubstituted olefins. Of note, the carbometalation reported here yields Z-configured allylic and homoallylic olefins. In contrast, the opposite olefin geometry is obtained from procedures based on carbometalation of terminal alkynes followed by trapping with aldehydes or epoxides.1
Supplementary Material
Complete experimental procedures and characterization data for Table 2 and Scheme 2. This material is available free of charge at http://pubs.acs.org.
Acknowledgments
Financial support from the NIGMS (GM074822) and UT Southwestern (J.M.R. is a Southwestern Medical Foundation Scholar in Biomedical Research).
References
- 1.Negishi E. Acc Chem Res. 1987;20:65–72.Lipshutz BH, Sengupta S. Org React. 1992;41:135–631.Marek I, Normant J. In: Metal-Catalyzed Cross-Coupling Reactions. Diederich F, Stang P, editors. Wiley-VCH; Great Britain: 1998. pp. 271–283.Zhang D, Ready JM. Org Lett. 2005;7:5681–5683. doi: 10.1021/ol052413g.see also Wipf P, Jahn H. Tetrahedron. 1996;52:12853–12910.
- 2.(a) Fallis AG, Forgione P. Tetrahedron. 2001;57:5899–5913. [Google Scholar]; (b) Forgione P, Fallis AG. Tetrahedron Lett. 2000;41:11–15. [Google Scholar]; (c) Forgione P, Wilson PD, Fallis AG. Tetrahedron Lett. 2000;41:17–20. [Google Scholar]; (d) Ryan J, Micalizio GC. J Am Chem Soc. 2006;128:2764–2765. doi: 10.1021/ja057352w. [DOI] [PubMed] [Google Scholar]; (e) Okada K, Oshima K, Utimoto K. J Am Chem Soc. 1996;118:6076–6077. [Google Scholar]; (f) Eisch JJ, Merkley JH. J Organomet Chem. 1969;20:P27–P31. [Google Scholar]; (g) Hojo M, Murakami Y, Aihara H, Sakuragi R, Baba Y, Hosomi A. Angew Chem Int Ed. 2001;40:621–623. doi: 10.1002/1521-3773(20010202)40:3<621::AID-ANIE621>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]; (h) Ewing JC, Ferguson GS, Moore DW, Schultz FW, Thompson DW. J Org Chem. 1985;50:2124–2128. [Google Scholar]
- 3.(a) Douboudin JG, Jousseaume B, Bonakdar A, Saux A. J Organomet Chem. 1979;168:227–242. [Google Scholar]; (b) Lu Z, Ma S. J Org Chem. 2006;71:2655–2660. doi: 10.1021/jo0524021. [DOI] [PubMed] [Google Scholar]
- 4.In some cases, dppe was not necessary.
- 5.We speculate that the CuBr in Table 2, entry 11 facilitates transmetalation from (vinyl)Fe to (vinyl)Cu. See Shirakawa E, Yamagami T, Kimura T, Yamaguchi S, Hayashi T. J Am Chem Soc. 2005;127:17164–17165. doi: 10.1021/ja0542136.
- 6.(a) Snider B, Karras M, Conn RSE. J Am Chem Soc. 1978;100:4624–4626. [Google Scholar]; (b) Sato F, Ishikawa H, Watanabe H, Miyake T, Sato M. Chem Commun. 1981:718–720. [Google Scholar]; (c) Smith JM, Lachicotte RJ, Holland PL. J Am Chem Soc. 2003;125:15752–15753. doi: 10.1021/ja038152s. [DOI] [PubMed] [Google Scholar]
- 7.Notes: (a) iPrMgCl provides the hydrogenated products in moderate yield. (b) No reaction is observed with tBuMgCl. (c) 4-Octyne does not react with CH3MgBr in the presence of Fe(III) catalysts while cis-hydrogenation is observed with EtMgBr in the presence of Fe(acac)3. (d) Reactions involving the methyl ether of 1a, CH3MgBr and Fe(acac)3 generate complex mixtures.
- 8.(a) Treating the alkoxide of 1a with 2 equiv Fe(CH3)3 (prepared as in ref 8b) afforded a 3:4 ratio of 2a:3a (100% conv). Fe(0), as prepared in ref 8b, did not catalyze the methylation of 1a. (b) Krafft ME, Holton RA. J Org Chem. 1984;49:3669–3670.
- 9.Bogdanovic B, Schwickardi M. Angew Chem Int Ed. 2000;39:4610–4612. [PubMed] [Google Scholar]
- 10.(a) Aleandri LE, Bogdanovic B, Bons P, Durr C, Gaidies A, Hartwig T, Huckett SC, Lagarden M, Wilczok U, Brand RA. Chem Mater. 1995;7:1153–1170. [Google Scholar]; (b) Furstner A, Leitner A, Mendez M, Krause H. J Am Chem Soc. 2002;124:13856–13863. doi: 10.1021/ja027190t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete experimental procedures and characterization data for Table 2 and Scheme 2. This material is available free of charge at http://pubs.acs.org.


