Abstract
Reactive oxygen species (ROS) produced by skeletal muscle stimulate adaptive responses to activity and mediate some degenerative processes. ROS activity is usually studied by measuring indirect end-points of their reactions with various biomolecules. In order to develop a method to measure the intracellular ROS generation in real-time in mature skeletal muscle fibers, these were isolated from the flexor digitorum brevis (FDB) muscle of mice and cultured on collagen-coated plates. Fibers were loaded with 5- (and 6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-DCFH DA) and measurements of 5- (and 6-) chloromethyl-2′,7′-dichlorofluorescin (CM-DCF) fluorescence from individual fibers obtained by microscopy over 45 min. The sensitivity of this approach was demonstrated by addition of 1 μM H2O2 to the extracellular medium. Contractions of isolated fibers induced by field electrical stimulation caused a significant increase in CM-DCF fluorescence that was abolished by pre-treatment of fibers with glutathione ethyl ester. Thus, CM-DCF fluorescence microscopy can detect physiologically relevant changes in intracellular ROS activity in single isolated mature skeletal muscle fibers in real-time, and contractions generated a net increase that was abolished when the intracellular glutathione content was enhanced. This technique has advantages over previous approaches because of the maturity of the fibers and the analysis of single cells which prevents contributions from nonmuscle cells.
INTRODUCTION
Reactive oxygen species (ROS) are continuously produced by skeletal muscle. They are thought to act to stimulate adaptive responses in skeletal muscle and may also mediate some degenerative processes (30, 34). Historically, ROS activities in tissues, including skeletal muscle, have usually been studied using indirect markers based on end-point products of the reactions of ROS and reactive nitrogen species with lipids, proteins, or DNA (14, 22). Other studies have obtained indirect information on the role of ROS in skeletal muscle by evaluation of the activities of the main antioxidant enzymes, such as manganese superoxide dismutase, copper, zinc superoxide dismutase, catalase, and glutathione peroxidase (34).
A variety of different technical approaches have been applied to study the activity and role of ROS in skeletal muscle (34). This tissue has been examined using both in vivo (36) and in vitro (39) studies. Our group has recently developed the technique of microdialysis to quantify ROS in the extracellular fluid of muscle in vivo, but cell types other than skeletal muscle such as endothelial cells, lymphocytes, and fibroblasts may contribute to the ROS detected in the extracellular fluid (13, 30). Other studies have reported data on ROS activity in contracting skeletal muscles using the ROS-sensitive fluorescent indicator 2′,7′ dichlorodihydrofluorescein (DCFH). These involved the measurement of 2′,7′ dichlorofluorescin (DCF) fluorescence from homogenized myotubes (42) or homogenized muscle (7), but disruption of the tissue during homogenization may lead to artifactual enhanced ROS generation (18). DCFH has also been used as an intracellular probe to examine ROS activity directly by measurement of epifluorescence from strips of isolated diaphragm (38), but again the DCF fluorescence may have also originated from other types of cells in addition to the muscle fibers.
Recently, studies of both extracellular and intracellular ROS generation have been undertaken using skeletal muscle myotubes in culture (30, 31, 37, 44) although since myotubes are immature skeletal muscle cells, their utility as a model to study ROS activities in mature skeletal muscle is limited.
A major aim of the current study was to develop and validate a new method, based on dichlorofluoroscein fluorescence microscopy, to monitor the generation of ROS in real-time in single mature skeletal muscle fibers. We argue that this approach may have significant advantages over current techniques, since the measurements are made from mature skeletal muscle fibers that have been isolated from adult muscle and hence are likely to reflect the situation in muscle in vivo to a greater extent than is seen with myotubes, the data are derived from a single isolated muscle fiber, avoiding any contribution to ROS generation from other cell types located within muscle tissue, and the isolated mature muscle fibers are viable in culture and retain excitability to electrical stimuli allowing for real-time monitoring of ROS generation during muscle contractile activity. A second aim of the study was to define whether manipulation of the content of the major intracellular reductant, glutathione in isolated fibers, would influence their ROS activity both at rest and following contractile activity.
METHODS
Isolation of single skeletal muscle fibers
Experiments were performed in accordance with UK Home Office guidelines under the UK Animals (Scientific Procedures) Act 1986. Female C57BL/6 mice, 2–4 months old, were humanely killed and the flexor digitorum brevis muscles were removed and placed into 0.4% type H collagenase (EC 3.4.24.3) (Sigma–Aldrich Co. St. Louis, MO) solution in culture medium. This was composed of minimum essential medium Eagle (MEM) (Sigma–Aldrich Co.) supplemented with 10 % fetal bovine serum (FBS) (Invitrogen Ltd, Paisley, UK) containing 2 mM glutamine, 50 i.u. penicillin, and 50 μg ml−1 streptomycin. Both FDB muscles from each mouse were incubated in collagenase solution at 37°C for 2 h, the mixture was manually shaken every 30 min to improve digestion of the connective tissue. Fiber bundles that had not been separated during the incubation were gently triturated by a wide-bore plastic pipette to separate the fibers. Free single muscle fibers were separated from damaged fibers and contaminating cells by centrifugation at low speed (600 g for 30 s). The fibers were washed four times in fresh culture medium. Cleaned fibers were plated onto 35-mm dishes pre-coated with 120 μl of a 1:6 mixture of Vitrogen collagen (Cohesion Technologies Inc., Palo Alto, CA) in 7 × Dulbecco's modified Eagle's medium (D-MEM) (Invitrogen Ltd.). Fibers were allowed to attach for 30 min at 37°C, 1 ml of fresh culture medium was added to each plate, and fibers were cultured for 18 h at 37°C in a 5% CO2 atmosphere (2, 41).
Viability of single skeletal muscle fibers
The viability of the isolated muscle fibers in culture was determined by examination of their morphology by bright-field microscopy and by their ability to exclude the vital stain, trypan blue. Fibers were incubated in a 0.04 mg/ml solution of trypan blue (Sigma-Aldrich Co.) in culture medium and examined under bright-field microscopy.
Loading of single skeletal muscle fibers with CM-DCFH DA
A solution of 5- (and 6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-DCFH DA) (Molecular Probes,™ Invitrogen, Eugene, OR) was prepared daily in absolute ethanol and kept at 4°C. Culture plates containing isolated fibers were washed with Dulbecco's Phosphate Buffered Saline (D-PBS) (Sigma-Aldrich Co.), and fibers were pre-incubated in D-PBS at 37°C for 30 min. The medium was then replaced by D-PBS containing CM-DCFH DA (17.5 μM) and incubated for 30 min at 37°C. The fibers were then washed with D-PBS and covered with MEM without Phenol Red (Sigma-Aldrich Co.) for fluorescence microscopy.
5- (and 6-) chloromethyl-2′,7′-dichlorodihydrofluorescein (CM-DCFH) is a derivative of DCFH modified to improve retention by cells. The diacetate form (CM-DCFH DA) is a non-polar molecule that diffuses into the fibers, and within the cytosol the acetate group is cleaved by cytosolic esterases to yield CM-DCFH, a hydrophilic nonfluorescent molecule that is retained by the cell. Intracellular ROS, particularly hydrogen peroxide, convert CM-DCFH to its fluorescent derivate, CM-DCF (49).
Fluorescence microscopy and image analysis
The imaging system consisted of a Zeiss Axiovert 200M epifluorescence microscope equipped with X10 and X20 objectives and filter sets: a 450–490 nm excitation/515–565 nm emission filter set that was used for CM-DCF fluorescence, and a 365 nm excitation/420 nm emission filter set that was used for 4′,6-diamidino-2-phenylindole (DAPI) fluorescence (Carl Zeiss Microimaging GmbH, Jena, Germany). Images were acquired and analyzed using a computer-controlled Zeiss HRc charged-coupled device (CCD) camera (Carl Zeiss Microimaging GmbH) and by AxioVision 4.4 image capture and analysis software (Carl Zeiss Microimaging GmbH) for quantification of changes in emission fluorescence. This software allows measurements to be made from user-defined areas of the microscope field; in this case, the fluorescence measurements were localized to selected areas of the muscle fibers.
CM-DCF fluorescence from fibers was recorded at 15 min intervals over 45 min at 25°C. Exposure of fibers to ultraviolet (UV) light was minimized by use of a 500 ms exposure time. Immediately after the measurement at 45 min, all remaining CM-DCFH in the fibers was photo-oxidized by continuous illumination with UV light for 15 min. During this time, images were recorded every 20 s. Overexposure of fibers to UV light caused oxidation of all remaining CM-DCFH and produced a maximum CM-DCF fluorescence emission that provides a measure of the total amount of available CM-DCFH in the portion of each fiber under study (31). The effect of temperature on endogenous CM-DCFH oxidation was examined by increasing the temperature of the cell culture to 37°C throughout the measurement.
In some studies, the CM-DCF fluorescence in fibers was examined by confocal microscopy in comparison with the fluorescence of mitotracker red (MitoTracker® Red CMXRos, (Molecular Probes™, Invitrogen). Fibers loaded with CM-DCFH (17.5 μM) and mitotracker red (20 nM) were examined using a Zeiss LSM 510 confocal microscope with a 63X/1.4 oil DIC objective and an argon ion laser. Excitation of CM-DCF was at 488 nm and the emission was monitored through a 505–530 nm filter reflected from a 545 nm dichroic mirror. Excitation of mitotracker red was achieved using a 543 nm helium neon laser and the emission was collected through a 545 nm dichroic mirror and a 560 nm long pass filter. Image data acquisition and analysis were carried out with Carl Zeiss Laser Scanning Systems LSM 510 software, version 3.2 (Carl Zeiss Microimaging GmbH).
Experimental protocol
Exposure of fibers to exogenous oxidant
The sensitivity of the experimental system to small changes in ROS was evaluated by addition of hydrogen peroxide to the culture wells at 15 min after the beginning of the experiment. Following preliminary titration experiments to determine the appropriate concentration of hydrogen peroxide for use (not shown in detail), a small volume of a concentrated solution of hydrogen peroxide was added to the 2 ml culture medium to produce a final concentration of 1 μM and the time-course and change in CM-DCF fluorescence monitored.
Electrical stimulation of contractions by fibers
Isolated fibers were stimulated to contract, as previously described for myotube cultures (31, 37). In brief, platinum electrodes were placed into the well and provided trains of biphasic square wave pulses of 2 ms in duration for 0.5 s repeated every 5 s at 50 Hz and 30 V/well. The total stimulation time was 15 min and this commenced at 15 min following the beginning of the experiment. Fibers contracted throughout the 15 min period (see video in supplementary material; for supplementary video, see www.liebertonline.com/ars)*.
Enhancement of intracellular glutathione content
Isolated fibers were incubated with 5 mM glutathione ethyl ester (GSHEE) (Sigma-Aldrich Co.) in the culture medium for 2 h prior to loading with CM-DCFH DA.
Statistical analyses
Data are presented as mean ± S.E.M. For multiple comparisons at any time point, analysis was by one-way ANOVA followed by the post hoc LSD test. Single comparisons between two experimental conditions at a time point were undertaken using the unpaired Student's t test. Comparisons between data from individual fibers at different time points were undertaken using Student's paired t test. Statistical significance was set at the α level of 0.05 for all of the tests.
RESULTS
Viability of single mature skeletal muscle fibers in culture
The majority of the isolated fibers showed good viability over 24 h in culture. Figures 1A and B show bright-field images of muscle fibers attached to the collagen layer which displayed typical morphology of skeletal muscle fibers with well-defined striations in the sarcolemma (Fig. 1A). Following staining with DAPI, nuclei showed peripheral distribution with no evidence of nuclear breakdown (Fig. 1B). The vast majority of fibers showed exclusion of trypan blue (Fig. 1C), although a small proportion showed both changes in morphology (shortening, thickening, and loss of striations) and an inability to exclude the dye. It is likely that these fibers were damaged during the isolation process (Fig. 1C).
FIG. 1. Viability of single mature skeletal muscle fibers in culture.
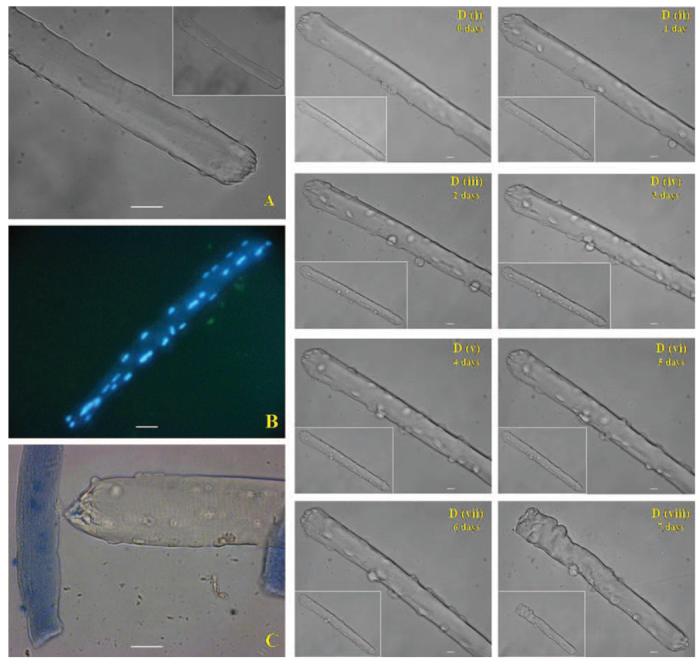
Light microscopic images of isolated fibers after 24 h in culture: (A) Bright field image, 32× original magnification. (B) Epifluorescence image of fibers stained with DAPI, 20× original magnification. (C) Bright-field image showing a viable fiber that excluded Trypan blue and parts of two damaged fibers. 32× original magnification. Scale bar = 10 μm. (D) (i–viii) Bright-field images of the same fiber acquired each day over 7 days. Main image: 32× original magnification. Scale bar = 10 μm. Inset image: 20× original magnification.
Images of a single isolated skeletal muscle fiber in culture taken daily over 7 days are shown in Fig. 1D. Typically fibers retained their morphology over 6–7 days (with changes of the culture medium every 48 h). In the example shown, there was no apparent morphological change over 6 days, but the fiber shortened by 7 days in culture and appeared to lose viability.
Optimization and standardization of CM-DCF fluorescence measurements
Confocal images of the CM-DCF fluorescence for the isolated fibers showed a homogeneous spread across the fiber in contrast to the localization of mitotracker red (Fig. 2). Mitotracker red has previously been shown to localize to the Z lines in skeletal muscle (46), and the appearance in Fig. 2C is compatible with this. Using the epifluorescence microscope (Zeiss Axiovert 200M) and AxioVision software, CM-DCF fluorescence emission measurements were obtained from user-defined areas of the image, and the image analysis system provided the mean gray value for the area selected. Fluorescence from the fibers appeared to be homogeneous and the area selected for measurements covered a wide surface of the fiber, avoiding the obvious nuclei or satellite cells. An area of the field without fibers was also selected to measure the background fluorescence. Fiber fluorescence was obtained by subtracting the background fluorescence from the fiber fluorescence.
FIG. 2. Confocal images of a single mature skeletal muscle fiber.
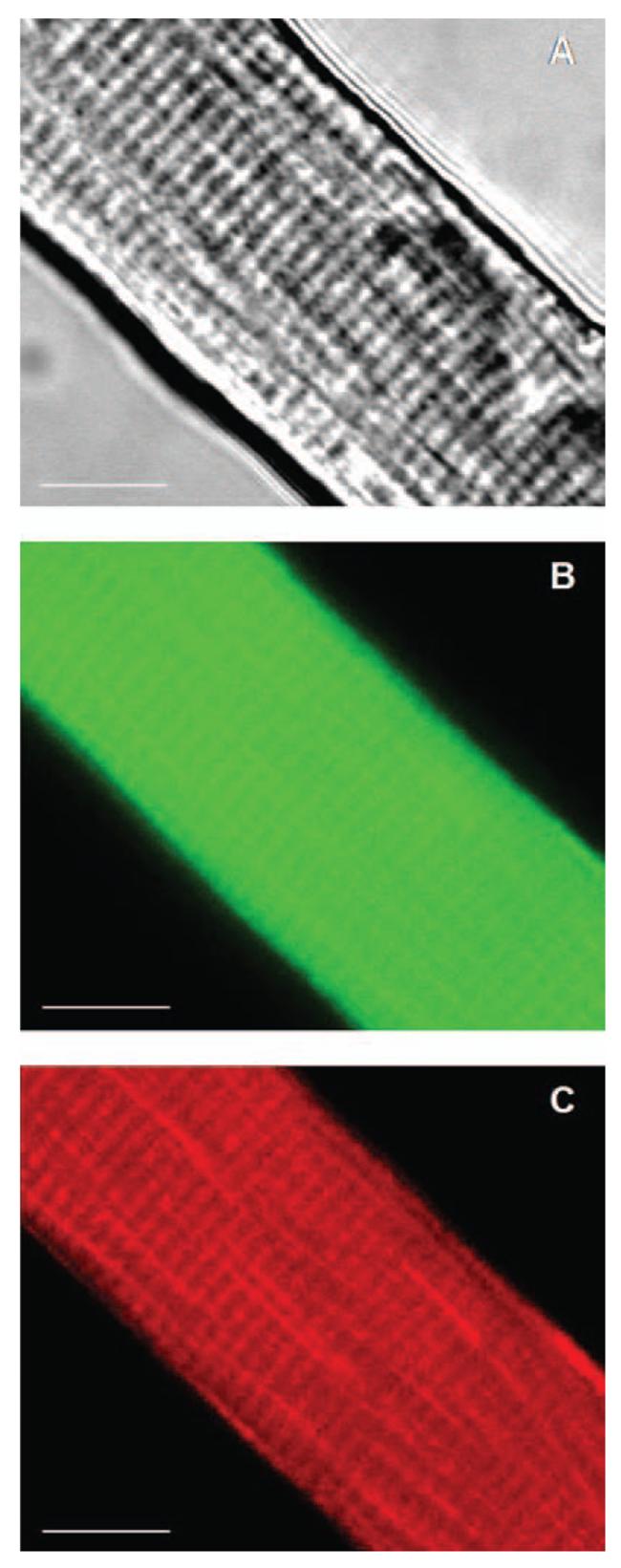
Selected area of a single mature skeletal muscle fiber loaded with the fluorophores CM-DCFH (17.5 μM) and Mitotracker red (20 nM). (A) Bright-field image. (B) and (C) Confocal images of the same optical section (thick = 2.61 μm) showing: CM-DCF fluorescence (B) and Mitotracker red fluorescence (C). 63× original magnification. Scale bar 10 = μm.
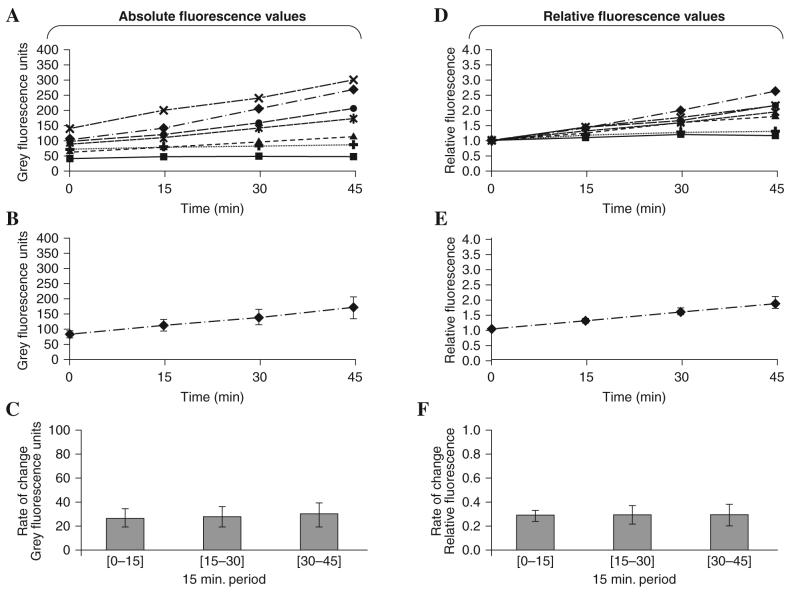
To minimize the photo-oxidation of CM-DCFH due to UV exposure, preliminary experiments were undertaken to determine the maximum frequency for fluorescence measurements and excitation intensity that would permit us to obtain a linear increase in fluorescence with time (31). These data (not shown in detail) indicated that fluorescence measurements at 15 min intervals with 500 ms exposure provided an appropriate protocol. Measurements of fluorescence intensity (expressed in gray scale values) from 7 individual quiescent fibers over 45 min are shown in Fig. 3A and the mean data derived from these values are presented in Fig. 3B. These data are recalculated to show the net change in fluorescence values over each 15 min interval (i.e., the rate of change) in Fig. 3C. Although all fibers showed an approximately linear increase in fluorescence over time, data from individual fibers varied in both the magnitude of fluorescence recorded and in the rate of increase with time (Fig. 3A) leading to a substantial variability in the group mean data (Fig. 3B and C).
FIG. 3. Optimization of measurements of CM-DCF fluorescence in single mature skeletal muscle fibers over a time course.
Data from seven individual quiescent fibers are presented. (A) Individual CM-DCF fluorescence values obtained from each fiber over 45 min. (B) Mean CM-DCF fluorescence values from the fibers at different time points (data are presented as mean ± SEM). (C) Rate of change in CM-DCF fluorescence each 15 min over the 45 min experiment (data are presented as mean ± SEM). (D) Fluorescence values relative to the initial fluorescence obtained for each fiber were calculated and these relative fluorescence values are presented for each fiber over the 45 min experimental period. (E) Mean relative fluorescence values from the group of fibers at different time points (data presented as mean ± SEM). (C) Rate of change in relative fluorescence each 15 min over the 45 min experiment (data presented as mean ± SEM).
Standardization of the CM-DCF fluorescence to account for variability in loading with CM-DCFH DA
It was argued that the main variable between fluorescence values obtained from individual fibers reflected different levels of loading of fibers with CM-DCFH DA and two approaches to standardization were examined.
The data presented in Fig. 3A-C were recalculated and expressed relative to the initial fluorescence value obtained for that fiber and these data are shown in Fig. 3D-F. The relative fluorescence of a fiber at time point “a” (rFa) was calculated as the fluorescence from the fiber (Fa) minus the fluorescence of the background at that time point (Fbackga) divided by the initial fluorescence from the fiber (F0) minus the initial fluorescence of the background (Fbackg0).
Standardization in this manner reduced the variability of the data in comparison with raw fluorescence values (see Fig. 3E in comparison with Fig. 3B). The rate of change in relative fluorescence over a 15 min period (ΔrF[a−b]) was calculated as the difference between the relative fluorescence at the end (rFb) and that at the beginning (rFa) of the 15 min period.
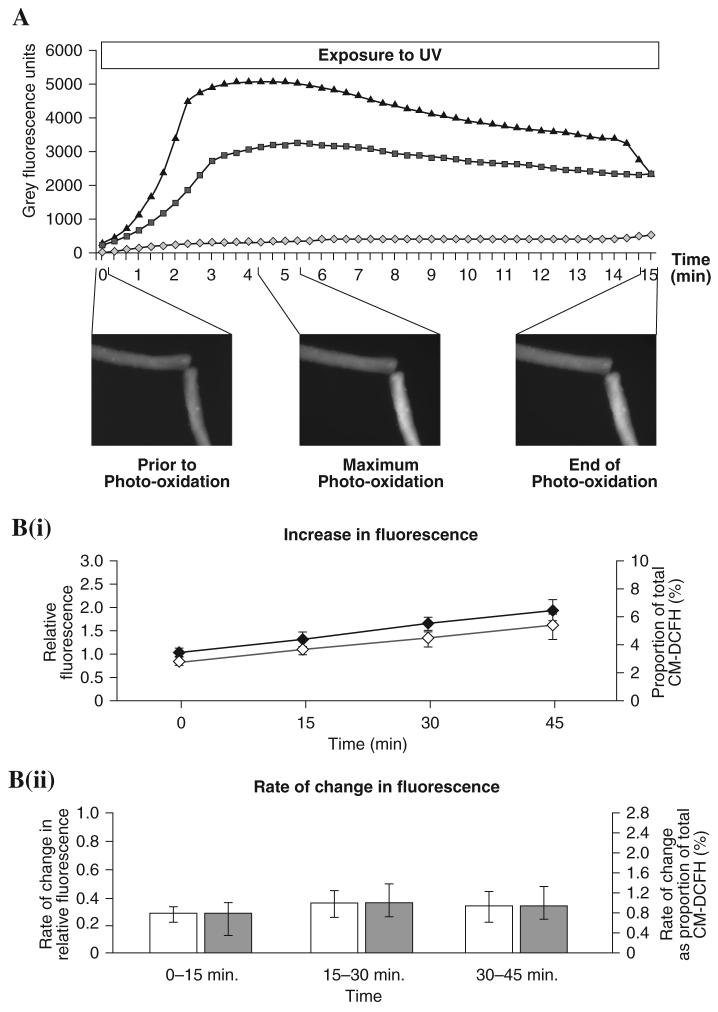
Previous studies of myotubes have used a protocol to standardize fluorescence measurements as a proportion of the total amount of DCFH available in the cell where this was calculated from the maximum fluorescence values obtained during photo-oxidation of the fiber (31, 44), and standardization against the initial value was compared with this approach. Fibers were exposed to continuous UV illumination for 15 min and during this period, images were obtained every 20 s. The maximum fluorescence emission was measured and taken to represent the total amount of CM-DCFH that was available in the portion of the fiber under study (31). Figure 4A shows examples of data from two fibers during photo-oxidation of the CM-DCFH. The maximum fluorescence emission was seen in one fiber after 260 s of continuous UV illumination, with a maximum fluorescence value of 5097 gray units, while the second fiber showed maximum fluorescence after 320 s with a value of 3229 gray units. After the maximum fluorescence was obtained, the values declined due to fluorophore photo-bleaching (43). At the end of the photo-oxidation, one of the fibers showed characteristic signs of damage induced by the high UV exposure (Fig. 4A: inset image). The maximum CM-DCF fluorescence value obtained during the photo-oxidation was used as a measure of the total CM-DCFH available in the fiber. The fluorescence data obtained from quiescent fibers during a 45 min experiment were recalculated as a percentage of the total CM-DCFH available and are shown in Fig. 4B in comparison with the same raw fluorescence data recalculated and expressed relative to the initial fluorescence value obtained for that fiber. Figure 4B(i) shows the increase in fluorescence standardized using both approaches, and Fig. 4B(ii) shows the rate of change of CM-DCF fluorescence. These data appear to show the same pattern regardless of the standardization method used.
FIG. 4. Assessment of CM-DCFH loading and comparison of methods to correct for differences in loading of CM-DCFH into single mature skeletal muscle fibers.
(A) Change in CM-DCF fluorescence in two fibers during continuous exposure to UV over 15 min. Fiber 1 fluorescence ( ), fiber 2 fluorescence (–▲–), background fluorescence (
), fiber 2 fluorescence (–▲–), background fluorescence ( ). Inset images show the morphology and fluorescence emission of the fibers prior to photo-oxidation, at the point when maximum photo-oxidation was observed and at the end of the UV exposure. (B) Comparison of two approaches to normalize values of fluorescence obtained from fibers. (B-i) Mean changes in fluorescence where data were normalized to the initial value from that fiber (◆), or data are expressed as a proportion of the total CM-DCFH within the fiber (◇). (B-ii) Rate of change in fluorescence where data were normalized to the initial value from that fiber (□), or data are expressed as a proportion of the total CM-DCFH within the fiber (
). Inset images show the morphology and fluorescence emission of the fibers prior to photo-oxidation, at the point when maximum photo-oxidation was observed and at the end of the UV exposure. (B) Comparison of two approaches to normalize values of fluorescence obtained from fibers. (B-i) Mean changes in fluorescence where data were normalized to the initial value from that fiber (◆), or data are expressed as a proportion of the total CM-DCFH within the fiber (◇). (B-ii) Rate of change in fluorescence where data were normalized to the initial value from that fiber (□), or data are expressed as a proportion of the total CM-DCFH within the fiber ( ). Values are expressed as mean ± S.E.M. for five fibers.
). Values are expressed as mean ± S.E.M. for five fibers.
When expressed as the rate of change of relative fluorescence, temperature had no influence on the CM-DCFH oxidation, although the absolute values for fluorescence were greater at 37°C in comparison with 25°C. Thus at 37°C, raw fluorescence values were increased by >50% compared with 25°C (52 ± 6 cf. 32 ± 6 gray scale units) but following calculation of the relative rate of change, the values did not differ (37°C: 0.06 ± 0.01%/15 min cf. 25°C: 0.10 ± 0.02%/15 min.)
Exposure of single mature skeletal muscle fibers to hydrogen peroxide
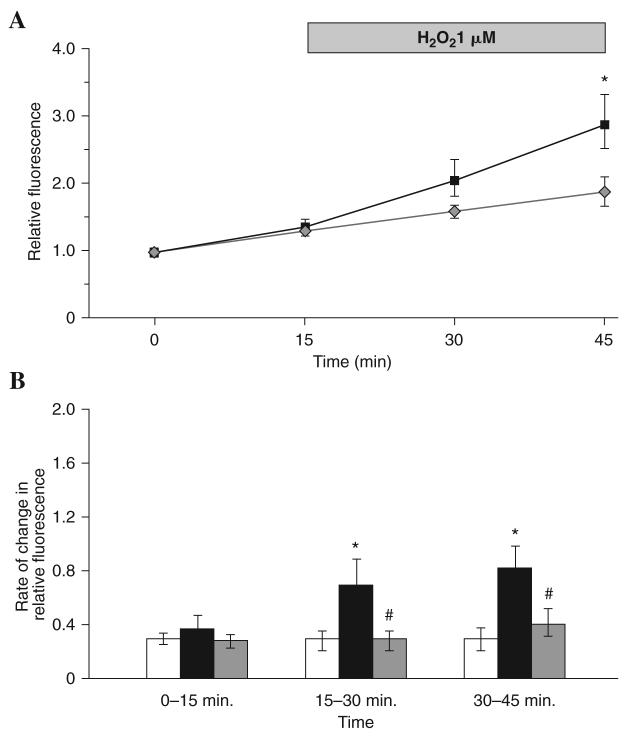
Figure 5 shows the CM-DCF fluorescence from fibers when hydrogen peroxide (H2O2) was added to the medium at a final concentration of 1 μM at 15 min. The fluorescence from these fibers increased after H2O2 addition and the fluorescence value at 45 min was significantly higher than those from control fibers with no H2O2 addition at the same time points (Fig. 5A). The rate of change in CM-DCF fluorescence from H2O2-treated fibers was increased by a mean of 133% over the first 15 min following treatment and a mean of 177% over the period 15–30 min following treatment compared with control fibers over the same time periods (Fig. 5B).
FIG. 5. CM-DCF fluorescence from single mature skeletal muscle fibers following exposure to H2O2 with and without glutathione loading.
(A). The relative fluorescence from control fibers that were not exposed to H2O2 ( ) compared with fibers exposed to 1 μM H2O2 at the 15 min time point (■). (B). Rate of change of relative DCF fluorescence in control fibers (□), fibers exposed to 1 μM H2O2 for 30 min starting at the 15 min time point (■) and fibers pre -treated with 5 mM glutathione ethyl ester (GSHEE) for 2 h prior to CM-DCFH DA loading and exposure to 1 μM H2O2 for 30 min starting at the 15 min time point (
) compared with fibers exposed to 1 μM H2O2 at the 15 min time point (■). (B). Rate of change of relative DCF fluorescence in control fibers (□), fibers exposed to 1 μM H2O2 for 30 min starting at the 15 min time point (■) and fibers pre -treated with 5 mM glutathione ethyl ester (GSHEE) for 2 h prior to CM-DCFH DA loading and exposure to 1 μM H2O2 for 30 min starting at the 15 min time point ( ). *Statistically significant compared with control fibers during the same time period; #statistically significant compared with fibers exposed to H2O2 during the same time period. Data were analyzed using a one-way ANOVA (p < 0.05), post hoc LSD (p < 0.05) and are shown as mean ± S.E.M, n 5–7 fibers in each group.
). *Statistically significant compared with control fibers during the same time period; #statistically significant compared with fibers exposed to H2O2 during the same time period. Data were analyzed using a one-way ANOVA (p < 0.05), post hoc LSD (p < 0.05) and are shown as mean ± S.E.M, n 5–7 fibers in each group.
Contractile activity in single mature skeletal muscle fibers
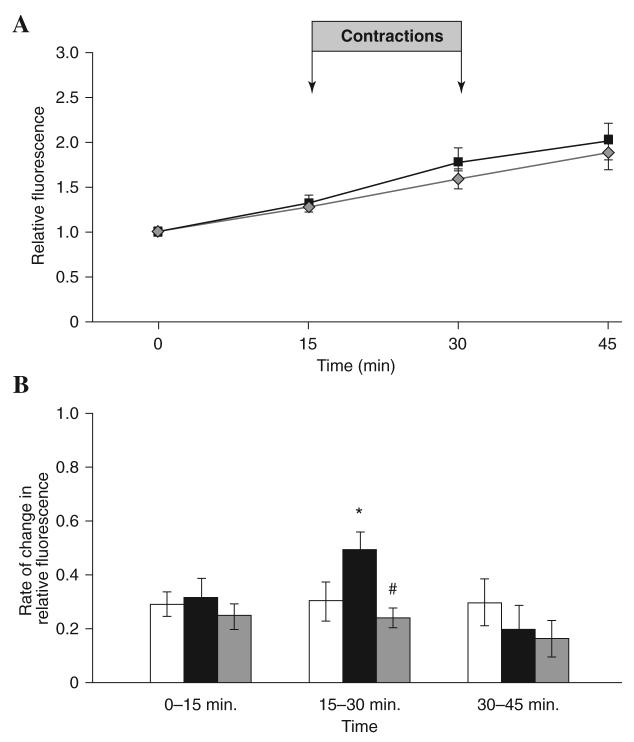
Figure 6 shows the CM-DCF fluorescence from fibers that were electrically stimulated to contract during the period 15–30 min and a control group of fibers which were not electrically stimulated. Control fibers showed a linear increase in fluorescence values over the time course (Fig. 6A) with no change in the rate of change of fluorescence during the experimental period (Fig. 6B). Electrically stimulated fibers showed a greater increase in fluorescence after contractile activity (Fig. 6A), and the rate of change in fluorescence was ∼58% higher and statistically different compared with the rate seen during the 15 min pre-stimulation period, and ∼63% higher than the rate seen from control nonstimulated fibers at the same time point (Fig. 6B). At 15 min post-stimulation, the rate of change in CM-DCF fluorescence had declined to similar levels to those seen in the control nonstimulated fibers (Fig. 6B).
FIG. 6. CM-DCF fluorescence from single mature skeletal muscle fibers subjected to a period of electrically stimulated contractile activity with and without prior glutathione loading.
(A). The relative fluorescence from control fibers ( ) compared with fibers that underwent contractile activity induced by electrical stimulation over the 15–30 min period (■). (B). Rate of change of relative DCF fluorescence in control fibers (□), fibers subjected to contractile activity induced by electrical stimulation over the 15–30 min period (■), and fibers pre -treated with 5 mM glutathione ethyl ester (GSHEE) for 2 h prior to CM-DCFH DA loading and subjected to contractile activity induced by electrical stimulation over the 15–30 min period (
) compared with fibers that underwent contractile activity induced by electrical stimulation over the 15–30 min period (■). (B). Rate of change of relative DCF fluorescence in control fibers (□), fibers subjected to contractile activity induced by electrical stimulation over the 15–30 min period (■), and fibers pre -treated with 5 mM glutathione ethyl ester (GSHEE) for 2 h prior to CM-DCFH DA loading and subjected to contractile activity induced by electrical stimulation over the 15–30 min period ( ). *Statistically significant compared with control fibers during the same time period; #statistically significant compared with fibers subjected to contractile activity induced by electrical stimulation over 15–30 min period. Data were analyzed using a one-way ANOVA (p < 0.05), post hoc LSD (p < 0.05) and are shown as mean ± S.E.M, n 7–13 fibers in each group.
). *Statistically significant compared with control fibers during the same time period; #statistically significant compared with fibers subjected to contractile activity induced by electrical stimulation over 15–30 min period. Data were analyzed using a one-way ANOVA (p < 0.05), post hoc LSD (p < 0.05) and are shown as mean ± S.E.M, n 7–13 fibers in each group.
Effect of prior glutathione loading of single mature skeletal muscle fibers on the response to hydrogen peroxide treatment and electrical stimulation
Figure 5B shows the effect of treatment with H2O2 (1 μM) on CM-DCF fluorescence from control untreated fibers and fibers treated with GSHEE (5 mM) for 2 h prior to loading with CM-DCFH. The rate of change in fluorescence did not increase after addition of hydrogen peroxide in fibers pre-treated with GSHEE and was significantly lower at 30 and 45 min compared with H2O2-treated fibers that had not been pre-treated with GSHEE. Figure 6B shows the effect of 15 min electrical stimulation of contractions on the fluorescence emission from fibers pre-treated with GSHEE (5 mM) for 2 h prior to loading with CM-DCFH compared with stimulated fibers without pre-treatment and control nonstimulated fibers. Following electrical stimulation for 15 min between 15–30 min, fibers treated with GSHEE showed no increase in the rate of change in fluorescence in contrast to the increase seen in stimulated fibers that had no pre-treatment
DISCUSSION
Isolation and culture of single mature skeletal muscle fibers
The first objective of this study was to isolate single mature muscle fibers from the FDB muscle and maintain them in culture with retention of appropriate morphological and physiological properties to facilitate measurements of intracellular ROS activity. Following digestion of muscle in collagenase solution, the released fibers were plated onto Vitrogen collagen and viable fibers remained attached to the collagen layer over 24 h at 37°C in 5% CO2. Isolated fibers showed an excellent morphological appearance (i.e, long and straight with well-defined striations and discrete nuclei; Fig. 1A and B) (10). Trypan blue staining revealed that the majority of the fibers excluded the dye although a small proportion of fibers were stained, probably due to damage during the isolation process.
Cultured fibers remained viable for at least 6 days and retained good morphological characteristics (Fig. 1D). By day 7, most fibers in culture showed some morphological changes. Several published studies have described the isolation of single mature fibers from the FDB for different purposes including studies of muscle contractility (9, 15, 17), muscle regeneration (2, 48, 50), and muscle gene expression (27). To our knowledge, this is the first study to use this model to detect and measure the intracellular ROS activity in situ by real-time fluorescence microscopy.
Optimization of measurements of CM-DCF fluorescence
CM-DCF fluorescence was found to be homogeneously distributed through the fiber (Fig. 2), and hence we quantified fluorescence from a wide area of the fiber excluding nuclei and satellite cells. Several authors have developed different experimental approaches in order to use DCFH to study ROS in skeletal muscle (7, 25, 31, 38, 42). Despite the excellent sensitivity of DCFH and CM-DCFH as probes for ROS, this approach has several drawbacks (1). In this study we examined different approaches to overcome the limitations and obtain a reliable method to detect and quantify the generation of ROS in single mature skeletal muscle fibers. DCFH and CM-DCFH are very susceptible to photo-oxidation (5, 12, 16, 28, 33, 38). We performed all experiments in a darkened laboratory to reduce incident light, and the exposure time of fibers to UV light was kept constant and the minimum necessary to record high quality images. This approach (a) helped maintain the viability of the fibers during the experiment, (b) allowed us to obtain fluorescence images without overexposing the fiber to UV, and (c) avoided excessive CM-DCFH photo-oxidation. In order to allow for nonuniform loading of fibers with CM-DCFH, we recalculated each fluorescence value relative to the initial value from that fiber. This approach allowed a reliable evaluation of the data in comparison with the method previously reported by McArdle et al. (31) in a study of myotubes, in which the fluorescence values were presented as a proportion of the total amount of DCFH available for oxidation in the myotube. The data obtained using these two approaches were essentially identical (Fig. 4).
The low UV exposure used for repetitive measurements and the normalization of data to correct for loading variability between fibers has allowed the development of a reliable method to assess the rate of change in CM-DCF fluorescence in real time from single fibers. Previous approaches have either involved single measurements from homogenized muscle cells (42), compared the relative values between untreated and treated muscle preparations (5, 34), or have assumed that fluorescence values are unchanged over time in untreated bundles of muscle fibers (40). The limitations of each of these alternative approaches can be seen in the variable data obtained from individual fibers that are shown in Fig. 3A. The techniques described here allow the effect of interventions to be studied in real time from single fibers, providing a more robust and reliable approach.
This approach to standardize the handling of fluorescence data provides differing results when compared with previous data for the effect of temperature on ROS generation (5). Thus, although absolute values of DCF fluorescence were greater at 37°C in comparison with 25°C, the rates of increase of the relative DCF fluorescence were unchanged, indicating a lack of dependence of ROS generation on temperature over the range studied.
Effect of exposure of single mature skeletal muscle fibers to hydrogen peroxide
Hydrogen peroxide has been frequently used as a positive control to examine the oxidation of DCFH and formation of DCF in different systems. Concentrations of hydrogen peroxide in the 100 μM range or higher have been applied to induce oxidation of DCFH after cellular disruption or tissue homogenization (7, 20, 47). Arbogast and colleagues used 100 μM H2O2 to treat bundles of fibers obtained from the diaphragm of mouse and fluorescence measurements were obtained immediately after H2O2 addition (5). In the present study, we undertook titration experiments with H2O2 in order to find optimal H2O2 concentrations in the medium that did not affect the viability of single muscle fibers over the period for fluorescence measurements (data not shown in detail). The optimal concentration of H2O2, where viability was unaffected, was found to be 1 μM, and the addition caused a progressive increase in CM-DCF fluorescence at 30 and 45 min (Fig. 5). H2O2 is able to diffuse across cellular membranes (11), although a recent report suggests that specific transport mechanisms might be involved in this process (8). Our data indicate that the model used in the present study was suitable to detect very small changes in intracellular H2O2 and possibly other ROS in single mature skeletal muscle fibers.
Effect of contractile activity in single mature skeletal muscle fibers
Many studies have demonstrated an increase in end-point indicators of the reactions of ROS in tissues during and following exercise, and the increase in ROS activity appears to be in major part due to generation by contracting skeletal muscle (21). Fifteen minutes of contractile activity in single muscle fibers produced a 58% mean increase in ROS activity compared with the 15 min pre-stimulation period and a 63% mean increase in ROS activity compared with nonstimulated fibers at the same time point (Fig. 6B). Reid and co-workers reported a significant 39% increase of intracellular DCFH oxidation in bundles of fibers from mouse diaphragm after 1 h of contractile activity (38), while Silveira et al. (42) reported that a strong stimulation protocol increased DCFH oxidation in both intracellular (171%) and extracellular (105%) compartments in primary myotubes derived from rat muscle. In contrast, they found that a moderate stimulation protocol did not induce any significant changes in DCFH oxidation which they primarily attributed to oxidation by H2O2 (42). Our group demonstrated that 10 min of contractile activity induced by electrical stimulation in myotubes derived from the H-2kb muscle cell line produced an approximately fourfold increase in the rate of DCFH oxidation (12), and we also reported an approximate doubling of DCFH oxidation following electrically stimulated contractile activity in mouse primary myotubes (44). Thus, all of these studies found a relatively modest increase in DCFH oxidation during contractile activity in muscle cells. Other workers have suggested that the increase in ROS activity during contractions should relate to the increase in oxygen consumption due to the increased energy demand by the contracting muscle cells (23), but this was clearly not seen. We hypothesize that the relatively small increase in intracellular ROS activity induced by contractile activity in our model of single muscle fibers compared with the previous studies of myotubes is due to the difference in maturity of the cells, with the mature muscle fibers having an increased capacity to regulate intracellular ROS activities. Silveira et al. (42) argued that muscle cells in culture rapidly export ROS during contractile activity, but whether this process varies between isolated fibers and myotubes is unclear.
CM-DCFH is a relatively nonspecific indicator in its ability to react with a variety of ROS (H2O2 and hydroxyl radical), nitric oxide, and reactive nitrogen species (such as peroxynitrite) (33), and hence cannot be used to monitor any specific ROS. Nevertheless, it is relevant to compare the relative increase in CM-DCF oxidation observed following 15 min contractile activity with that seen following exposure to 1 μM H2O2. This specific ROS has been widely used as a positive control for such experiments because of its relative stability in comparison with other ROS. The protocol of contractions used here has been shown to induce release of superoxide and nitric oxide from muscle cells in culture and mice in vivo (13, 37), to lead to a fall in muscle glutathione and protein thiol content (45), and to induction of redox-regulated adaptive responses (30) when applied to intact muscles in vivo, but the degree of increase in intracellular CM-DCF fluorescence induced by the contraction protocol was less that that seen following exposure of the fibers to 1 μM H2O2. The extent to which extracellular H2O2 might influence intracellular levels has been examined by Antunes and Cadenas (4) who calculated that, in Jurkat cells, an extracellular:cytosolic gradient of ∼7:1 would be rapidly established following addition of external H2O2. Thus, if these data are also applicable to skeletal muscle fibers, the likely change in intracellular H2O2 following addition of 1 μM H2O2 to the extracellular medium is ∼0.1 μM H2O2 and the rise in CM-DCFH oxidation seen following contractile activity was lower than that induced by addition of 1 μM H2O2 to the medium. Thus, it can be inferred that the absolute level of cytosolic ROS activity in muscle fibers that was achieved following contractile activity was small and equivalent to <1 μM H2O2, potentially as low as 0.1 μM H2O2. Many previous studies have used addition of H2O2 to muscle fibers or isolated organelles in vitro as an approach to mimic the effects of endogenous oxidants in tissues (for examples, see refs. 3, 19, 24, 32, 35, 40) and concentrations of H2O2 ranging from 100 pM to 10 mM have been used in previous studies of skeletal muscle. The current data indicate that only the lower range of concentrations is likely to be physiologically relevant for skeletal muscle.
Effect of prior GSH loading on ROS activity of single muscle fibers subjected to H2O2 addition or contractile activity
The ability of glutathione to reduce several ROS is well established. Strenuous physical exercise can cause a disturbance of GSH homeostasis with a decrease in tissue concentrations (e.g., see ref. 45). This has been claimed to be (at least in part) caused by oxidation by increased production of ROS. Thus, it may be beneficial for the cell to increase GSH levels to protect against ROS, but most tissues cannot synthesize GSH and import it from the circulation (26). Treatment of myogenic cells with glutathione ethyl ester (GSHEE) has been proposed as an experimental approach to increase intracellular GSH (6). GSHEE is able to cross the plasma membrane and inside the cytosol is converted to GSH (29). An in vivo study demonstrated that the supplementation of mice with GSHEE increased the intracellular GSH content level, attenuated exercise-induced stress, and improved endurance exercise performance (26). Ardite and co-workers examined C2C12 myotubes in vitro and increased intracellular GSH by treatment of cells with GSHEE after the intracellular GSH had previously been depleted (6). One of the objectives of our study was to determine whether loading of fibers with GSH by GSHEE treatment influenced intracellular ROS activity in isolated single muscle fibers. Data indicate that pre-treatment of fibers with GSHEE prevented the increase in CM-DCF fluorescence induced by exposure of fibers to 1 μM H2O2 (Fig. 5B) or to contractile activity (Fig. 6B). The mechanism of action of the GSHEE supplement is not entirely clear, but an increased intracellular GSH might directly reduce superoxide or peroxynitrite or provide additional substrate to reduce H2O2 through the action of glutathione peroxidase (18). Silveira and co-workers previously reported that a protocol of intense contractile activity in primary myotubes derived from rat muscle increased intracellular DCFH oxidation, but this was abolished if the cells had been previously incubated with GSH (42).
A potential drawback of the model used here may be the exposure of muscle fibers to nonphysiological levels of oxygen in the cell culture media, although other researchers examining DCF fluorescence from muscle cells have not controlled for this (e.g., refs. 5, 42). In previous studies of myotubes in culture, we discussed the evidence that standard culture conditions might not be optimal, leading to either excessive oxygen exposure or hypoxia dependent upon the metabolic activity of the muscle cells under study (31) and we standardized the volume of cell culture media and well size to attempt to minimize potential differences in the oxygen concentrations of the different cultures; this procedure was again followed in the current work.
In summary, this study reports a new model to examine ROS activity in skeletal muscle. It describes a method to isolate and culture single mature skeletal muscle fibers and also an imaging technique, based in CM-DCF fluorescence microscopy, to measure the intracellular ROS activity of cells subjected to different experimental conditions. In addition, the study demonstrates that contractile activity in skeletal muscle fibers generated only a small increase in intracellular ROS activity, which was abolished when the intracellular glutathione was enhanced. The method and experimental model are suitable for other specific fluorescence probes to facilitate the study of different ROS and reactive nitrogen species in the regulation of the redox status of skeletal muscle fibers.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Frank McArdle and Dr. Anne McArdle for helpful discussions and the Wellcome Trust for generous financial support (Grant No: 073263/Z/03).
ABBREVIATIONS
- CM-DCF
5- (and 6-) chloromethyl-2′,7′-dichlorofluorescin
- CM-DCFH DA
5- (and 6-) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate
- CM-DCFH
5- (and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein
- DAPI
4′,6-diamidino-2-phenylindole
- DCF
2′,7′ dichlorofluorescin
- DCFH
2′,7′ dichlorodihydrofluorescein
- D-MEM
Dulbecco's modified Eagle's medium
- D-PBS
Dulbecco's phosphate buffered saline
- FBS
fetal bovine serum
- FDB
flexor digitorum brevis
- GSH
glutathione
- GSHEE
glutathione ethyl ester
- MEM
minimum essential medium Eagle
- ROS
reactive oxygen species
- UV
ultraviolet
Footnotes
Supplementary Material: Video of contracting isolated mature skeletal muscle fiber. Contractile activity was induced by electrical stimulation. Platinum electrodes were placed into the medium to provide trains of biphasic square wave pulses of 2 ms in duration for 0.5 s repeated every 5 s at 50 Hz and 30 V/well.
REFERENCES
- 1.Afzal M, Matsugo S, Sasai M, Xu B, Aoyama K, Takeuchi T. Method to overcome photoreaction, a serious drawback to the use of dichlorofluorescin in evaluation of reactive oxygen species. Biochem Biophys Res Commun. 2003;304:619–624. doi: 10.1016/s0006-291x(03)00641-7. [DOI] [PubMed] [Google Scholar]
- 2.Anderson J, Pilipowicz O. Activation of muscle satellite cells in single-fiber cultures. Nitric Oxide Biol Chem. 2002;7:36–41. doi: 10.1016/s1089-8603(02)00011-3. [DOI] [PubMed] [Google Scholar]
- 3.Andrade F, Reid MB, Westerbald H. Contractile responses of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target of redox modulation. FASEB J. 2000;15:309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- 4.Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. doi: 10.1016/s0014-5793(00)01638-0. [DOI] [PubMed] [Google Scholar]
- 5.Arbogast S, Reid MB. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2004;287:R698–R705. doi: 10.1152/ajpregu.00072.2004. [DOI] [PubMed] [Google Scholar]
- 6.Ardite E, Barbera JA, Roca J, Fernandez–Checa JC. Glutathione depletion impairs myogenic differentiation of murine skeletal muscle C2C12 cells through sustained NF-kappaB activation. Am J Pathol. 2004;165:719–728. doi: 10.1016/s0002-9440(10)63335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejma J, Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 8.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Bruton JD, Dahlstedt AJ, Abbate F, Westerblad H. Mitochondrial function in intact skeletal muscle fibers of creatine kinase deficient mice. J Physiol. 2003;552:393–402. doi: 10.1113/jphysiol.2003.050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruusgaard JC, Liestol K, Gundersen K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J Appl Physiol. 2006;100:2024–2030. doi: 10.1152/japplphysiol.00913.2005. [DOI] [PubMed] [Google Scholar]
- 11.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 12.Chignell CF, Sik RH. A photochemical study of cells loaded with 2′,7′-dichlorofluorescin: implications for the detection of reactive oxygen species generated during UVA irradiation. Free Radic Biol Med. 2003;34:1029–1034. doi: 10.1016/s0891-5849(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 13.Close GL, Ashton T, McArdle A, Jackson MJ. Microdialysis studies of extracellular reactive oxygen species in skeletal muscle: factors influencing the reduction of cytochrome c and hydroxylation of salicylate. Free Radic Biol Med. 2005;39:1460–1467. doi: 10.1016/j.freeradbiomed.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 15.Edman KA. Contractile properties of mouse single muscle fibers, a comparison with amphibian muscle fibers. J Exp Biol. 2005;208:1905–1913. doi: 10.1242/jeb.01573. [DOI] [PubMed] [Google Scholar]
- 16.Gomes A, Fernandes E, Lima JL. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez E, Messi ML, Zheng Z, Delbono O. Insulin-like growth factor-1 prevents age-related decrease in specific force and intracellular Ca2+ in single intact muscle fibers from transgenic mice. J Physiol. 2003;552:833–844. doi: 10.1113/jphysiol.2003.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. Oxford: Oxford Univ Press; 1989. [Google Scholar]
- 19.Haycock JW, Rowe SJ, Cartledge S, Wyatt A, Ghanem G, Morandini R, Rennie IG, MacNeil S. Alpha-melanocyte-stimulating hormone reduces impact of proinflammatory cytokine and peroxide-generated oxidative stress on keratinocyte and melanoma cell lines. J Biol Chem. 2000;275:15629–15636. doi: 10.1074/jbc.275.21.15629. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh HJ, Cheng CC, Wu ST, Chiu JJ, Wung BS, Wang DL. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J Cell Physiol. 1998;175:156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Jackson MJ. Reactive oxygen species and redox-regulation of skeletal muscle adaptations to exercise. Philos Trans R Soc Lond B. 2005;360:2285–2291. doi: 10.1098/rstb.2005.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochim Biophys Acta. 1985;847:185–190. doi: 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- 23.Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol. 1992;73:1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- 24.Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibers of the rat. J Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- 26.Leeuwenburgh C, Ji LL. Glutathione and glutathione ethyl ester supplementation of mice alter glutathione homeostasis during exercise. J Nutr. 1998;128:2420–2426. doi: 10.1093/jn/128.12.2420. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Schneider MF. Fiber type-specific gene expression activated by chronic electrical stimulation of adult mouse skeletal muscle fibers in culture. J Physiol. 1998;512:337–344. doi: 10.1111/j.1469-7793.1998.337be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchesi E, Rota C, Fann YC, Chignell CF, Mason RP. Photoreduction of the fluorescent dye 2′-7′-dichlorofluorescein: a spin trapping and direct electron spin resonance study with implications for oxidative stress measurements. Free Radic Biol Med. 1999;26:148–161. doi: 10.1016/s0891-5849(98)00174-9. [DOI] [PubMed] [Google Scholar]
- 29.Martensson J, Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci USA. 1989;86:471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physio Cel Physiol. 2001;280:C621–C627. doi: 10.1152/ajpcell.2001.280.3.C621. [DOI] [PubMed] [Google Scholar]
- 31.McArdle F, Pattwell DM, Vasilaki A, McArdle A, Jackson MJ. Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med. 2005;39:651–657. doi: 10.1016/j.freeradbiomed.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 32.McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol. 2004;561:233–244. doi: 10.1113/jphysiol.2004.069914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murrant CL, Andrade FH, Reid MB. Exogenous reactive oxygen and nitric oxide alter intracellular oxidant status of skeletal muscle fibers. Acta Physiol Scand. 1999;166:111–121. doi: 10.1046/j.1365-201x.1999.00555.x. [DOI] [PubMed] [Google Scholar]
- 34.Murrant CL, Reid MB. Detection of reactive oxygen and nitrogen species in skeletal muscle. Microsc Res Tech. 2001;55:236–248. doi: 10.1002/jemt.1173. [DOI] [PubMed] [Google Scholar]
- 35.Oba T, Kurono C, Nakajima R, Takaishi T, Ishida K, Fuller GA, Klomkleaw W, Yamaguchi M. H2O2 activates ryanodine receptor but has little effect on recovery of releasable Ca2+ content after fatigue. J Appl Physiol. 2002;93:1999–2008. doi: 10.1152/japplphysiol.00097.2002. [DOI] [PubMed] [Google Scholar]
- 36.O'Neill CA, Stebbins CL, Bonigut S, Halliwell B, Longhurst JC. Production of hydroxyl radicals in contracting skeletal muscle of cats. J Appl Physiol. 1996;81:1197–1206. doi: 10.1152/jappl.1996.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 37.Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med. 2004;37:1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- 39.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 40.Sandstrom ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol. 2006;575:251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shefer G, Yablonka–Reuveni Z. Isolation and culture of skeletal muscle miofibers as a means to analyze satellite cells. In: Helgason CD, Miller CL, editors. Methods in Molecular Biology. Basis Cell Culture Protocols. Vol. 290. Totowa, NJ: Humana Press Inc; 2005. pp. 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silveira LR, Pereira–Da-Silva L, Juel C, Hellsten Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med. 2003;35:455–464. doi: 10.1016/s0891-5849(03)00271-5. [DOI] [PubMed] [Google Scholar]
- 43.Song L, Hennink EJ, Young IT, Tanke HJ. Photobleaching kinetics of fluorescein in quantitative fluorescence microscopy. Biophys J. 1995;68:2588–2600. doi: 10.1016/S0006-3495(95)80442-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vasilaki A, Csete M, Pye D, Lee S, Palomero J, McArdle F, Van Remmen H, Richardson A, McArdle A, Faulkner JA, Jackson MJ. Genetic modification of the manganese superoxide dismutase/glutathione peroxidase 1 pathway influences intracellular ROS generation in quiescent, but not contracting, skeletal muscle cells. Free Radic Biol Med. 2006;41:1719–1725. doi: 10.1016/j.freeradbiomed.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Vasilaki A, Mansouri A, Van Remmen H, Van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 46.Vendelin M, Beraud N, Guerrero K, Andrienko T, Kuznetsov AV, Olivares J, Kay L, Saks VA. Mitochondrial regular arrangement in muscle cells: a “crystal-like” pattern. Am J Physiol Cell Physiol. 2005;288:C757–C767. doi: 10.1152/ajpcell.00281.2004. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 48.Wozniak AC, Pilipowicz O, Yablonka–Reuveni Z, Greenway S, Craven S, Scott E, Anderson JE. C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem. 2003;51:1437–1445. doi: 10.1177/002215540305101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intra-cellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- 50.Yablonka–Reuveni Z, Seger R, Rivera AJ. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem. 1999;47:23–42. doi: 10.1177/002215549904700104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.