Abstract
We investigated the effects of rivastigmine (a cholinesterase inhibitor) and selegiline ((-)deprenyl, an irreversible inhibitor of monoamineoxidase-B), alone and in combination, on brain acetylcholinesterase (AChE), (Na+, K+)-, Mg2+-ATPase activities, total antioxidant status (TAS), and learning performance, after long-term drug administration in aged male rats. The possible relationship between the biochemical and behavioral parameters was evaluated.
Methods
Aged rats were treated (for 36 days) with rivastigmine (0.3 mg/kg rat/day ip), selegiline (0.25 mg/kg rat/day im), rivastigmine plus selegiline in the same doses and way of administration as separately. Aged and adult control groups received NaCl 0.9% 0.5 ml ip.
Results
TAS was lower in aged than in adult rats, rivastigmine alone does not affect TAS, decreases AChE activity, increases (Na+, K+)-ATPase and Mg2+-ATPase activity of aged rat brain and improves cognitive performance. Selegiline alone decreases free radical production and increases AChE activity and (Na+, K+)-ATPase activity, improving cognitive performance as well. In the combination: rivastigmine seems to cancel selegiline action on TAS and AChE activity, while it has additive effect on (Na+, K+)-ATPase activity. In the case of Mg2+-ATPase selegiline appears to attenuate rivastigmine activity. No statistically significant difference was observed in the cognitive performance.
Conclusion
Reduced TAS, AChE activity and learning performance was observed in old rats. Both rivastigmine and selesiline alone improved performance, although they influenced the biochemical parameters in a different way. The combination of the two drugs did not affect learning performance.
Keywords: aged rat, brain enzymes, TAS, learning, rivastigmine, selegiline
Introduction
Brain aging in most cases is characterized by cognitive deficits and a central cholinergic hypofunction (Bartus et al 1982). Alzheimer’s disease (AD), a neurodegenerative disorder, is characterized by loss of memory and other cognitive abilities. It is also characterized by a prominent loss of cholinergic neurons in the basal forebrain (Davis and Maloney 1976), leading to decreased amounts of acetylcholine and decreased activities of cholinacetyltransferase (ChAT) and acetylcholinesterase (AChE) in almost the entire neocortex (Coyle et al 1983). The observed association between the loss of cholinergic neurons, receptors, reduction of cholinergic markers, cognitive and executive function impairments in AD was the base for the development of cholinergic hypothesis (Bartus 2000) and the introduction of AChE inhibitors as the main therapeutic approach for this disease. Rivastigmine is a second-generation carbamatebased pseudo-irreversible AChE and butyrylcholinesterase (BuChE) inhibitor, indicated for treatment of mild to moderate AD (Anand et al 1996; Corey-Bloom et al 1998; Eskander et al 2005; Caltagirone et al 2005; Takeda et al 2006; Gonzalez-Gutierrez and Gobbart 2007) or for patients with rapid disease progression (Farlow et al 2005). As the disease and age progress, significant loss of other neurons (noradrenergic, dopaminergic) is observed as well (Davies and Wolozin 1987; Strong 1998). The pathology of the disorder may involve oxidative stress and accumulation of free radicals, leading to excessive lipid peroxidation and neuronal degeneration in the brain (Smith et al 1991; Strong 1998; Pratico and Delanty 2000). Selegiline, (-)deprenyl, an irreversible monoamineoxidase-B (MAO-B) inhibitor, has been used in depression and in Parkinson’s disease in combination with L-dopa (Birkmayer et al 1985; Lieberman and Fazzini 1991; Knoll 2000; Negrotti et al 2001; Kitani et al 2002). Selegiline enhances the release of dopamine, blocks the reuptake of dopamine and produces an amphetamine-like effect (Ebadi et al 2002). Pretreatment with selegiline can protect neurons against a variety of neurotoxins as MPTP, DSP-4, 5,6-dihydroserotonin and AF64A, which damage dopaminergic, adrenergic, serotonergic, and cholinergic neurons respectively (Walsh et al 1984; Ricci et al 1992; Mayar and Haberle 1999; Matsubara et al 2001). In patients with moderate impairment from AD, treatment with selegiline slowed the progression of disease (Sano et al 1997; Filip and Colibas 1999; Knoll 2003). However, according to recent data from a meta-analysis, selegiline significantly improved cognition and activities of daily living at an earlier time point, but not at a later assessment time (Wilcock et al 2002; Birks and Flicher 2003). Selegiline prevents the effects of oxidative stress in a variety of models both in vitro and in vivo (Youdim et al 2001).
The underlying mechanism of the beneficial effect of selegiline on neuronal function is believed to be associated with enhanced activity of free radical scavenging enzymes (Carrillo et al 1994; Kitani et al 2002; Kiray et al 2006), diminished production of hydrogen peroxide through MAO-B inhibition (Cohen and Spina 1989; Takahata et al 2006), some trophic-like effects that increase the survival of degenerating motoneurons (Ju et al 1994), restoration of ChaT reduced activity (Koutsilieri 2001), the number of neurons in the hippocampus (Kiray et al 2006), or enhancement of neuroplastic status (Murphy et al 2006). Recently Ono and colleagues (2006) also reported an in vitro antiamyloidogenic activity of selegiline.
We have previously shown that the administration of selegiline in a dose of 0.25 mg kg-1 rat/other day for 50 days in old rats increased whole brain TAS, stimulated (Na+, K+)-ATPase activity and although it increased AChE activity, it improved the learning performance of aged rats (Carageorgiou et al 2003).
The (Na+, K+)-ATPase, or Na+ pump, is an energy transducting ion pump first described by Skou in 1952 (Skou 1998). In recent years, research on (Na+, K+)-ATPase revealed that interactions of (Na+, K+)-ATPase with other proteins not only are important for regulation of pumping function, but also make it possible for the enzyme to function as a single transducter (Xie and Cai 2003). Long-term pharmacological interruption of cholinergic transmission can decrease the postsynaptic membrane potential by altering (Na+, K+)-ATPase activity. This can be seen as a decline in [3H] ouabaine binding (Henning et al 1994). Age-associated impairments in a test of attention and evidence of involvement of cholinergic systems was referred by Jones and colleagues (1995). It is known that inhibition of (Na+, K+)-ATPase induces neurotransmitter release in several experimental models (Rodríguez de Lores Arnaiz and Pellegrino de Iraldi 1991). Furthermore, studies suggest that (Na+, K+)-ATPase might play a role on memory formation (dos Reis-Lunardelli et al 2007). According to Gorini and colleagues (2002) (Na+, K+)-ATPase is a particular age-related enzyme. The (Na+, K+)-ATPase activity is lower in all plasma membrane subfractions (rat frontal cerebral cortex) at 22 months of age, than in 5 months (where reduction is already evident at 10 months of age). Similar reductions in (Na+, K+)-ATPase activity were observed by Kaur and colleagues (1998) in different brain regions of 24-month-old rats. However, old age and selective loss of cholinergic basal frontal cells did not significantly alter the presynaptic second messenger system that influences (Na+, K+)-ATPase activity. On the other hand the lesions had a much greater effect on performance than old age alone did (Stoehr et al 1997).
Little is known about the activity of Mg2+-ATPase in old age, an enzyme that is of primary importance in phosphorylation reactions and the maintenance of high brain intracellular Mg2+. Its change can control rates of protein synthesis and growth of the cell (Sanui and Rubin 1982). Decreased Mg2+-ATPase activity in the frontal cortex of old age rats was reported by Gorini and colleagues (2002).
Oxidative stress has been implicated in aging and age-related neurodegenerative diseases (Atamna and Frey 2007; Tahirovic et al 2007; Weinreb et al 2007) and the proposal of the “free radical theory of aging” (Harman 1956). Impaired total antioxidant capacity in different structures from aged rat brain was observed by Siqueira and colleagues (2005). A decrease of the total antioxidant status in the brain of old male rats has been observed by Carageorgiou and colleagues (2003).
Based on the aforementioned selegiline study in aged rats, the foreseen trend of combining drugs with a different mechanism of action in AD therapy (Youdim and Weinstock 2002; Ucar et al 2005; Dantoine et al 2006; Groner et al 2007; Tahirovic et al 2007) and since multiple factors contribute to AD pathology (van Dyck 2004; Liu and Ames 2005) we decided to investigate the effect of the combination of the two agents, rivastigmine and selegiline, on brain TAS, AChE, (Na+, K+)-ATPase, Mg2+-ATPase activities and on cognitive capacity of aged rats. We also considered evaluating the possibility of correlations between biochemical and behavioral data. It should be mentioned that there were no previous in vivo data about the effect of a) rivastigmine alone on the activities of brain (Na+, K+)-ATPase, Mg2+-ATPase or TAS. and b) rivastigmine plus selegiline combined administration on all the biochemical and behavioral parameters studied.
Materials and methods
Animals
Fifty two (52) aged male Wistar rats (24 months old) and 485 ± 23 g BW were used. A group of 11 adult rats (8 months old) and 391 ± 12 g BW was also used as an adult control. The rats were housed five or six in a cage at a constant room temperature (22 ± 1 °C) under a 12-h light: 12-h dark (light 08:00–20:00 h) cycle. Food and water were provided ad libitum. Animals were cared for in accordance with the principles of the Guide for the Care and Use of Experimental Animals (Committee on Care and Use of Laboratory Animals 1985).
Drugs in vivo administration
Rats were divided into five groups, according to the procedure followed in the object recognition test: 1) Group (R) was treated with rivastigmine (0.3 mg kg−1 rat day-1 ip) for 36 consecutive days, 2) Group (S) was treated with selegiline (0.25 mg kg−1 rat day−1 im) for the same period, 3) Group (R + S) was treated with the combination of the two drugs at the doses and way of administration mentioned before for each drug separately and for the same period of time, 4) a group was treated with equal volumes (0.5 ml) of NaCl 0.9% ip (aged control group) and 5) a group was also treated with equal volumes (0.5 ml) of NaCl 0.9% ip (adult control group) for every of the 36 consecutive days.
Tissue preparation
Animals were sacrificed by decapitation (right after the last performance test and 90 minutes after the last drug administration) and the whole brain was rapidly removed. The tissue was homogenized and centrifuged as described earlier (Tsakiris et al 2000; Antoniades et al 2002). In the resulting supernatant, the protein content was determined according to the method of Lowry and colleagues (1951) and the enzyme activities and TAS were evaluated.
Determination of enzyme activities
AChE activity was determined according to Ellman and colleagues (1961) and (Na+, K+)-ATPase, Mg2+-ATPase activities according to Bowler and Tirri (1974). The enzyme reaction mixture and assay conditions of these enzyme activities were previously described in detail (Tsakiris et al 2000; Antoniades et al 2002).
Determination of brain total antioxidant status
TAS was evaluated in each fresh homogenized rat brain. The total antioxidant capacity was measured spectrophotometrically by a commercial kit (Randox Laboratories Ltd., Cat. No. NX2332) as previously reported (Tsakiris et al 2000). 2,2′-Azino-di-[3-ethylbenzthiazoline sulphonate] (ABTS) was incubated with a peroxidase (metmyoglobin) and H2O2 in order to produce the radical cation ABTS+. The latter had a relatively stable blue-green color, which was measured at 600 nm. Inhibited values of TAS reflect the increase of brain free radical production whereas stimulated TAS values show the decrease of free radical production and the protective antioxidant effect of the drug in the brain.
Cognitive capacities tests
Cognitive capacities were evaluated using two different tasks: object recognition test (ORT) and passive avoidance conditioned response (PA). The ORT was carried out according to the procedure described by Vannucchi and colleagues (Ennaceur and Delacour 1988; Scali et al 1994; Vannucchi et al 1997). The apparatus was an open white polyvinylchloride arena (70 × 60 × 30 cm3) illuminated by a 75 W lamp suspended 50 cm above the arena. The objects to be distinguished were made of polyvinylchloride, grey-colored and were in two different shapes: cubes (8 × 8 cm2 side) or pyramids (8 cm height). Apparently they had no significance for the rats. For the procedure, the rat was submitted to a session of two trials, each of which had a 5-min duration. The intertribal interval (ITI) was 60 min. In the first trial (T1) two identical objects were presented in two opposite corners of the box and the amount of time spent by each animal for the object exploration was recorded. Exploration was considered to be directing the nose at a distance <2 cm to the object and/or touching it with the nose. During the second trial (T2), one of the objects presented in T1 was replaced by a new (differently-shaped) one. To reduce place preference effects, the positions of the two different objects were randomly changed during T2 for each rat. The times spent on exploration of the familiar (F) and new (N) object during T2 were recorded separately and a discrimination index (D) was calculated (N - F/N + F). An animal was defined as impaired if the D was <0.20. This procedure took place twice for each animal. The first session was one day before the beginning of drugs administration. Among the 52 aged rats studied, 42 (81%) showed impaired performances with a D <0.10, four rats were unimpaired (D <0.50), while six rats were discarded because they did not explore. The 42 impaired aged rats were subdivided into the four aforementioned groups: Aged control group (10 rats), Group (R) (10 rats), Group (S) (11 rats), and Group (R + S) (11 rats). Among the 11 adult rats studied none was discarded, as all of them sufficiently explored. The second session was on the 34th day of the drugs administration, in order to evaluate the cognitive capacity of the animals practically at the end of the experiment. The task took place one hour after the drugs’ administration.
The passive avoidance training was started 24 h after the last object recognition session. It was carried out according to the procedure described by Riekkinen and colleagues (1997) with some modifications (the testing trial took place 24 h after the training trial and not 72 h after it) and consisted of two trials. The passive avoidance box had a light and a dark compartment of equal size, which were separated by a sliding guillotine door. During the first trial (training trial), which took place at the 35th day of drugs administration, the rats were placed in the light compartment. Thirty seconds later the door was opened. After the rat entered the dark compartment, the door was closed and a foot shock of 1.0 mA (3 s) was given. The latency to enter the dark compartment was measured (360 s maximum latency). During the second trial (testing trial), which took place on the 36th day of drugs administration (last day of the experiment), the rat was placed in the light compartment again and the latency to enter the dark compartment was measured. Passive avoidance most likely involves both working memory and reference memory (Myhrer 2003).
Statistical analysis
The biochemical data were analyzed by a two-tailed Student’s t-test. The object recognition data were analyzed by a nonparametric Mann-Whitney test and the passive avoidance data were analyzed by an one-way ANOVA test and a post-hoc test (Bonferroni test).
Drugs
Rivastigmine; Novartis Ltd., Basle, Switzerland. Selegiline; Sigma-Aldrich. St. Louis, MO, USA.
Results
Total antioxidant status (TAS)
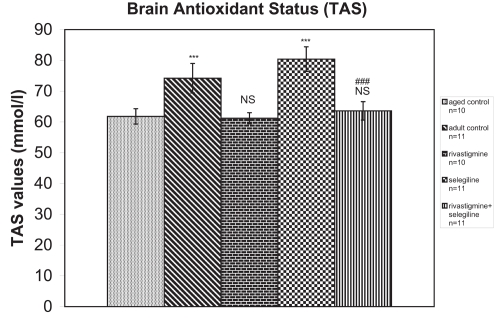
Group (S) (received selegiline) showed a significant increase in TAS compared with the control group of aged rats (+30%, P < 0.001, tvalue = 9.66). Groups (R) (received rivastigmine) and (R + S) (received rivastigmine + selegiline) did not show any difference in comparison to the aged control group (tvalues = 0.48 and 1.13 respectively). TAS was significantly decreased in rivastigmine + selegiline-treated rats compared with selegiline-treated rats (-21%, P < 0.001, tvalue = 16.66) (Figure 1).
Figure 1.
Effects of rivastigmine, selegiline, and rivastigmine + selegiline on brain antioxidant status (TAS). TAS values were determined in each homogenized rat whole brain. Values of the groups of aged control rats and of rivastigmine indicate the mean ± standard error (SE) of ten independent experiments (ten rats). Values of the groups of adult control rats, of selegiline, and of rivastigmine + selegiline indicate the mean ± SE. of eleven independent experiments (eleven rats). The average value of each experiment arises from three determinations.
Notes: NS, nonstatistical significance; ***P < 0.001 compared with aged control group; ###P < 0.001 compared with selegiline-treated group.
Brain AChE activity
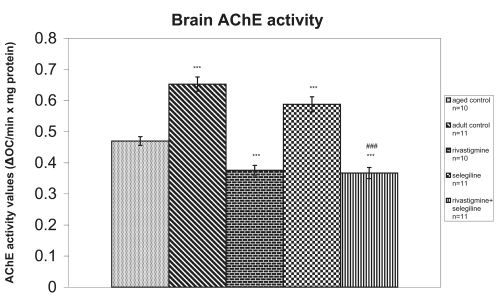
Selegiline-treated rats revealed a significant increase in brain AChE activity compared with the aged control rats (+25%, P < 0.001, tvalue = 10.73). Contrary to this, rivastigmine alone and rivastigmine+selegiline co-administration induced a significant decrease in brain AChE activity in comparison to the aged saline-treated rats (−20%, P < 0.001, tvalue = 10.80 and −22%, P < 0.001, tvalue = 11.08, respectively). Brain AChE activity was also significantly decreased in rats treated with rivastigmine+selegiline in comparison to the rats treated with selegiline alone (-38%, P < 0.001, tvalue = 36.99) (Figure 2).
Figure 2.
Effects of rivastigmine, selegiline, and rivastigmine + selegiline on brain AChE activity. AChE activities were determined in each homogenized rat whole brain. Values of the groups of aged control rats and of rivastigmine indicate the mean ± standard error (SE) of ten independent experiments (ten rats). Values of the groups of adult control rats, of selegiline and of rivastigmine + selegiline indicate the mean ± SE of eleven independent experiments (eleven rats). The average value of each experiment arises from three determinations.
Notes: ***P < 0.001 compared with aged control group; ###P < 0.001 compared with selegiline-treated group.
(Na+, K+)-ATPase activity
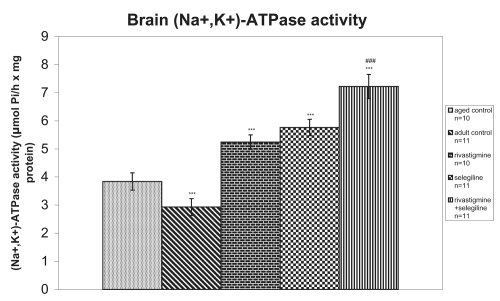
(Na+, K+)-ATPase activity was significantly increased in rats which received selegiline (+50%, P < 0.001, tvalue = 11.08) or rivastigmine (+36%, P < 0.001, tvalue = 8.48) in comparison with aged saline-treated rats. Furthermore, in the case of rivastigmine+selegiline co-administration, an additive action of the two drugs concerning the increase of (Na+, K+)-ATPase activity was revealed in comparison to the aged control group (+88%, P < 0.001, tvalue = 15.62). A significant increase of (Na+, K+)-ATPase activity in (R+S) group in comparison to the (S) group was also observed (+25%, P < 0.001, tvalue = 14.84) (Figure 3).
Figure 3.
Effects of rivastigmine, selegiline, and (rivastigmine + selegiline) on brain (Na+, K+)-ATPase activity. (Na+, K+)-ATPase activities were determined in each homogenized rat whole brain. Values of the groups of aged control rats and of rivastigmine indicate the mean ± standard error (SE) of ten independent experiments (ten rats). Values of the groups of adult control rats, of selegiline and of rivastigmine + selegiline indicate the mean ± SE of eleven independent experiments (eleven rats). The average value of each experiment arises from three determinations.
Notes: ***P < 0.001 compared with aged control group; ###P < 0.001 compared with selegiline-treated group.
Mg2+-ATPase activity
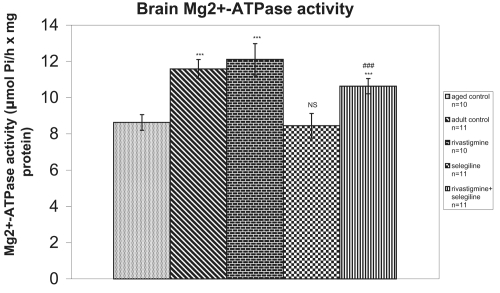
Mg2+-ATPase activity was significantly increased in (R) group and (R + S) group in comparison to the aged control group (+40%, P < 0.001, tvalue = 8.78 and +23%, P < 0.001, tvalue = 8.15, respectively), while no difference in Mg2+-ATPase activity was observed in the selegiline-treated group (tvalue = 0.55). The rivastigmine+selegiline co-administration induced a significant increase in Mg2+-ATPase activity in comparison to selegiline alone administration (+26%, P < 0.001, tvalue = 13.45) (Figure 4).
Figure 4.
Effects of rivastigmine, selegiline, and (rivastigmine + selegiline) on brain Mg++-ATPase activity. Mg++-ATPase activities were determined in each homogenized rat whole brain. Values of the groups of aged control rats and of rivastigmine indicate the mean ± standard error (SE) of ten independent experiments (ten rats). Values of the groups of adult control rats, of selegiline and of rivastigmine + selegiline indicate the mean ± SE of eleven independent experiments (eleven rats). The average value of each experiment arises from three determinations.
Notes: NS, nonstatistical significance; ***P < 0.001 compared with aged control group; ###P < 0.001 compared with selegiline-treated group.
Object recognition test
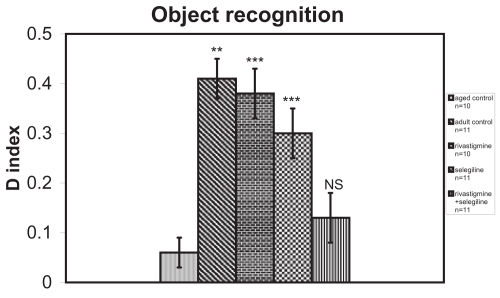
In the object recognition test the discrimination between familiar and novel objects was significantly better in selegiline-treated (P < 0.001, Mann-Whitney U = 4.50) or rivastigmine-treated rats (P < 0.001, Mann-Whitney U = 0.50) than aged saline-treated rats. There was no statistically significant difference in the discrimination index between (R + S) group (Mann-Whitney U = 12.00) and aged control group (Figure 5).
Figure 5.
Effects of rivastigmine, selegiline, and rivastigmine + selegiline on object recognition task on day 34 of the administration of the drugs. Values of the groups of aged control rats and of rivastigmine indicate the mean ± standard error (SE) of ten independent experiments (ten rats). Values of the groups of adult control rats, of selegiline and of rivastigmine + selegiline indicate the mean ± (SE) of eleven independent experiments (eleven rats).
Notes: NS, nonstatistical significance; **P < 0.05; ***P < 0.001 compared with aged control group.
Passive avoidance procedure
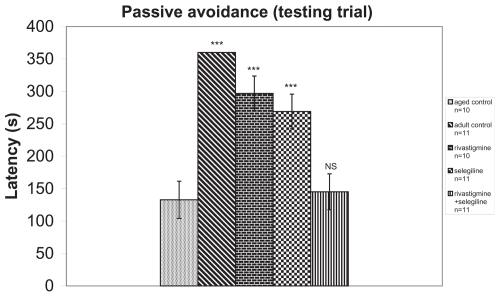
In passive avoidance procedure, during the training trial the mean latency (42 s) was not significantly different among the groups. During the testing trial, a significantly better performance was observed in groups (R)(F = 59.18) and (S)(F = 59.18), in comparison with the aged control group. The combination of rivastigmine + selegiline did not show any statistically significant difference from the aged control group (F = 59.18) (Figure 6). These results are similar to those of the object recognition test.
Figure 6.
Effects of rivastigmine, selegiline, and rivastigmine + selegiline on passive avoidance test in testing trial (day 36 of the administration of the drugs). Values of the groups of aged control rats and of rivastigmine indicate the mean ± standard error (SE) of ten independent experiments (ten rats). Values of the groups of adult control rats, of selegiline, and of rivastigmine + selegiline indicate the mean ± SE of eleven independent experiments (eleven rats).
Notes: NS, nonstatistical significance; ***P < 0.001 compared with aged control group.
Adult versus aged rats
An increase of total antioxidant status (TAS) was observed in adult control group compared with the aged control group (+20%, P < 0.001, tvalue = 5.62) (Figure 1). Adult controls revealed a significant increase in brain AChE activity compared with the aged control rats (+39%, P < 0.001, tvalue = 25.07) (Figure 2). (Na+, K+)-ATPase activity was significantly decreased in adult controls compared to the aged control group (−24%, P < 0.001, tvalue = 5.17) (Figure 3). Mg2+-ATPase activity was significantly increased in adult control group in comparison with the aged control group (+34%, P < 0.001, tvalue = 10.69) (Figure 4). In the object recognition test the adult control group performed significantly better than the aged one (P < 0.05, Mann-Whitney U = 9.00) (Figure 5). In passive avoidance procedure, during the testing trial, a better performance was observed in adult control group in comparison with the aged control group (F = 59.18) (Figure 6).
Discussion
We have examined: (a) the long term effects of rivastigmine in combination with selegiline on the activity of AChE, (Na+, K+)-ATPase, Mg2+-ATPase and TAS in whole brain homogenate of aged male rats, as well as their cognitive capacity; and (b) the possible relationship between biochemical and behavioral findings.
Rivastigmine decreased AChE activity in the aged rat whole brain as expected (Anand et al 1996; Corey-Bloom et al 1998), in comparison with the adult and aged control group (Figure 2). It is worth noticing that the adult control has the highest value of AChE activity. On the contrary, selegiline alone significantly increased AChE activity in the aged rats. The latter data are in accordance with other studies (Ricci et al 1992; Lakshmana et al 1998; Zhu et al 2000; Carageorgiou et al 2003). In particular Zhu and colleagues (2000) found increased AChE activity in the rat brain following 1 week of selegiline administration and Lakshmana and colleagues (1998) observed increased AChE activity in certain brain areas of adult monkeys following selegiline treatment with most pronounced effect in the region of hippocampus. Enhanced AChE activity in rat hippocampus was also reported by Ricci and colleagues (1992) following intracerebroventricular administration of selegiline. In the combination of rivastigmine + selegiline, the rivastigmine effect appears to prevail leading to a significant decrease in brain AChE activity compared with the (S) group and with the two control groups (Figure 2).
Reduced activity of AChE and ChAT has been reported in the cortex and the hippocampus of AD patients (Fishman et al 1986) and is also associated with the decline in cognitive function (DeKosky et al 1992). In our study, brain AChE activity was decreased in aged rats, in parallel with the object recognition and passive avoidance performance (Figure 2, 5-6). On the contrary, while rivastigmine (as expected) decreases AChE activity, it improves cognitive performance. Here a question could arise as to whether the 20% inhibition of AChE by rivastigmine is enough to produce an increase in cortical and hippocampal ACh release. Many studies in the literature using different doses of rivastigmine revealed a strong correlation between AChE inhibition and ACh increase in the aforementioned rat brain areas (Tanaka et al 1994; Chen et al 1998; Kozaka 1999; Trabace et al 2000; Scali et al 2002; Amenta et al 2006; Liang and Tang 2006). Considering the improved performance in learning and memory tests by rivastigmine group of rats, we suggest that this percentage reduction of AChE was enough for the needed cortical and hippocampal ACh release. Also, while selegiline increases AChE activity, it improves cognitive performance. In the combination of rivastigmine + selegiline treatment no improved performance was observed despite decreased AChE activity. Concerning AChE activity, it is obvious that the mechanism by which a better performance is attained in the behavioral tests following administration of rivastigmine or selegiline (given separately) is different. Rivastigmine is used in AD according to the cholinergic hypothesis, which improves or delays to some extent the deterioration of AD patients (Anand et al 1996; Corey-Bloom et al 1998; Bartus 2000). At the same time selegiline was found to improve cognitive function after long-term administration in rats (Knoll 2000) and to slow the progression of AD in man (Sano et al 1997). In our past experiments, although selegiline increased AChE activity, it improved avoidance performance (Carageorgiou et al 2003). The present study also shows improvement in both learning parameters. One can only speculate that the increased expression of ChAT and AChE in the hippocampus by selegiline (Ricci et al 1992) may have also caused an increase of ACh (which is mainly involved in learning and memory) provided that ACh release is not affected by selegiline in the hippocampus (Knoll 1989; Nowakowska et al 2001; de Lima et al 2005). Furthermore, it has been reported that increases in dopamine levels enhance a compensatory release of acetylcholine in the frontal cortex (Nilsson et al 1992; Shimazu et al 1996) and that the forebrain dopaminergic system is related to cognitive function (Marie and Defer 2003; Remy and Samson 2003). According to Koutsilieri and colleagues (2001) selegiline (given in a dose of 2 mg/kg) completely restores ChAT activity deficits in simian immunodeficiency infection in brain regions containing cholinergic neurons. It is unclear whether selegiline acted on the expression of ChAT (directly or through increased dopamine availability) thereby increasing protein synthesis or as a neuroprotective agent on cholinergic and other neurons through its antioxidant effects (Kitani et al 2002). In addition, Appleyard (1995) reported that AChE induced long-term potentiation in hippocampal pyramidal neurons, suggesting that AChE per se might enhance cognitive performance. We could refer to Shen’s (1994, 2004) hypotheses on the design of agents that could enhance the neuronal AChE activity in order to delay the degeneration of brain AChE system in the development of dementia and AD. Furthermore, Frolich (2002) has come to a similar conclusion concerning the cholinergic hypothesis in its present form and the use of AChE-inhibitors. According to Kaduszkiewicz and colleagues (2005), the scientific basis for recommendations of cholinesterase inhibitors for the treatment of Alzheimer’s disease is questionable because of flawed methods and small clinical benefits; even though the AChE inhibitors and memantine is the only available drug treatment until now (Lane 2006; Birks 2006).
In any case, one cannot exclude the neuronal adaptations to diminished synaptic ACh metabolism in acetylcholinesterase knockout mice (Volpicelli-Daley et al 2003) and the possibility of AChE involvement of the senile plaque (Rees and Brimijoin 2003; Castro and Martinez 2006).
The synaptic plasma membrane enzyme (Na+, K+)-ATPase is very important for neurotransmission-neuronal excitability (Sastry and Philips 1977), metabolic energy production (Mata et al 1980), the uptake and release of catecholamines (Bogdanski et al 1968; Swann 1984), serotonin (Hernandez 1987), glutamate (Lees et al 1990), and at least partial ACh release (Meyer and Cooper 1981). Studies suggest that (Na+, K+)-ATPase might play a role on memory formation (dos Reis-Lunardelli et al 2007) and that it is a particular age-related enzyme (Gorini et al 2002).
Increased activity of whole brain (Na+, K+)-ATPase was observed after the administration of rivastigmine, selegiline, or their combination (Figure 3). In the latter, the effect seems to be additive. Similar results concerning increased enzyme activity in aged and selegiline-treated rats were observed in our previous studies (Tsakiris et al 1996; Carageorgiou et al 2003).
Dickey and colleagues (2005) reported a decreased overall (Na+, K+)-ATPase enzyme activity in the amyloid containing hippocampi of the APP + PSI mice. They also reported absence of (Na+, K+)-ATPase staining in the zone surrounding congophilic plaques, which was occupied by dystrophic neurites and that cerebral (Na+, K+)-ATPase can be directly inhibited by high concentrations of soluble Ab. It has been also reported that (Na+, K+)-ATPase protein levels are decreased in AD tissue but not in normal aged tissue (Harik et al 1989; Liguri et al 1990). Our study deals with normally aged rats in which (Na+, K+)-ATPase activity was actually and significantly increased in comparison with the adult control, but their performance was decreased. Considering that ouabain, a (Na+, K+)-ATPase inhibitor, has been shown to impair memory consolidation, it was suggested that increasing (Na+, K+)-ATPase activity and maintaining ionic balance of the neurons may benefit AD patients by delaying the onset of neuritic dystrophia and memory dysfunction (Watts and Mark 1971; Mark and Watts 1971).
In our study with aged rats, in spite of enhanced (Na+, K+)-ATPase activity in comparison with the adult control, the aged animals had an impaired learning performance. In addition, in the (S) and (R) groups (with enhanced (Na+, K+)-ATPase activity) a better learning performance was observed, while in the combination group (in which an even higher increase of (Na+, K+)-ATPase activity was observed) no statistically significant difference in its learning performance was noticed (Figures 3, 5-6). According to all the above, we come to the conclusion that increased (Na+, K+)-ATPase activity is not relevant to the cognition enhancement by rivastigmine, selegiline, or their combination, at least not under our experimental conditions.
The role of Mg2+-ATPase is to maintain high brain intracellular Mg2+, the changes of which can control rates of protein synthesis and growth of the cell (Sanui and Rubin 1982). Decreased Mg2+-ATPase activity in the frontal cortex of old age rats was reported by Gorini and colleagues (2002). In our experiments, decreased Mg2+-ATPase activity was observed in old rats in parallel with decreased learning performance. Mg2+-ATPase activity was found to be higher in the groups (R), (R+S) and young controls (Figure 4). Consequently, a positive association between increased Mg2+-ATPase activity and learning performance could be supported for rivastigmine, while selegiline improves learning performance without affecting brain Mg2+-ATPase activity. In the combination, although there was an increased Mg2+-ATPase activity, no significant improvement in the behavioral parameters was observed. It is likely that the addition of selegiline in some way reduced the effect of rivastigmine alone on Mg2+-ATPase activity and this could influence the better performance of rivastigmine alone. This association is questionable as well.
The observed decrease of brain TAS during aging is in accordance with our previous studies (Tsakiris et al 1996; Carageorgiou et al 2003) and is associated with the decreased learning performance (object recognition and passive avoidance test) (Figures 1, 5-6). Although rivastigmine administration in aged rats did not affect TAS, it resulted in a better learning performance. On the contrary, selegiline administration (as previously shown by Carageorgiou and colleagues [2003]) increased TAS and this effect can be linked with better learning performance (Figures 1, 5-6). Similar results were also reported by Kiray and colleagues (2006): increase of spatial memory performance in aged male rats after selegiline administration for 21 days, suppression of lipid peroxidation, and alleviation of the age-related decrease of the number of neurons in the hippocampus. Improved performance after long term selegiline administration has also been observed by Knoll (1989) and other investigators (Nowakowska et al 2001; de Lima et al 2005). In the combination of rivastigmine + selegiline no statistically significant differences were observed either in TAS or in learning performance although there is a tendency in the object recognition test (Figures 1, 5). It is rather obvious that selegiline effect on TAS is blunted by rivastigmine (Figures 1, 5-6). Selegiline is a MAO-B inhibitor enhancing dopamine levels and after its chronic administration long-term postsynaptic changes have most likely occurred. The dopaminergic activity for example could be inhibitory to cholinergic striatal inter neurons. This probably explains the negation of the behavioral effects of rivastigmine by selegiline. In the study of Sagi and colleagues (2005) the chronic treatment of rats with ladostigil, a novel drug derived from the combination of rivastigmine and a MAO-B inhibitor rasagiline, resulted in activation of both striatal cholinergic and dopaminergic activity, attenuation of stereotyped motor behavior and maintenance of normal spontaneous motor performance. However, Takahata and colleagues (2005) (donepezil and selegiline in acute and high dose in scopolamine + chlorophenylalanine – induced memory deficits) and Dringenberg and colleagues (2000) (tacrine and selegiline with electroencephalographic and behavioral evidence) who used totally different experimental protocols observed that the combination of selegiline and another AChE inhibitor (donepezil or tacrine) acted synergistically and improved reversal of memory impairment in rats.
Conclusions
The overall analysis of our data revealed that rivastigmine when given alone decreases AChE, does not influence TAS, increases (Na+, K+)-ATPase and Mg2+-ATPase activities, and improves learning performance of the aged rats. In the combination the effect of rivastigmine on AChE activity (reduced) appears to prevail that of selegiline (increased) and the result is reduced activity of AChE. In the case of TAS, although rivastigmine when given alone, did not affect TAS, in the combination decreases the enhanced by selegiline old rat brain TAS and increases Mg2+-ATPase activity. There is also an improved learning performance by each drug alone, but not in the combination. It is obvious that the better performance of rivastigmine and selegiline given separately in the object recognition and in the passive avoidance test is attributed to a different mechanism of action: Selegiline possibly acts through its antioxidant effect and increased levels of catecholamines and rivastigmine by its anticholinesterase activity and increased levels of acetylcholine. Finally, the combination of the two drugs does not appear to be beneficial for the declining memory of aged rats at least not under our experimental conditions. Reduced Mg2+-ATPase activity is correlated with old age and reduced learning performance. Rivastigmine is correlated with increased Mg2+-ATPase activity and increased learning performance. Decreased TAS is correlated with old age and in parallel with decreased performance. Selegiline is correlated with increased TAS and increased performance. In the combination, rivastigmine+selegiline did not affect either TAS or learning performance. Several transmitter systems can probably have a primary function in some cognitive processes and among which are the cholinergic and dopaminergic ones, but the extent of interactions is difficult to be elucidated. The subject therefore requires further investigation.
To our knowledge this is the first report about a) the effect of rivastigmine on (Na+, K+)-ATPase and Mg2+-ATPase activities, TAS, on rat whole brain and b) the combined administration of rivastigmine and selegiline and its effect on all the studied parameters.
Acknowledgments
This work was funded by the University of Athens. The authors report no conflicts of interest.
References
- Amenta F, Tayebati SK, Vitali D, et al. Association with the cholinergic precursor choline alphoscerate and the cholinesterase inhibitor rivastigmine: An approach for enhancing cholinergic neurotransmission. Mech Ageing Dev. 2006;127:173–9. doi: 10.1016/j.mad.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Anand R, Gharabawi G, Enz A. Efficacy and safety results of the early phase studies with Exelon (ENA-713) in Alzheimer’s disease: an overview. J Drug Dev Clin Pract. 1996;8:109–116. [Google Scholar]
- Antoniades C, Carageorgiou H, Tsakiris S. Effects of (-)deprenyl (selegiline) on acetylcholinesterase and (Na+, K+)-ATPase activities in adult rat whole brain. Pharmacol Res. 2002;46:165–9. doi: 10.1016/s1043-6618(02)00090-7. [DOI] [PubMed] [Google Scholar]
- Appleyard ME. Acetylcholinesterase induces long-term potentiation in CA1 pyramidal cells by a mechanism dependent on metabotropic glutamate receptors. Neurosci Lett. 1995;190:25–8. doi: 10.1016/0304-3940(95)11491-e. [DOI] [PubMed] [Google Scholar]
- Atamna H, Frey WH., II Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer’s disease. Mitochondrion. 2007;7:297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, et al. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Birkmayer W, Knoll J, Riedeter P, et al. Increased life expectancy resulting from addition of L-deprenyl to Madopar treatment in Parkinson’s disease:a long term study. J Neural Transm. 1985;64:113–27. doi: 10.1007/BF01245973. [DOI] [PubMed] [Google Scholar]
- Birks J. AChE-inhibitors for Alzheimer’s disease. Cohrane Database Syst Rev. 2006;1:CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birks J, Flicker L. Selegiline for Alzheimer’s disease. Cochrane Database Syst Rev. 2003;1:CD000442. doi: 10.1002/14651858.CD000442. [DOI] [PubMed] [Google Scholar]
- Bogdanski DF, Tissari A, Brodie BB. Role of sodium, potassium, ouabain and reserpine in uptake, storage and metabolism of biogenic amines in synaptosomes. Life Sci. 1968;7:419–28. doi: 10.1016/0024-3205(68)90013-1. [DOI] [PubMed] [Google Scholar]
- Bowler K, Tirri R. The temperature characteristics of synaptic membrane ATPases from immature and adult rat brain. J Neurochem. 1974;23:611–13. doi: 10.1111/j.1471-4159.1974.tb06068.x. [DOI] [PubMed] [Google Scholar]
- Caltagirone C, Bianchetti A, Di Luca M, et al. Guidelines for the treatment of Alzheimer’s disease from the Italian Association of Psychogeriatrics. Drugs Aging. 2005;22(Suppl 1):1–26. doi: 10.2165/00002512-200522001-00002. [DOI] [PubMed] [Google Scholar]
- Carageorgiou H, Zarros A, Tsakiris S. Selegiline long-term effects on brain acetylcholinesterase, (Na+, K+)-ATPase activities, antioxidant status and learning performance of aged rats. Pharmacol Res. 2003;48:245–51. doi: 10.1016/s1043-6618(03)00149-x. [DOI] [PubMed] [Google Scholar]
- Carrillo MC, Kitani K, Kanai S, et al. (-)Deprenyl increases activities of superoxide dismutase and catalase in certain brain regions in old male mice. Life Sci. 1994;54:975–81. doi: 10.1016/0024-3205(94)00499-4. [DOI] [PubMed] [Google Scholar]
- Castro A, Martinez A. Targeting beta-amyloid pathogenesis through acetylcholinesterase inhibitors. Curr Pharm Res. 2006;12:4377–87. doi: 10.2174/138161206778792985. [DOI] [PubMed] [Google Scholar]
- Chen Y, Shohami E, Bass R, et al. Cerebro-protective effects of ENA713, a novel acetylcholinesterase inhibitor, in closed head injury in the rat. Brain Res. 1998;784:18–24. doi: 10.1016/s0006-8993(97)00982-7. [DOI] [PubMed] [Google Scholar]
- Cohen G, Spina MB. Deprenyl suppresses the oxidant stress associated with increased dopamine turnover. Ann Neurol. 1989;26:689–90. doi: 10.1002/ana.410260518. [DOI] [PubMed] [Google Scholar]
- Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Washington, DC: Institute of Laboratory Animal Resources, National Research Council; 1985. p. 83. [Google Scholar]
- Corey-Bloom J, Anand R, Veach J. A randomized trial evaluating the efficacy and safety of Ena 713 (rivastigmine tartrate), a new acetyl-ChE inhibitor, in patients with mild to moderately severe Alzheimer’s disease. Int J Geriatr Psychopharmacol. 1998;1:55–65. [Google Scholar]
- Coyle JT, Price DL, De Long MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–90. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Dantoine T, Auriacombe S, Sarazin M, et al. Rivastigmine monotherapy and combination therapy with memantine in patients with moderately severe Alzheimer’s disease who failed to benefit from previous cholinesterase inhibitor treatment. Int J Clin Pract. 2006;60:110–18. doi: 10.1111/j.1368-5031.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- Davies P, Wolozin BL. Recent advances in the neurochemistry of Alzheimer’s disease. J Clin Psychiatry. 1987;48(Suppl):23–30. [PubMed] [Google Scholar]
- DeKosky ST, Harbaugh RE, Schmitt FA, et al. Cortical biopsy in Alzheimer’s disease: diagnostic accuracy and neurochemical, neuropathological, and cognitive correlations. Intraventricular Bethanecol Study Group. Ann Neurol. 1992;32:625–32. doi: 10.1002/ana.410320505. [DOI] [PubMed] [Google Scholar]
- de Lima MN, Laranja DC, Caldana F, et al. Reversal of age – related deficits in object recognition memory in rats with L-deprenyl. Exp Gerontol. 2005;40:506–11. doi: 10.1016/j.exger.2005.03.004. [DOI] [PubMed] [Google Scholar]
- dos Reis-Lunardelli EA, Castro CC, Bavaresco C, et al. Effects of thyroid hormones on memory and on Na(+), K(+)-ATPase activity in rat brain. Curr Neurovasc Res. 2007;4:184–93. doi: 10.2174/156720207781387204. [DOI] [PubMed] [Google Scholar]
- Dickey CA, Gordon MN, Wilcock DM, et al. Dysregulation of (Na+, K+)-ATPase by amyloid in APP+PS1 transgenic mice. BMC Neurosci. 2005;6:7. doi: 10.1186/1471-2202-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Laporte PP, Diavolitsis P. Increased effectiveness of tacrine by deprenyl co-treatment in rats: EEG and behavioral evidence. Neuroreport. 2000;11:3513–16. doi: 10.1097/00001756-200011090-00022. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Sharma S, Shavali S, et al. Neuroprotective actions of selegiline. J Neurosci Res. 2002;67:285–9. doi: 10.1002/jnr.10148. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney D, Andres D, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Eskander MF, Nagykery NG, Leung EY, et al. Rivastigmine is a potent inhibitor of acetyl-and butyrylcholinesterase in Alzheimer’s plaques and tangles. Brain Res. 2005;1060:144–52. doi: 10.1016/j.brainres.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Farlow MR, Small GW, Quarg P, et al. Efficacy of rivastigmine in Alzheimer’s disease patients with rapid disease progression: results of a meta-analysis. Dement Geriatr Cogn Disord. 2005;20:192–7. doi: 10.1159/000087301. [DOI] [PubMed] [Google Scholar]
- Filip V, Kolibas E. Selegiline in the treatment of Alzheimer’s disease: a long-term randomized placebo-controlled trial. Czech and Slovak Senile Dementia of Alzheimer type Study Group. J Psychiatry Neurosci. 1999;24:234–43. [PMC free article] [PubMed] [Google Scholar]
- Fishman EB, Siek GC, MacCallum RD, et al. Distribution of the molecular forms of acetylcholinesterase in human brain: alterations in dementia of the Alzheimer type. Ann Neurol. 1986;19:246–52. doi: 10.1002/ana.410190305. [DOI] [PubMed] [Google Scholar]
- Frolich L. The cholinergic pathology in Alzheimer’s disease – discrepancies between clinical experience and pathophysiological findings. J Neural Transm. 2002;109:1003–13. doi: 10.1007/s007020200083. [DOI] [PubMed] [Google Scholar]
- González-Gutiérrez JL, Gobartt AL, en representación del grupo de investigadores RIVASOL Rivastigmine solution prescribing habits in patients with Alzheimer-type dementia in Spain (RIVASOL study) Rev Neurol. 2007;44:705–10. [PubMed] [Google Scholar]
- Gorini A, Canosi U, Devecchi E, et al. ATPases enzyme activities during ageing in different types of somatic and synaptic plasma membranes from rat frontal cerebral cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:81–90. doi: 10.1016/s0278-5846(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Groner E, Ashani Y, Schorer-Apelbaum D, et al. The kinetics of inhibition of human acetylcholinesterase and butyrylcholinesterase by two series of novel carbamates. Mol Pharmacol. 2007;71:1610–17. doi: 10.1124/mol.107.033928. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harik SI, Mitchell MJ, Kalaria RN. Ouabain binding in the human brain. Effects of Alzheimer’s disease and aging. Arch Neurol. 1989;46:951–4. doi: 10.1001/archneur.1989.00520450021013. [DOI] [PubMed] [Google Scholar]
- Henning RH, Nelemans SA, van den Akker J, et al. Induction of Na+/K+-ATPase activity in long- term stimulation of nicotinic acetylcholine receptors in C2C12 myotubes. Br J Pharmacology. 1994;111:1271–89. doi: 10.1111/j.1476-5381.1994.tb14758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J. Brain (Na+,K+)-ATPase activity possibly regulated by a specific serotonin receptor. Brain Res. 1987;408:399–402. doi: 10.1016/0006-8993(87)90414-8. [DOI] [PubMed] [Google Scholar]
- Ju WY, Holland DP, Tatton WG. (-)-Deprenyl alters the time course of death of axotomised facial motoneurons and the hypertrophy of neighboring astrocytes in immature rats. Exp Neurol. 1994;126:233–46. doi: 10.1006/exnr.1994.1061. [DOI] [PubMed] [Google Scholar]
- Jones DNC, Barnes JC, Kirby DL, et al. Age – associated impairments in a test of attention: Evidence for involvement of cholinergic systems. J Neurosci. 1995;15:7282–92. doi: 10.1523/JNEUROSCI.15-11-07282.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaduszkíewicz H, Zimmermann T, Beck-Bornholdt HP, et al. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomized clinical trials. BMJ. 2005;331:321–7. doi: 10.1136/bmj.331.7512.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Sharma D, Singh R, et al. Regional effects of ageing on (Na+, K+)-ATPase activity in rat brain and correlation with multiple unit action potentials and lipid peroxidation. Indian J Biochem Biophys. 1998;35:364–71. [PubMed] [Google Scholar]
- Kiray M, Bagriyianik HA, Pekcetin C, et al. Deprenyl and the relationship between its effects on spatial memory, oxidant stress and hippocampal neurons in aged male rats. Physiol Res. 2006;55:205–12. doi: 10.33549/physiolres.930742. [DOI] [PubMed] [Google Scholar]
- Kitani K, Minami C, Isobe K, et al. Why (-)deprenyl prolongs survivals of experimental animals: increase of anti-oxidant enzymes in brain and other body tissues as well as mobilization of various humoral factors may lead to systemic anti-aging effects. Mech Ageing Dev. 2002;123:1087–100. doi: 10.1016/s0047-6374(01)00392-x. [DOI] [PubMed] [Google Scholar]
- Knoll J. Enhancer regulation/endogenous and synthetic enhancer compounds: a neurochemical concept of the innate and acquired drives. Neurochem Res. 2003;28:1275–97. doi: 10.1023/a:1024224311289. [DOI] [PubMed] [Google Scholar]
- Knoll J. (-)Deprenyl (selegiline): past, present, and future. Neurobiology. 2000;8:179–99. [PubMed] [Google Scholar]
- Knoll J. The pharmacology of selegiline ((-)deprenyl). New aspects. Acta Neurol Scand Suppl. 1989;126:83–91. doi: 10.1111/j.1600-0404.1989.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Kosasa T, Kuriya Y, Matsui K, et al. Effect of donepezil hydrochloride (E2020) on basal concentration of extracellular acetylcholine in the hippocampus of rats. Eur J Pharmacol. 1999;380:101–7. doi: 10.1016/s0014-2999(99)00545-2. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Scheller C, Sopper S, et al. Selegiline completely restores choline acetyltransferase activity deficits in simian immunodeficiency infection. Eur J Pharmacol. 2001;411:R1–R2. doi: 10.1016/s0014-2999(00)00874-8. [DOI] [PubMed] [Google Scholar]
- Lakshmana MK, Rao BS, Dhingra NK, et al. Chronic (-)deprenyl administration increases dendritic arborization in CA3 neurons of hippocampus and AChE activity in specific regions of the primate brain. Brain Res. 1998;796:38–44. doi: 10.1016/s0006-8993(98)00312-6. [DOI] [PubMed] [Google Scholar]
- Lane RM, Potkin SG, Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol. 2006;9:101–24. doi: 10.1017/S1461145705005833. [DOI] [PubMed] [Google Scholar]
- Lees GJ, Lehmann A, Sandberg M, et al. The neurotixicity of ouabain, a sodium-potassium ATPase inhibitor, in the rat hippocampus. Neurosci Lett. 1990;120:159–162. doi: 10.1016/0304-3940(90)90027-7. [DOI] [PubMed] [Google Scholar]
- Liang YQ, Tang XC. Comparative studies, donepezil and rivastigmine on brain acetylcholine, dopamine, norepinephrine, and 5-hydroxytryptamine levels in freely-moving rats. Acta Pharmacol Sin. 2006;27:1127–36. doi: 10.1111/j.1745-7254.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- Lieberman A, Fazzini E. Experience with selegiline and levodopa in advanced Parkinson’s disease. Acta Neurol Scand Suppl. 1991;136:66–9. doi: 10.1111/j.1600-0404.1991.tb05022.x. [DOI] [PubMed] [Google Scholar]
- Liguri G, Taddei N, Nassi P, et al. Changes in (Na+, K+)-ATPase, Ca2+-ATPase and some soluble enzymes related to energy metabolism in brains of patients with Alzheimer’s disease. Neurosci Lett. 1990;112:338–42. doi: 10.1016/0304-3940(90)90227-z. [DOI] [PubMed] [Google Scholar]
- Liu J, Ames BN. Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer’s disease, and Parkinson’s disease. Nutr Neurosci. 2005;8:67–89. doi: 10.1080/10284150500047161. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- Magyar K, Haberle D. Neuroprotective and neuronal rescue effects of selegiline: review. Neurobiology. 1999;7:175–90. [PubMed] [Google Scholar]
- Marie RM, Defer GL. Working memory and dopamine: clinical and experimental clues. Curr Opin Neurol. 2003;16(Supp l):S29–S35. doi: 10.1097/00019052-200312002-00006. [DOI] [PubMed] [Google Scholar]
- Mark RF, Watts ME. Drug inhibition of memory formation in chickens. I. Long term memory. Proc R Soc Lond B Biol Sci. 1971;178:439–54. doi: 10.1098/rspb.1971.0074. [DOI] [PubMed] [Google Scholar]
- Mata M, Fink DJ, Gainer H, et al. Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem. 1980;34:213–15. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- Matsubara K, Senda T, Uezono T, et al. L-Deprenyl prevents the cell hypoxia induced by dopaminergic neurotoxins, MPP(+) and beta-carbolinium: a microdialysis study in rats. Neurosci Lett. 2001;302:65–8. doi: 10.1016/s0304-3940(01)01601-9. [DOI] [PubMed] [Google Scholar]
- Meyer ME, Cooper RJ. Correlations between (Na+, K+)-ATPase activity and acetylcholine release in rat cortical synaptosomes. J Neurochem. 1981;36:467–75. doi: 10.1111/j.1471-4159.1981.tb01616.x. [DOI] [PubMed] [Google Scholar]
- Murphy KJ, Foley AG, O’Connell AW, et al. Chronic exposure of rats to cognition enhancing drugs produces a neuroplastic response identical to that obtained by complex environment rearing. Neuropsychopharmacology. 2006;31:90–100. doi: 10.1038/sj.npp.1300810. [DOI] [PubMed] [Google Scholar]
- Myhrer T. Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res Rev. 2003;41:268–87. doi: 10.1016/s0165-0173(02)00268-0. [DOI] [PubMed] [Google Scholar]
- Negrotti A, Bizzarri G, Calzetti S. Long-term persistence of symptomatic effect of selegiline in Parkinson’s disease. A two-months placebo-controlled withdrawal study. J Neural Transm. 2001;108:215–19. doi: 10.1007/s007020170089. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Leanza G, Bjorklund A. Acetylcholine release in the hippocampus: regulation by monoaminergic afferents as assessed by in vivo microdialysis. Brain Res. 1992;584:132–40. doi: 10.1016/0006-8993(92)90886-e. [DOI] [PubMed] [Google Scholar]
- Nowakowska E, Kus K, Chodera A, et al. Investigating potential anxiolytic, antidepressant and memory enhancing activity of deprenyl. J Physiol Pharmacol. 2001;52(4 Pt 2):863–73. [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, et al. Anti-Parkinsonian agents have anti-amyloidogenic activity for Alzheimer’s beta amyloid fibrils in vitro. Neurochem Int. 2006;48:275–85. doi: 10.1016/j.neuint.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Pratico D, Delanty N. Oxidative injury in diseases of the central nervous system: focus on Alzheimer’s disease. Am J Med. 2000;109:577–85. doi: 10.1016/s0002-9343(00)00547-7. [DOI] [PubMed] [Google Scholar]
- Rees TM, Brimijoin S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today (Barc) 2003;39:75–83. doi: 10.1358/dot.2003.39.1.740206. [DOI] [PubMed] [Google Scholar]
- Remy P, Samson Y. The role of dopamine in cognition: evidence from functional imaging studies. Curr Opin Neurol. 2003;16(Suppl 2):S37–S41. doi: 10.1097/00019052-200312002-00007. [DOI] [PubMed] [Google Scholar]
- Ricci A, Mancini M, Strocchi P, et al. Deficits in cholinergic neurotransmission markers induced by ethylcholine mustard aziridium (AF64A) in the rat hippocampus: sensitivity to treatment with the monoamine oxidase-B inhibitor L-deprenyl. Drugs Exp Clin Res. 1992;18:163–71. [PubMed] [Google Scholar]
- Riekkinen M, Schmidt B, Kuitunen J, et al. Effects of combined chronic nimodipine and acute metrifonate treatment on spatial and avoidance behavior. Eur J Pharmacol. 1997;322:1–9. doi: 10.1016/s0014-2999(96)00976-4. [DOI] [PubMed] [Google Scholar]
- Rodríguez de Lores Arnaiz G, Pellegrino de Iraldi A. The release of catecholamines by an endogenous factor that inhibits neuronal Na+,K(+)-ATPase. Microsc Electron Biol Celular. 1991;15:93–105. [PubMed] [Google Scholar]
- Sagi Y, Drigués N, Youdim BHM. The neurochemical and behavioral effects of the novel cholinesterase-monoamine oxidase inhibitor, ladostigil, in response to L-dopa and L-tryptophan, in rats. Br J Pharmacol. 2005;146:553–60. doi: 10.1038/sj.bjp.0706355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. New Engl J Med. 1997;336:1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Sanui H, Rubin H. The role of magnesium in cell proliferation and transformation. In: Boynton AL, McKeehan WL, Whitfield JP, editors. Ions, cell proliferation and cancer. Palgrave, New York: Academic Pr; 1982. pp. 517–37. [Google Scholar]
- Sastry BS, Philips JW. Antagonism of biogenic amine-induced depression of celebral cortical neurones by (Na+, K+)-ATPase inhibitors. Can J Physiol Pharmacol. 1977;55:170–80. doi: 10.1139/y77-025. [DOI] [PubMed] [Google Scholar]
- Scali C, Casamenti F, Bellucci A, et al. Effect of subchronic administration of metrifonate, rivastigmine and donepezil on brain acetylcholine in aged F344 rats. J Neural Transm. 2002;109:1067–80. doi: 10.1007/s007020200090. [DOI] [PubMed] [Google Scholar]
- Scali C, Casamenti F, Pazzagli M, et al. Nerve growth factor increases extracellular acetylcholine levels in the parietal cortex and hippocampus of aged rats and restores object recognition. Neurosci Lett. 1994;170:117–20. doi: 10.1016/0304-3940(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Shen ZX. Brain Cholinesterases: III. Future perspectives of AD research and clinical practice. Med Hypotheses. 2004;63:298–307. doi: 10.1016/j.mehy.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Shen ZX. Acetylcholinesterase provides deeper insights into Alzheimer’s disease. Med Hypotheses. 1994;43:21–30. doi: 10.1016/0306-9877(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Shimazu S, Sekimoto H, Tanida N, et al. Effect of Selegiline on cholinergic system of brain and learning behavior. Neurobiol Aging. 1996;17:S29–S30. [Google Scholar]
- Siqueira IR, Fochesatto C, de Andrande A, et al. Total antioxidant capacity is impaired in different structures from aged rat brain. Int J Devl Neurosci. 2005;23:663–71. doi: 10.1016/j.ijdevneu.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Skou JC. Nobel Lecture. The identification of the sodium pump. Biosci Rep. 1998;18:155–69. doi: 10.1023/a:1020196612909. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci USA. 1991;88:10540–3. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoer JD, Mobley SL, Roice D, et al. The effects of selective cholinergic basal forebrain lesions and aging upon expectancy in the rat. Neurobiol Learn Mem. 1997;67:214–27. doi: 10.1006/nlme.1997.3768. [DOI] [PubMed] [Google Scholar]
- Strong R. Neurochemical changes in the aging human brain: implications for behavioral impairment and neurodegenerative disease. Geriatrics. 1998;53(Suppl 1):S9–S12.S. [PubMed] [Google Scholar]
- Swann AC. (Na+, K+)-adenosine triphosphatase regulation by the sympathetic nervous system: effects of noradrenergic stimulation and lesion in vivo. J Pharmacol Exp Ther. 1984;228:304–11. [PubMed] [Google Scholar]
- Tahirovic I, Sofic E, Sapcanin A, et al. Brain antioxidant capacity in rat models of betacytotoxic-induced experimental sporadic Alzheimer’s disease and diabetes mellitus. Neurochem Res. 2007;32:1709–17. doi: 10.1007/s11064-007-9410-1. [DOI] [PubMed] [Google Scholar]
- Takahata K, Shimazu S, Katsuki H, et al. Effects of selegiline on antioxidant systems in the nigrostriatum in rat. J Neural Transm. 2006;113:151–8. doi: 10.1007/s00702-005-0309-1. [DOI] [PubMed] [Google Scholar]
- Takahata K, Minami A, Kusumoto H, et al. Effects of selegiline alone or with donepezil on memory impairment in rats. Eur J Pharmacol. 2005;518:140–4. doi: 10.1016/j.ejphar.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Takeda A, Loveman E, Clegg A, et al. A systematic review of the clinical effectiveness of donepezil, rivastigmine and galantamine on cognition, quality of life and adverse events in Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:17–28. doi: 10.1002/gps.1402. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ogawa N, Asanuma M, et al. Chronic administration of acetylcholinesterase inhibitor in the senescent rat brain. Neurobiol Aging. 1994;15:721–5. doi: 10.1016/0197-4580(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Trabace L, Coluccian A, Gaetani S, et al. In vivo neurochemical effects of the acetylcholinesterase inhibitor ENA713 in rat hippocampus. Brain Res. 2000;865:268–71. doi: 10.1016/s0006-8993(00)02266-6. [DOI] [PubMed] [Google Scholar]
- Tsakiris S, Angelogianni P, Schulpis KH, et al. Protective effect of L-cysteine and glutathione on rat brain (Na+,K+)-ATPase inhibition induced by free radicals. Z Naturforsch. 2000;55:271–7. doi: 10.1515/znc-2000-3-421. [DOI] [PubMed] [Google Scholar]
- Tsakiris S, Angelogianni P, Stavridis JC. Correlations between activities of (Na+, K+)-ATPase and acetylcholinesterase in postnatally developing rat brain. Med Sci Res. 1996;24:155–6. [Google Scholar]
- Ucar G, Gokhan N, Yesilada A, et al. 1-N-Substituted thiocarbamoyl-3-phenyl-5-thienyl-2-pyrazolines: a novel cholinesterase and selective monoamine oxidase B inhibitors for the treatment of Parkinson’s and Alzheimer’s diseases. Neurosci Lett. 2005;382:327–31. doi: 10.1016/j.neulet.2005.03.028. [DOI] [PubMed] [Google Scholar]
- van Dyck CH. Understanding the latest advances in pharmacologic interventions for Alzheimer’s disease. CNS Spectr. 2004;9(7 Suppl 5):24–8. doi: 10.1017/s1092852900024780. [DOI] [PubMed] [Google Scholar]
- Vannucchi MG, Scali C, Kopf SR, et al. Selective muscarinic antagonists differentially affect in vivo acetylcholine release and memory performances of young and aged rats. Neuroscience. 1997;79:837–46. doi: 10.1016/s0306-4522(97)00091-2. [DOI] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Hrabovska A, Duysen EG, et al. Altered striatal function and muscarinic cholinergic receptors in acetylcholinesterase knockout mice. Mol Pharmacol. 2003;64:1309–16. doi: 10.1124/mol.64.6.1309. [DOI] [PubMed] [Google Scholar]
- Walsh TJ, Tilson HA, DeHaven DL, et al. AF64A, a cholinergic neurotoxin, selectively depletes acetylcholine in hippocampus and cortex, and produces long-term passive avoidance and radial-arm maze deficits in the rat. Brain Res. 1984;321:91–102. doi: 10.1016/0006-8993(84)90684-x. [DOI] [PubMed] [Google Scholar]
- Watts ME, Mark RF. Separate actions of ouabain and cycloheximide on memory. Brain Res. 1971;25:420–3. doi: 10.1016/0006-8993(71)90450-1. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Drigues N, Sagi Y, et al. The application of proteomics and genomics to the study of age-related neurodegeneration and neuroprotection. Antioxid Redox Signal. 2007;9:169–79. doi: 10.1089/ars.2007.9.169. [DOI] [PubMed] [Google Scholar]
- Wilcock GK, Birks J, Whitehead A, et al. The effect of selegiline in the treatment of people with Alzheimer’s disease: a meta-analysis of published trials. Int J Geriatr Psychiatry. 2002;17:175–83. doi: 10.1002/gps.545. [DOI] [PubMed] [Google Scholar]
- Xie Z, Cai T. Na+-K+ – ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3:157–68. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Wadia A, Tatton W, et al. The anti-Parkinson drug rasagiline and its cholinesterase inhibitor derivatives exert neuroprotection unrelated to MAO inhibition in cell culture and in vivo. Ann N Y Acad Sci. 2001;939:450–8. doi: 10.1111/j.1749-6632.2001.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Weinstock M. Novel neuroprotective anti-Alzheimer drugs with anti-depressant activity derived from the anti-Parkinson drug, rasagiline. Mech Ageing Dev. 2002;123:1081–6. doi: 10.1016/s0047-6374(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Zhu J, Hamm RJ, Reeves TM, et al. Postinjury administration of L-deprenyl improves cognitive function and enhances neuroplasticity after traumatic brain injury. Exp Neurol. 2000;166:136–52. doi: 10.1006/exnr.2000.7484. [DOI] [PubMed] [Google Scholar]