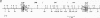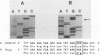Abstract
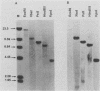
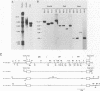
The molecular basis has been determined for differences in infectivity and XC phenotype of endogenous ecotropic murine leukemia virus of the low-leukemia mouse strain C3H/He, its relative in the high-leukemia mouse strain AKR, and highly infectious, XC-positive C3H virus variants selected in vitro. Endogenous ecotropic type C virus induced by iododeoxyuridine from the nontransformed C3H/10T1/2 cell line is XC negative and replication deficient. In contrast, viruses produced late after iododeoxyuridine induction in chemically transformed C3H/10T1/2 cells (MCA5) are XC positive and infectious. XC-negative viruses can be converted to XC-positive viruses by being grown in certain transformed cell lines. We have cloned the endogenous ecotropic provirus of C3H/He from MCA5 cells, which is XC negative and replication deficient, as well as two XC-positive C3H proviruses derived by in vitro conversion. Fragment exchange between the XC-negative molecular clone p110 and the XC-positive AKR virus clone p623 revealed that the defect in p110 lies 3' of the SalI site located in the pol region. Nucleotide sequencing established that the C3H p110 provirus was integrated within the R region of an endogenous VL30 long terminal repeat (LTR) in reverse orientation and that the virus differed from the infectious AKR p623 provirus by a point mutation, substituting Lys for Arg at the potential precursor cleavage site for gp70 and p15E. In vitro-converted XC-positive C3H proviral clones 3211 and 4211 are identical to XC-negative C3H p110, except that they have Arg at this site and the normal cleavage site is thus regenerated in these clones. The XC-negative C3H p110 was blocked in processing of Pr85env, whereas clones 3211 and 4211 had normal cleavage of the env precursor into gp70. Both the XC-negative C3H provirus and the in vitro-converted XC-positive C3H proviruses had a single copy of a 99-base-pair enhancer element in the LTR, whereas two copies of this sequence are present in the AKR proviral LTR. Substitution of Arg for Lys at the envelope precursor processing site of C3H p110 by site-directed mutagenesis is sufficient by itself to convert the virus to the XC-positive replication-competent phenotype. Thus, we have established that a single point mutation at the processing site of the envelope precursor protein Pr85 is responsible for the difference in the infectivity and XC phenotype of endogenous ecotropic murine leukemia virus from C3H/He and AKR mice and that the basis for in vitro conversion is a mutation at this site.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Dunn C. Y. Endogenous C-type viruses of BALB-c cells: frequencies of spontaneous and chemical induction. J Virol. 1974 Jan;13(1):181–185. doi: 10.1128/jvi.13.1.181-185.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch F. X., Garten W., Klenk H. D., Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology. 1981 Sep;113(2):725–735. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov I., Stuhlmann H., Harbers K., Jaenisch R. Cloning of two genetically transmitted Moloney leukemia proviral genomes: correlation between biological activity of the cloned DNA and viral genome activation in the animal. J Virol. 1982 Jun;42(3):1088–1098. doi: 10.1128/jvi.42.3.1088-1098.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Bedigian H. G., Thomas C. Y., Jenkins N. A. DNAs of two molecularly cloned endogenous ecotropic proviruses are poorly infectious in DNA transfection assays. J Virol. 1984 Feb;49(2):437–444. doi: 10.1128/jvi.49.2.437-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Larco J. E., Rapp U. R., Todaro G. J. Cell surface receptors for ecotropic MuLV: detection and tissue distributions of free receptors in vivo. Int J Cancer. 1978 Mar 15;21(3):356–360. doi: 10.1002/ijc.2910210317. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Rommelaere J., Ellis R. W., Hopkins N. Nucleotide sequences associated with differences in electrophoretic mobility of envelope glycoprotein gp70 and with GIX antigen phenotype of certain murine leukemia viruses. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1642–1645. doi: 10.1073/pnas.77.3.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L., Sternberg N. In vitro packaging of lambda Dam vectors and their use in cloning DNA fragments. Methods Enzymol. 1979;68:281–298. doi: 10.1016/0076-6879(79)68020-5. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Risser R. The role of envelope glycoprotein processing in murine leukemia virus infection. J Virol. 1987 Sep;61(9):2852–2856. doi: 10.1128/jvi.61.9.2852-2856.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., Bye J., Skehel J., Waterfield M. Cloning and DNA sequence of double-stranded copies of haemagglutinin genes from H2 and H3 strains elucidates antigenic shift and drift in human influenza virus. Nature. 1980 Sep 25;287(5780):301–306. doi: 10.1038/287301a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Capps W. I., Huebner R. J. Isolation of naturally occurring viruses of the murine leukemia virus group in tissue culture. J Virol. 1969 Feb;3(2):126–132. doi: 10.1128/jvi.3.2.126-132.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. W., Steffen D., Gusella J., Tabin C., Bird S., Cowing D., Weinberg R. A. DNA methylation affecting the expression of murine leukemia proviruses. J Virol. 1982 Oct;44(1):144–157. doi: 10.1128/jvi.44.1.144-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N., Jolicoeur P. Variants of N-tropic leukemia virus derived from BALB/c mice. J Virol. 1975 Oct;16(4):991–999. doi: 10.1128/jvi.16.4.991-999.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Holland G. D., King S. R., Risser R. Germ line integration of a murine leukemia provirus into a retroviruslike sequence. J Virol. 1987 Mar;61(3):701–707. doi: 10.1128/jvi.61.3.701-707.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Risser R. A locus that enhances the induction of endogenous ecotropic murine leukemia viruses is distinct from genome-length ecotropic proviruses. J Virol. 1982 Dec;44(3):950–957. doi: 10.1128/jvi.44.3.950-957.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Risser R. Molecular and biological characterization of the endogenous ecotropic provirus of BALB/c mice. J Virol. 1985 Dec;56(3):798–806. doi: 10.1128/jvi.56.3.798-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Diverse long terminal repeats are associated with murine retroviruslike (VL30) elements. Mol Cell Biol. 1986 Apr;6(4):1276–1282. doi: 10.1128/mcb.6.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin A., Keshet E. Nucleotide sequence analysis of the long terminal repeat of murine virus-like DNA (VL30) and its adjacent sequences: resemblance to retrovirus proviruses. J Virol. 1983 Sep;47(3):656–659. doi: 10.1128/jvi.47.3.656-659.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Chattopadhyay S. K., Teich N. M., Rowe W. P., Levine A. S. AKR murine leukemia virus genome: frequency of sequences in DNA of high-, low-, and non-virus-yielding mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3555–3559. doi: 10.1073/pnas.71.9.3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rowe W. P., Teich N., Hartley J. W. Murine leukemia virus: high-frequency activation in vitro by 5-iododeoxyuridine and 5-bromodeoxyuridine. Science. 1971 Oct 8;174(4005):155–156. doi: 10.1126/science.174.4005.155. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Allelism and linkage studies of murine leukemia virus activation genes in low leukemic strains of mice. J Exp Med. 1982 Apr 1;155(4):1233–1238. doi: 10.1084/jem.155.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Genetic interactions in induction of endogenous murine leukemia virus from low leukemic mice. Cell. 1982 Apr;28(4):881–888. doi: 10.1016/0092-8674(82)90067-8. [DOI] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Rowe W. P., Lilly F. A major genetic locus affecting resistance to infection with murine leukemia viruses. II. Apparent identity to a major locus described for resistance to friend murine leukemia virus. J Exp Med. 1971 Jun 1;133(6):1234–1241. doi: 10.1084/jem.133.6.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Rands E., Lowy D. R., Lander M. R., Chattopadhyay S. K. Restriction endonuclease mapping of ecotropic murine leukemia viral DNAs: size and sequence heterogeneity of the long terminal repeat. Virology. 1981 Jan 30;108(2):445–452. doi: 10.1016/0042-6822(81)90451-7. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Birkenmeier E., Bonner T. I., Gonda M. A., Gunnell M. Genome structure of mink cell focus-forming murine leukemia virus in epithelial mink lung cells transformed vitro by iododeoxyuridine-induced C3H/MuLV cells. J Virol. 1983 Feb;45(2):740–754. doi: 10.1128/jvi.45.2.740-754.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R. Kinetics of expression of infectious ecotropic, xenotropic, and mink cell focus-forming murine leukemia virus after 5-iododeoxyuridine induction of cells from high- and low-leukemia mouse strains. J Virol. 1983 Feb;45(2):755–765. doi: 10.1128/jvi.45.2.755-765.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Marshall T. H. Cell surface receptors for endogenous mouse type C viral glycoproteins and epidermal growth factor: tissue distribution in vivo and possible participation in specific cell-cell interaction. J Supramol Struct. 1980;14(3):343–352. doi: 10.1002/jss.400140308. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C. Endogenous ecotropic mouse type C viruses deficient in replication and production of XC plaques. J Virol. 1976 May;18(2):411–417. doi: 10.1128/jvi.18.2.411-417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R., Nowinski R. C., Reznikoff C. A., Heidelberger C. Endogenous oncornaviruses in chemically induced transformation. I. Transformation independent of virus production. Virology. 1975 Jun;65(2):392–409. doi: 10.1016/0042-6822(75)90045-8. [DOI] [PubMed] [Google Scholar]
- Rodland K. D., Brown A. M., Magun B. E. Individual mouse VL30 elements transferred to rat cells by viral pseudotypes retain their responsiveness to activators of protein kinase C. Mol Cell Biol. 1987 Jun;7(6):2296–2298. doi: 10.1128/mcb.7.6.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Hartley J. W. Studies of genetic transmission of murine leukemia virus by AKR mice. II. Crosses with Fv-1 b strains of mice. J Exp Med. 1972 Nov 1;136(5):1286–1301. doi: 10.1084/jem.136.5.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P. Studies of genetic transmission of murine leukemia virus by AKR mice. I. Crosses with Fv-1 n strains of mice. J Exp Med. 1972 Nov 1;136(5):1272–1285. doi: 10.1084/jem.136.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Genetic factors influencing C-type RNA virus induction. J Exp Med. 1972 Jul 1;136(1):175–184. doi: 10.1084/jem.136.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Segregation of loci for C-type virus induction in strains of mice with high and low incidence of leukemia. Science. 1973 May 25;180(4088):865–866. doi: 10.1126/science.180.4088.865. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]