Abstract
Chronic pain is a common, disabling problem in older adults. Pain self-management training is a multimodal therapy that has been found to be effective in young to middle-aged adult samples; however, few studies have examined the effectiveness of this therapy in older adults. In this randomized, controlled trial, we evaluated a pain self-management training group (SMG) intervention as compared with an education-only (BOOK) control condition. Participants, 65 years of age or older who experienced persistent, noncancer pain that limited their activities, were recruited from 43 retirement communities in the Pacific Northwest of the United States. The primary outcome was physical disability, as measured by the Roland-Morris Disability Questionnaire. Secondary outcomes were depression (Geriatric Depression Scale), pain intensity (Brief Pain Inventory), and pain-related interference with activities (Brief Pain Inventory). Randomization occurred by facility to minimize cross-contamination between groups. Two-hundred and fifty-six individuals, mean age=81.8 (SD: 6.5), enrolled and 218 completed the study. No significant differences in outcomes were found between groups at post-intervention, 6-month follow-up, or 12-month follow-up. The SMG group showed a significantly greater increase over time, relative to the BOOK group, in two process measures, as measured by the Chronic Pain Coping Inventory: use of relaxation and use of exercise/stretching. In both cases, the increase was greatest from baseline to the post-intervention assessment. Study findings indicate that additional research is needed to determine the most effective content and delivery methods for self-management therapies targeted at older adults with chronic pain.
1. Introduction
Chronic pain is a common problem in older adults, and is often associated with depression, sleep disturbance, decreased mobility, increased health care utilization, and physical and social role dysfunction (American Geriatrics Society, 2002). As the population in developed countries grows older, there will be a growing need for therapies that are effective in decreasing pain, suffering, and pain-related disability in this group. Medical and surgical therapies are only moderately helpful and can be associated with significant risks. Thus, there is a need to develop and evaluate the effectiveness of non-drug and non-surgical therapies for persistent pain in older adults.
Increasingly, self-management is recognized as an effective therapeutic strategy for chronic pain (Smith and Elliott, 2005). Self-management is an approach in which the patient is an active participant in treatment (Creer et al., 1976). Lorig and colleagues identified five core self-management skills: problem-solving, decision-making, resource utilization, formation of a patient-provider partnership, and adoption of actions to manage the health condition. Tailoring the invention to the individual person in treatment also is a distinguishing characteristic of self-management (Lorig and Holman, 2003). These skills are taught in a variety of settings (inpatient, outpatient, community) and delivered to groups or individuals.
Preliminary support for self-management approaches to chronic pain comes from reports of improvements in pain among individuals with arthritis (Lorig and Holman, 1993), back pain (Von Korff et al., 1998), and diverse chronic pain conditions (LeFort et al., 1998), after participating in pain self-management training programs. Furthermore, individuals who participate in such programs show increases in active pain coping and in self-efficacy for managing pain, and decreases in negative cognitive responses to pain (LeFort et al., 1998; Lorig et al., 1998; Moore et al., 2000), suggesting that the effects of self-management programs on pain outcomes may be mediated by these cognitive and behavioral changes.
Retirement communities are becoming an increasingly common residential site for older adults. These communities serve, on average, mid-old (i.e., 75–84 yrs) to old-old adults (i.e., 85 yrs), who commonly experience persistent pain and activity limitations due to pain. Thus, such communities are a promising setting in which to conduct pain self-management programs. Potentially, self-management programs could reach a far broader audience if offered in retirement communities than in health care facilities, where transportation problems and other barriers to access may exist.
We conducted a randomized controlled trial (RCT) to evaluate the efficacy of a pain self-management group intervention (SMG), as compared with an education control condition (BOOK), in decreasing physical disability, pain, pain-related interference with activities, and depressive symptoms in older retirement community residents with chronic pain. We hypothesized that SMG participants, as compared with BOOK participants, would show greater improvement in these outcomes at the primary endpoint of one year. We also hypothesized that the SMG intervention, as compared with the control condition, would be associated with increased self-efficacy for managing pain and use of pain coping strategies, and with decreased negative cognitive responses to pain (catastrophizing).
2. Methods
2.1 Participants and settings
Recruitment and study procedures have been reported previously (Ersek et al., 2004b). In brief, study participants were recruited from 43 retirement communities in the Puget Sound area of Washington State. Study inclusion criteria were age 65 years or older, pain lasting more than three months that interfered with daily activities, average pain in the past week greater than two on a 0–10 scale, ability to complete study questionnaires, and ability to attend seven weekly sessions at the participant’s retirement facility. Exclusion criteria were active cancer, surgery within the past six months, and surgery planned in the next six months.
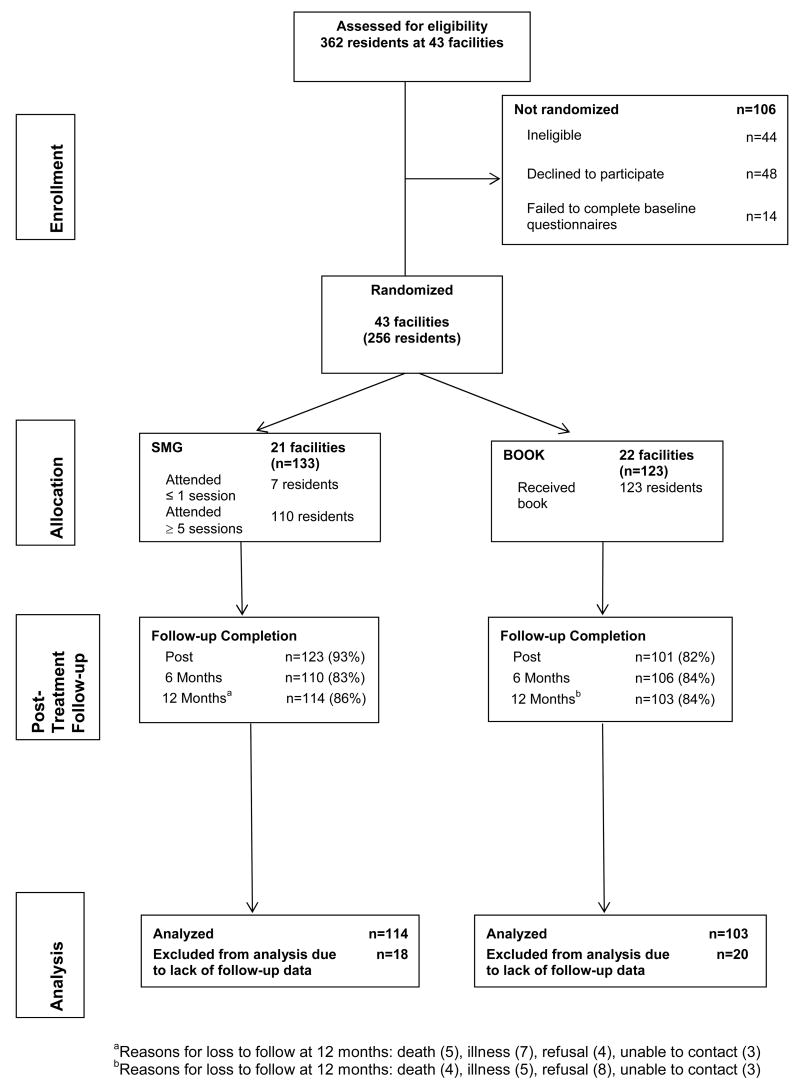
Among 362 individuals screened for the study, 106 were ineligible or declined to participate (Figure 1). The 256 study participants did not differ significantly from those who were eligible but declined to enroll (n=48) or failed to complete baseline measures (n=14) in gender, race, education, income, living arrangements (alone or with someone), or 0–10 ratings of average pain intensity and activity interference in the past week. However, as compared with nonparticipants, participants were significantly younger [age mean (SD) = 83.7 (6.0) years for nonparticipants and 81.8 (6.5) years for participants, P = .04].
Fig. 1.
Participant flow through the study.
2.2 Procedures
Seventy-seven retirement facilities were approached concerning participation in the study. Two facilities declined to participate. Residents in the remaining 75 facilities were surveyed concerning interest in participating in the study. Of these facilities, 32 were excluded because fewer than four residents expressed interest. In the remaining 43 facilities, recruitment efforts were made at two facilities at a time (to facilitate randomization; see randomization procedures, below) whenever possible, in multiple waves from September 2002 to March 2005.
All study procedures were conducted at the participants’ facilities. Study participants completed measures at baseline (prior to randomization), at the end of the intervention (or comparable period for the BOOK group), and at 6 and 12 months after the end of the group intervention. At 6 months, only the primary outcome measure of physical disability and the secondary outcome measures of pain intensity and interference were administered. Most participants completed the measures independently; however, a few participants with impaired sight and/or lack of fine motor control required assistance to complete the instruments. Beginning approximately one year after initial study enrollment, we provided grocery and department store gift cards to participants for completion of study measures ($10 cards after the post-treatment assessment and $25 gift cards after the 12-month assessment); this change was made to ensure high response rates at long-term follow-up. Study procedures and measures were approved by the Swedish Medical Center Institutional Review Board and all participants provided written informed consent. Figure 1 shows participant flow through the study.
2.3 Study measures
2.3.1 Baseline measures: demographics, cognitive functioning, co-morbidity
At baseline, participants provided sociodemographic (age, race, ethnicity, gender, marital status, education) and pain history information (sites and duration of pain). We also administered the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) to screen for cognitive impairment. The MMSE score is the total number of correct answers (out of 30 possible); scores of 24–30 indicate no cognitive impairment, scores of 18–23 suggest mild cognitive impairment, and scores from 0–17 suggest severe cognitive impairment (Tombaugh and McIntyre, 1992). Participants also completed a self-report version of the Charlson index (CI), which has been demonstrated to be reliable and valid as an index of comorbid medical conditions in older adults (Katz et al., 1996).
2.3.2 Outcome measures
2.3.2.1. Primary outcome: physical disability
The Roland-Morris Disability Questionnaire (RDQ) (Roland and Morris, 1983) is widely used to assess pain-related physical disability. The RDQ has been demonstrated to be valid, reliable, and responsive to change (Roland and Morris, 1983; Deyo, 1986; Jensen et al., 1992; Beurskens et al., 1996; Underwood et al., 1999; Roland and Fairbank, 2000). Although developed as a measure of physical disability related to back pain, the RDQ, re-worded without reference to the back, has been found to be a reliable and valid measure of physical disability for patients with other chronic pain problems as well (Jensen et al., 1992). The RDQ is scored from 0–24, with higher scores indicating more severe physical disability.
2.3.2.2. Secondary outcome measures: pain intensity and pain interference
Study participants completed the Brief Pain Inventory (BPI), a widely-used, reliable, valid instrument that assesses pain history, location, intensity, and interference with activities (Cleeland and Ryan, 1994; Caraceni et al., 2002). Pain intensity was measured by calculating the mean of ratings of average pain, current pain, and worst pain during the past week, using a scale of 0 (“No pain”) to 10 (“Pain as bad as you can imagine”). Pain-related interference is a composite measure of the degree to which pain limits a person’s general function (Cleeland and Ryan, 1994). This measure was calculated as the mean of 0 (“does not interfere”) to 10 (“completely interferes”) ratings of pain interference with general activity, mood, walking ability, work (including housework), relations with others, sleep, and enjoyment of life during the past week.
2.3.2.2. Secondary outcome measure: depression
The Geriatric Depression Scale (GDS) is a 30-item self-report measure designed to assess depressive symptoms in older persons. It has demonstrated reliability and validity. Scores of 11 or higher suggest possible depression (Yesavage et al., 1983; Montorio and Izal, 1996). The tool demonstrates adequate sensitivity and specificity in detecting depression in geriatric psychiatric and medical outpatients (Olin et al., 1992; Logsdon and Teri, 1995).
2.3.3. Measures of pain-related cognitions and coping
2.3.3.1 Self-efficacy
Participants completed the 8-item Arthritis Self-Efficacy Scale (Gonzalez et al., 1995; Lorig et al., 1996) which was modified by replacing the word ‘arthritis’ with ‘pain.’ The Arthritis Self-Efficacy Scale has been demonstrated to have high internal consistency, adequate test-retest reliability, and validity (Gonzalez et al., 1995). Participants rated on a scale from 1 (“very uncertain”) to 10 (“very certain”) their confidence that they can decrease their pain, keep pain from interfering with sleep, keep discomfort from interfering with the things they want to do, regulate activity to remain active, keep fatigue from interfering with activities, do something to feel better if they are feeling blue, manage pain during daily activities, and deal with the frustration of pain. Scores for the scale are reported as the mean of the eight ratings. We found the scale to have good psychometric characteristics in a subsample of the current study participants (Turner et al., 2005).
2.3.3.2. Catastrophizing
The Coping Strategies Questionnaire (CSQ) catastrophizing subscale was used to measure this concept (Rosenstiel and Keefe, 1983). The CSQ has demonstrated reliability and validity in several samples of older adults, including those who are older than 75 years, and is one of the most widely used measures of pain catastrophizing (Keefe et al., 1987a; Keefe et al., 1987b; Keefe et al., 1992).
2.3.3.3. Coping
Study participants also completed the Chronic Pain Coping Inventory (CPCI). The CPCI assesses cognitive and behavioral coping strategies used by people to manage chronic pain. It contains nine subscales: guarding, resting, asking for assistance, relaxation, task persistence, exercise/stretch, seeking support, coping self-statements, and medication use (Jensen et al., 1995). Respondents are asked to report how many days in the past week they used various strategies to cope with chronic pain. Subscale scores are calculated by averaging the responses to items on each scale; higher scores indicate more frequent use of the coping strategy. The CPCI scales have been shown to have acceptable internal consistency and test-retest reliability, and to be associated significantly with physical disability and depression (Jensen et al., 1995; Hadjistavropoulos et al., 1999; Nielson et al., 2001). Further development and psychometric testing have supported the reliability and validity of an additional activity pacing subscale (Nielson et al., 2001), which was included in the CPCI used in this study.
2.3.3.4 Medication use
At each timepoint, participants were asked to write down all medications they had taken for pain in the previous two weeks. We instructed them to report all over-the-counter medications as well as prescribed medicines such as opioids, antidepressants, and anti-seizure medications. We then created four “yes/no” variables reflecting medication use: acetaminophen, nonsteroidal anti-inflammatory drugs (NSAIDs), opioids (which included tramadol), and other/adjuvant agents, which included agents such as antidepressants used specifically for pain, gabapentin and other anticonvulsants, corticosteroids, and topical anesthetic and capsaicin preparations (American Geriatrics Society, 2002; American Pain Society, 2002).
2.4 Randomization
Randomization was done by facility to minimize cross-contamination between SMG and BOOK participants. In all cases, allocation was determined by a statistician (KC). The principal investigator (ME) contacted the statistician with the name or names of the facilities that were to be randomized along with limited information that was necessary to ensure similarity between SMG and BOOK facilities. Facilities that were similar in size (≤100 apartments or >100 apartments), ownership (for-profit or not-for-profit), and residents (middle class to upper class or lower class/subsidized housing) were paired, and recruitment took place at the facilities in each pair simultaneously. The facilities within each pair were randomized after the end of participant recruitment and baseline measure completion at both facilities. Allocation was determined using a computer random number generator. This procedure of forming matched pairs and then randomizing was used for 36 of the facilities. However, seven facilities were randomized individually because there was no matching facility available at the time these facilities were ready to be randomized. In this situation, a 50:50 randomization was used when there was balance in the number of control and intervention institutions up to that point. If there was an imbalance, then a biased randomization was used with there being a 2/3 probability of the new facility being randomized to the group (intervention or control) with fewer facilities enrolled so far. This biased randomization only applied when a single institution was being randomized; pairs were always randomized with one going into the control group and the other going into the intervention group regardless of imbalance. Twenty-one facilities with a total of 133 study participants were allocated to the intervention and 22 facilities with 123 participants were randomized to the control group.
2.5 SMG and BOOK protocols
2.5.1. Pain self-management group (SMG)
The SMG intervention consisted of seven weekly 90-minute group sessions that incorporated basic education about persistent pain as well as training in and practice of pain self-management techniques (Table 1). These included progressive muscle relaxation; selected range of motion, strengthening, and balance exercises; and application of heat and cold. Presentations and discussion also focused on pacing activities, challenging negative thoughts, dealing with pain flare-ups and setbacks in pain management activities, and pain medicines and complementary therapies. Participants received a class syllabus, relaxation compact disc (CD), and two hot/cold gel packs. Groups were facilitated by one of three leaders (two nurses and one clinical psychologist) trained in the protocol. All three had expertise in geriatrics and/or pain management and experience in facilitating therapeutic groups.
Table 1.
Summary of the Self-Management Group Intervention
| Topics and Activities |
|---|
Session 1: Introduction, Basic Principles of Pain
|
Session 2: Exercise and Physical Activity as Pain Management
|
Session 3: Engaging in Pleasant, Meaningful Activities; Pacing Activities
|
Session 4: Challenging Negative Thoughts and Dealing with Pain Flare-Ups and Setbacks
|
Session 5: Non-Drug Pain Therapies; Heat & Cold; Dealing with Pain Flare-Ups and Setbacks (Cont.)
|
Session 6: Pain Medications & Complementary Therapies
|
Session 7: Review and Wrap-up
|
SMG participants developed personalized pain management plans in which they chose pain management strategies that were presented in class to include in their own repertoire. Group facilitators worked with participants to develop pain management action plans that were specific and realistic, and that participants felt confident they could accomplish. All participants were encouraged to incorporate exercise and relaxation into their pain management plan in a way that was consistent with their goals and lifestyle. For example, some participants chose to begin or increase participation in a walking routine or exercise class, while others initiated a regimen of balance and/or strengthening exercises. A few participants developed their exercise plan with assistance from a physical therapist or activities director and others initiated their program independently. Sometimes, members of the SMG formed partnerships to stay motivated or increase safety while exercising. Facilitators instructed participants to practice pain management skills between classes and to document successes in and roadblocks to the use of pain management strategies on a weekly goal-setting and progress report. The facilitator and group reviewed members’ progress reports at the beginning of each class. Problems implementing pain management plans were discussed, and advice was solicited from group members on ways to address barriers to incorporating pain relief strategies into daily routines.
The intervention was manualized using a facilitator protocol to ensure consistent delivery. A copy of the protocol is available from the corresponding author.
2.5.2. Educational book control condition (BOOK)
The BOOK condition was designed to control for information about chronic pain and its management. Participants received a copy of The Chronic Pain Workbook (Catalano and Hardin, 1996) or Managing Your Pain Before It Manages You (Caudill, 2002). The former book was used during the first part of the study but was replaced by the Caudill book to ensure that BOOK participants received current information about pain management. Both books included similar self-management approaches to chronic pain and were treated as equivalent in providing a control condition to the SMG group. Facilitators telephoned participants 1 and 4 weeks after they received the book and, using a standard script, asked general questions about participants’ current pain and functioning. They also asked whether participants had read any part of the book and, if so, what chapters or sections they had read. Participants were asked if they had questions about book content or general questions about pain; if so, the facilitator addressed these issues. There was no specific therapeutic component in the phone calls and facilitators did not help BOOK participants identify goals or develop a pain management plan.
2.5.3. Booster and follow-up phone calls
The SMG group facilitator telephoned each participant at 12, 16, 22, and 30 weeks after the final group session. Depending on the participants’ questions and facilitator feedback, calls varied in length from approximately 5 to 20 minutes. During the booster phone calls, facilitators used a standardized script to inquire about pain and functioning, current pain management plans, and successes and obstacles in meeting pain management goals. They also provided encouragement and assistance in problem-solving obstacles encountered in pain management.
BOOK participants received follow-up phone calls at the same intervals. Similar to the SMG calls, BOOK participants were asked about their pain and current pain management strategies. However, the conversation was more general than in the SMG calls and did not focus on pain management plans and problem-solving.
2.5.4. Ensuring facilitator adherence to SMG protocol
As noted above, group facilitators followed detailed protocol manuals developed for the study. Also, all sessions were audio-taped. A random sample of 20% of the audio-tapes was audited by a research nurse who listened to the tapes and followed a detailed checklist to document that 8–13 (depending on the class) key topics were covered and addressed as detailed in the protocol manual. The overall percentage of items that were covered in accordance with the protocol in the audited classes (i.e., adherence to the protocol) exceeded 95%. Any deviations from the protocol were discussed with the facilitator.
2.6. Statistical power
Power calculations were based on the number of participants needed to detect a moderate effect size (i.e., 0.5) (Cohen, 1977) at 1-year follow-up, assuming a 20% attrition rate during that period. Randomization by facility necessitated estimation of the intraclass correlation (ICC) since power varied with the correlation of individuals within site and the effect size. Using data from an earlier pilot study (Ersek et al. 2003), we conservatively estimated an ICC = 0.1. Given these parameters, enrollment of 273 individuals from 34 facilities (17 Treatment and 17 Control; n=6–7 participants at each site) would yield a final sample size of 218 and yield 84% power to detect an effect size of 0.5. The actual final analysis sample size was 218 participants from 43 facilities. ICC estimates for the four outcome variables ranged from 0 to 0.06, and none were significantly different from 0. Based on the final sample size obtained, power is 92% for detecting an effect size of 0.5 if ICC=.05 and power is 87% for detecting an effect size of 0.5 if ICC=.10. Therefore, even with a conservative estimate of ICC, there was adequate power to identify significant differences.
2.7 Statistical analyses
We first compared the two study groups on baseline characteristics using chi-square analyses (for categorical variables) and t-tests (for continuous variables). We also used chi-square and t-tests to compare study participants who did, versus did not, complete the final follow-up assessment. Inspections of the distributions of scores on the outcome measures confirmed assumptions of normality for the outcomes (RDQ, BPI intensity and interference, GDS).
To test the study hypotheses, we conducted mixed effects analyses of covariance (ANCOVA) (Laird and Ware, 1982) to compare SMG and BOOK groups on the outcome and process measures across time. An intent-to-treat approach was used. Data from all participants were analyzed regardless of the number of classes attended (SMG) or the amount of book that was read (BOOK). Hypothesis testing to compare the effects of SMG and BOOK on the primary outcome was repeated with imputed values for missing data. Results did not differ; therefore, we present only the results using observed The analysis sample consisted of all participants (115 SMG participants and 103 BOOK participants) who completed the 12-month assessment.
The analytic model had two random effects, site and person nested within site, and two fixed effects, group (i.e., treatment or control) and time. The repeated scores for the RDQ at post-intervention, 6 months, and 1 year were the primary outcome measure, and age, gender, and the baseline value of the dependent measure were included as covariates. Similar analyses were conducted for the secondary outcomes (pain intensity, pain-related interference, and depression) and the process measures. Significance for the test of the primary outcome was set at p <.05.
We compared the two treatment groups in terms of clinically meaningful improvement at one year on the primary outcome measure, the RDQ, using chi-square analysis. We defined clinically meaningful improvement as a decrease of at least 4 points from baseline (Beurskens et al., 1996). Finally, we compared pain medication use in the two study groups at each assessment using chi-square analyses. All analyses were conducted using SPSS version 13.0 or 14.0.
3. Results
3.1 Sample characteristics, session attendance, and follow-up response
The final sample (n=256, 133 in SMG and 123 in BOOK) consisted largely of white, non-Hispanic females who lived alone (Table 2). Most participants (84.6%) were 75 years or older. This profile is similar to that reported in other studies conducted among residents of retirement communities (Mossey and Gallagher, 2004). The most common sites of pain were the back, legs and/or feet, and hips and/or buttocks. These patterns are consistent with the predominantly musculoskeletal sources of pain in older adults (American Geriatrics Society, 2002). The only significant difference between the two study groups in any demographic or baseline outcome measure was the higher percentage of head pain in the BOOK group (11.4%) compared with the SMG group (4.5%), (χ2 =4.12, p=0.042).
Table 2.
Baseline characteristics of study participants
| Characteristic | SMG
(n = 133) |
BOOK
(n = 123) |
P-value* |
|---|---|---|---|
| Age, mean (SD) years | 81.9 (6.3) | 81.8 (6.7) | 0.88 |
| Female, % | 87.2 | 82.1 | 0.26 |
| Education, % | |||
| High school or less | 29.3 | 19.8 | 0.08 |
| Post-secondary education | 70.7 | 80.2 | |
| Caucasian, % | 93.2 | 93.5 | 0.93 |
| Living alone, % | 74.4 | 71.3 | 0.58 |
| Income, annual USD % | |||
| < $30,000 | 45.6 | 49.6 | 0.46 |
| $30,000 – $45,000 | 35.7 | 28.0 | |
| > $45,000 | 18.7 | 22.4 | |
| Charlson co morbidity score, % | 0.59 | ||
| 0 | 43.1 | 40.7 | |
| 1–2 | 40.8 | 38.2 | |
| ≥3 | 16.1 | 21.1 | |
| MMSE score, mean (SD) | 28.3 (1.5) | 28.3 (1.9) | 0.95 |
| Pain sites % | |||
| Neck | 18.9 | 23.6 | 0.37 |
| Back | 53.0 | 61.0 | 0.20 |
| Shoulder | 37.9 | 46.3 | 0.17 |
| Arms and/or hands | 34.8 | 31.7 | 0.59 |
| Buttocks/hips | 50.8 | 57.7 | 0.27 |
| Abdomen | 6.1 | 4.9 | 0.68 |
| Legs and/or feet | 72.0 | 74.0 | 0.72 |
| Chest | 5.3 | 6.5 | 0.68 |
| Head | 4.5 | 11.4 | 0.04 |
t-test for age, MMSE; Chi-square test for other variables
MMSE=Mini-Mental State Exam
Figure 1 provides information about session attendance in the SMG group and follow-up assessment completion. In general, attendance was high: 83% of the SMG attended five or more sessions. The SMG and BOOK groups did not differ significantly in completion of the 12-month follow-up assessment (SMG: 86%, BOOK: 85%). Study participants who did (n=218) versus did not (n=38) complete the 12-month assessment did not differ significantly on any outcome measure at baseline. However, a greater percentage of non-white participants as compared with white participants did not complete the final assessment (47% versus 13%, χ2 =14.95, p <.001). Participants who lived alone (versus those who lived with someone else) were also more likely to not complete the final assessment (18% versus 6%, χ2 = 6.13, p=.01).
3.2. Effectiveness of SMG relative to the control condition: changes on outcome measures
Study participants at baseline showed moderate levels of pain and pain-related disability (Table 3). Scores on each outcome measure decreased only slightly in each group over time between the baseline and one-year assessments, with similar scores in the two groups at each assessment. There were no statistically significant treatment group effects in the analyses examining change over time in each outcome measure, controlling for age, gender, and baseline value of the outcome measure.
Table 3.
Outcome Measures at Baseline, Post-intervention, and 1 year
| Measure (possible range) | SMG
Mean (SD) |
BOOK
Mean (SD) |
Estimated effect size (95% CI)2 |
|---|---|---|---|
| Roland Morris Disability Questionnaire (0–24) | .08 (−0.72 to 0.88) | ||
| Baseline | 12.2 (4.7) | 13.0 (4.5) | |
| Post-intervention | 11.8 (4.9) | 12.4 (5.4) | |
| 6 months | 11.4 (5.3) | 12.0 (5.1) | |
| 1 year | 11.6 (5.7) | 11.9 (5.6) | |
| Pain Intensity (0–10) | .03 (−0.36 to 0.42) | ||
| Baseline | 5.4 (1.9) | 5.4 (1.8) | |
| Post-intervention | 4.9 (1.9) | 5.0 (2.1) | |
| 6 months | 4.8 (2.2) | 4.6 (2.0) | |
| 1 year | 5.0 (2.1) | 4.5 (2.1) | |
| Pain Interference (0–10) | −.09 (−.046 to 0.28) | ||
| Baseline | 4.2 (2.0) | 4.5 (2.0) | |
| Post-intervention | 4.1 (2.0) | 4.2 (2.2) | |
| 6 months | 3.7 (2.2) | 4.1 (2.1) | |
| 1 year | 3.7 (2.2) | 3.9 (2.3) | |
| Geriatric Depression Scale (0–30)1 | 0.26 (−0.31 to 0.83) | ||
| Baseline | 11.1 (2.8) | 11.0 (3.0) | |
| Post-intervention | 11.1 (2.9) | 10.9 (3.3) | |
| 1 year | 11.2 (3.1) | 10.8 (2.7) |
Note: The two groups did not differ significantly (P < .05) at baseline on any of these measures, as analyzed by t-tests. Furthermore, there were no statistically significant treatment group effects in the analyses examining change over time in each outcome measure, controlling for age, gender, and baseline value of the outcome measure.
Not administered at 6 months.
Unstandardized estimates from the mixed effects models, apply to all outcome time points
The two groups also did not differ significantly in the proportion of participants who met our criteria for clinically significant change on the primary outcome measure (decrease of at least 4 on the RDQ) at 12 months (21% in SMG, 27% in BOOK). In addition, 12% of SMG participants and 19% of BOOK participants reported increases of 4 or more on the RDQ at 12 months; these rates did not differ significantly between groups (χ2 =2.09; p=.10).
3.3. Effects of SMG relative to the control condition on process measures
As would be expected given the emphasis of the SMG on using relaxation to cope with pain, the SMG group showed a significantly greater increase over time, relative to the BOOK group, in this coping strategy. On average, SMG participants reported using one or more relaxation techniques 3 days per week (SD: 1.5 days) compared with 2.4 days per week (SD: 1.5 days) for BOOK participants Similarly, the SMG group showed a greater increase over time in the use of exercise and stretching to cope with pain. In both cases, the increase was greatest from baseline to the post-intervention assessment, with scores falling back some at one year (Table 4).
Table 4.
Process Measures at Baseline, Post-intervention, and 1 year
| Measure (possible range) | SMG
Mean (SD) |
BOOK
Mean (SD) |
P-value* |
|---|---|---|---|
| Self-efficacy Scale (1–10) | 0.19 | ||
| Baseline | 5.7 (2.0) | 5.5 (1.9) | |
| Post-intervention | 6.3 (1.8) | 5.7 (1.9) | |
| 1 year | 6.2 (2.2) | 6.0 (2.0) | |
| Coping Strategies Questionnaire - catastrophizing (0–6) | 0.89 | ||
| Baseline | 1.3 (1.1) | 1.4 (1.2) | |
| Post-intervention | 1.0 (1.1) | 1.2 (1.2) | |
| 1 year | 1.1 (1.1) | 1.1 (1.2) | |
| CPCI Guarding (0–7)§ | 0.18 | ||
| Baseline | 3.5 (1.7) | 3.7 (1.6) | |
| Post-intervention | 3.5 (1.7) | 3.4 (1.8) | |
| 1 year | 3.4 (1.8) | 3.2 (1.7) | |
| CPCI Resting (0–7) | 0.81 | ||
| Baseline | 3.5 (1.6) | 3.4 (1.6) | |
| Post-intervention | 3.5 (1.7) | 3.4 (1.6) | |
| 1 year | 3.4 (1.7) | 3.4 (1.7) | |
| CPCI Asking for Assistance (0–7) | |||
| Baseline | 0.91 | ||
| Post-intervention | 1.7 (1.9) | 1.9 (2.0) | |
| 1 year | 1.8 (2.0) | 1.9 (1.9) | |
| 1.7 (2.0) | 1.9 (1.9) | ||
| CPCI Relaxation (0–7) | 0.003 | ||
| Baseline | 2.0 (1.5) | 2.0 (1.5) | |
| Post-intervention | 3.0 (1.5) | 2.4 (1.5) | |
| 1 year | 2.5 (1.5) | 2.1 (1.4) | |
| CPCI Task Persistence (0–7) | 0.75 | ||
| Baseline | 4.3 (1.7) | 4.4 (1.6) | |
| Post-intervention | 4.2 (1.6) | 4.2 (1.7) | |
| 1 year | 3.9 (1.8) | 4.4 (1.8) | |
| CPCI Exercise/stretch (0–7) | 0.03 | ||
| Baseline | 2.7 (2.0) | 2.7 (1.7) | |
| Post-intervention | 3.4 (1.8) | 3.0 (1.7) | |
| 1 year | 3.0 (1.9) | 2.5 (1.8) | |
| CPCI Seeking Support (0–7) | 0.12 | ||
| Baseline | 2.4 (1.7) | 2.3 (1.6) | |
| Post-intervention | 2.6 (1.7) | 2.2 (1.5) | |
| 1 year | 2.5 (1.8) | 2.3 (1.5) | |
| CPCI Coping Self-statements (0–7) | 0.12 | ||
| Baseline | 3.8 (1.8) | 3.9 (1.7) | |
| Post-intervention | 3.9 (2.0) | 3.6 (1.7) | |
| 1 year | 3.7 (2.1) | 3.5 (1.7) | |
| CPCI Pacing (0–7) | 0. 05 | ||
| Baseline | 4.1 (1.8) | 4.3 (1.7) | |
| Post-intervention | 4.4 (1.9) | 4.1 (1.7) | |
| 1 year | 4.3 (2.0) | 3.9 (1.8) |
Note: The two groups did not differ significantly (P < .05) at baseline on any of these measures, as analyzed by t-tests.
From analyses examining change over time in each measure, controlling for age, gender, and baseline value of the measure.
The range for all CPCI subscales scores refers to 0–7 days/week that the respondent reports using specific coping strategies. Subscale scores are calculated by averaging responses on all subscale items.
3.4. Changes in medication use
Table 5 shows the percentage of BOOK and SMG participants who reported use of the four categories of medications during the previous two weeks. At baseline, the only significant difference between the groups was in NSAID use (BOOK: 43.1% versus SMG: 27.8%; χ2 =6.54, p=.01). Logistic regression analyses were conducted to identify significant differences in analgesic use post-treatment (timepoint2) and 1 year follow-up, controlling for baseline values. No significant differences were found between BOOK and SMG groups on any of the four types of medications.
Table 5.
Medication Use at Baseline, Post-intervention, and 1 year Follow-up
| Medication | SMG
% using |
BOOK
% using |
P-value
(differences at baseline)* |
|---|---|---|---|
| Acetaminophen | 0.48 | ||
| Baseline | 54.1 | 58.5 | |
| Post-intervention | 60.5 | 66.7 | |
| 1 year | 56.1 | 58.3 | |
| Opioids | 0.28 | ||
| Baseline | 26.3 | 32.5 | |
| Post-intervention | 24.2 | 28.4 | |
| 1 year | 36.8 | 33.0 | |
| Nonsteroidal Anti-inflammatory Drugs (NSAIDs) | 0.01 | ||
| Baseline | 27.8 | 43.1 | |
| Post-intervention | 32.3 | 43.1 | |
| 1 year | 29.8 | 32.4 | |
| Other | 0.76 | ||
| Baseline | 18.0 | 19.5 | |
| Post-intervention | 19.4 | 15.7 | |
| 1 year | 12.3 | 16.5 |
Pearson’s Chi-square
4. Discussion
The purpose of this clustered, randomized controlled trial was to compare the effects of a self-management group intervention with a control condition in which participants received a book about chronic pain management. At post-intervention, and 6 and 12 month follow-up, we found no significance differences between the two groups on pain, pain-related disability, or depression in this sample of older adults with persistent pain. The percentage of participants in the SMG and BOOK groups who reported clinically significant improvement in the primary outcome of pain-related disability was similar (21% v. 27%, respectively).
These study results were somewhat surprising because our pilot study comparing SMG and BOOK demonstrated significant differences between the two conditions on physical role functioning and pain intensity post-intervention (Ersek et al., 2003). Based on the pilot we altered the SMG to increase content and discussion about exercise and overcoming obstacles to practicing pain coping strategies. In addition, we added booster sessions to enhance long-term outcomes. Different measures of physical functioning/disability were used in each study which may have affected outcomes; however, pain intensity was measured using the same instrument. These inconsistent findings are reflected in the literature; some studies have found benefits for individuals who participate in self-management courses for painful conditions (Lorig et al., 1985; Lorig and Holman, 1993; LeFort et al., 1998), other studies have failed to find benefits (Solomon et al. 2002). A recent systematic review concluded that self-management programs resulted in small to moderate effects for selected chronic diseases (diabetes, hypertension), but that arthritis self-management programs were not associated with statistically significant effects (although there was a trend towards statistical significance) (Warsi et al., 2004). The authors of this review also concluded that there was evidence of publication bias.
We can only speculate as to the reasons for the negative results in the current study. One possibility is that including persons with diverse chronic pain conditions may have diluted the overall efficacy of the SMG condition. For example, focusing on a more limited chronic pain problem such as knee osteoarthritis or low back pain would have allowed us to assist participants in learning and practicing specific exercises targeting the painful areas. Given the diverse pain problems that affected study participants, we presented a more general approach to exercise, one that included discussion and practice of basic strengthening and balance exercises with assistance in developing a plan for incorporating regular, appropriate aerobic exercise. Although SMG participants significantly increased their use of exercise, this general approach may have been less helpful than one that focused on a specific joint or body area. The diversity of pain issues also affected our choice of primary outcome measure; a generic measure of disability such as the RDQ may have been less sensitive in detecting change than a more specific measure such as the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Another factor affecting outcomes was our decision to include people with neuropathic pain as well as musculoskeletal pain. Most nondrug pain management approaches have not been systematically evaluated in patients with neuropathic pain, and thus positive results on musculoskeletal pain may have been obscured by lack of effectiveness in neuropathic pain (Dworkin et al., 2003). Unfortunately, the current study lacked statistical power to test this hypothesis.
Another possible explanation for the negative results has to do with the characteristics of the study participants. In general, they were educated and had access to financial, social, and health care resources. These individuals might already be functioning at a relatively high level, and thus might have had little room for improvement. Evidence for higher level of functioning is suggested by comparing pain and functioning measures of our sample versus those reported by Tan et al (Tan et al., 2004); these investigators also used the BPI average pain intensity, BPI interference scale, and the RDQ to describe pain and disability in a large sample of mostly male, intractable pain patients referred to a pain clinic. Baseline total sample means (SD) for the current and Tan studies were, respectively, BPI average pain intensity of 6.0(1.8) versus 6.9(2.0); BPI Pain Interference of 4.3(2.0) versus 7.6(2.0); RDQ of 12.6(4.6) versus 16.4(5.3). Although one would expect higher pain and disability in Tan et al’s sample, the average percentage difference in scores (i.e., 9% average pain, 16% disability, and 33% pain interference) indicates that pain in our sample of older adults was associated with less disability and pain-related interference than in a sample of younger, predominantly male patients. Similarly, the total sample mean Coping Strategies Questionnaire, Catastrophizing Subscale, score in our sample was (1.4, SD= 1.2), which is considerably lower (by observation) than mean scores reported by other investigators whose samples were predominantly female and/or younger chronic pain patients (range: 2.2–2.5) (Rosenstiel and Keefe, 1983; Geisser et al., 1994; Jensen et al., 1995; Turner et al., 2000; Jensen et al., 2001; Ersek et al., 2004a).
These differences between age groups also reveal the lack of knowledge about pain in older adults. For example, there are no age-related norms for the RDQ and other measures. Thus, we were unable to determine if our study sample was representative of all older adults. Also, little is known about the course of pain-related disability in this population. For this reason, it is difficult to interpret our finding that 12–19% of the entire sample reported significantly greater disability at the 1-year follow-up. Would this percentage have been higher without any intervention? Is it reasonable to expect that we can enhance function in the oldest old, or should our pain interventions focus on preventing further deterioration?
Another factor that may have diminished the difference in outcomes between groups was the nature of the control condition. Prior to randomization, 17.6% of all study participants responded that, given a choice, they preferred receiving the book. Furthermore, the majority of BOOK participants (93.6%) reported that they read at least some of the book, and rated the content they read as moderately helpful [mean=2.9(SD=1.2); scale of 0 (“not at all useful”) to 5 (“very useful”)]. The positive response of the BOOK group to the control condition might have predisposed them to good outcomes over time.
The number of therapeutic strategies covered in the course also may have limited its effectiveness. Although all topics covered in the SMG intervention conformed to Lorig et al.’s recommendations (Lorig and Holman, 2003) and clinical practice guidelines for persistent pain (American Geriatrics Society, 2002; American Pain Society, 2002), some self management strategies have stronger empirical support than others. For example, there is strong empirical evidence for exercise in pain management, whereas the support for applications of heat and cold is largely based on expert opinion and methodologically flawed studies (American Geriatrics Society, 2002; American Pain Society, 2002). Similarly, Blyth and colleagues found that use of medications and hot/cold packs are associated with pain-related disability and increased health care utilization (Blyth et al., 2005). Although this study is cross-sectional, resulting in the inability to make causal attributions, the authors argue that more emphasis should be placed on increasing active self-management strategies for pain, such as exercise and relaxation. Our study reinforces the need to examine multi-model therapies and identify the most effective components of these programs, and possibly eliminate the less effective or useful components to enhance outcomes and minimize costs (Lorig et al., 1998).
Effective pain management for older adults might also require a more extensive, integrated approach that includes both self-management and targeted health care treatment. Such an approach has been successful in treating late-life depression. Unutzer and colleagues tested the effectiveness of the Improving Mood-Promoting Access to Collaborative Treatment (IMPACT), a multi-disciplinary team intervention in which patients were assigned to a depression care manager for 12 months. In collaboration with primary care providers and a consulting psychiatrist, the care manager offered education and support, regular monitoring and outcomes assessment, antidepressant medication management, brief psychotherapy, behavioral activation, and referrals to medical, psychological and/or social services as needed (Oishi et al, 2003; Unutzer et al, 2002). Outcomes at 12 and 24 months showed that, compared to usual care, patients receiving IMPACT reported significant improvement in depressive symptoms, physical functioning, and quality of life as well as greater adherence to antidepressant therapy and satisfaction with treatment (Hunkeler et al, 2006; Unutzer et al, 2002). Secondary analyses revealed that chronic pain also decreased significantly among patients who received the IMPACT intervention (Lin et al, 2003). This study suggests that treatment for complex problems such as pain and depression requires an aggressive, comprehensive approach that goes beyond self-management.
While the lack of a significant treatment effect is disappointing, this study adds to our knowledge about self-management programs in older adults, a growing population in which persistent pain is common. Findings from this trial underscore the need for additional studies to identify effective approaches to managing pain in older persons and also provide insight into treatment components that might affect treatment efficacy.
Acknowledgments
Support for this study was provided by grant #R01 NR007787 from the National Institute of Nursing Research, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Geriatrics Society. The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:S205–224. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- American Pain Society. Guideline for the management of pain in osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis [Google Scholar]
- Beurskens AJ, de Vet HC, Koke AJ. Responsiveness of functional status in low back pain: a comparison of different instruments. Pain. 1996;65:71–76. doi: 10.1016/0304-3959(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Blyth FM, March LM, Nicholas MK, Cousins MJ. Self-management of chronic pain: a population-based study. Pain. 2005;113:285–92. doi: 10.1016/j.pain.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Caraceni A, Cherny N, Fainsinger R, Kaasa S, Poulain P, Radbruch L, De Conno F. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23:239–255. doi: 10.1016/s0885-3924(01)00409-2. [DOI] [PubMed] [Google Scholar]
- Catalano EM, Hardin KN. The Chronic Pain Control Workbook. New York: MJF Books; 1996. [Google Scholar]
- Caudill MA. Managing pain before it manages you. New York: Guilford Press; 2002. [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1977. [Google Scholar]
- Creer TL, Renne CM, Christian WP. Behavioral contributions to rehabilitation and childhood asthma. Rehabil Lit. 1976;37:226–232. 247. [PubMed] [Google Scholar]
- Deyo RA. Comparative validity of the sickness impact profile and shorter scales for functional assessment in low-back pain. Spine. 1986;11:951–954. doi: 10.1097/00007632-198611000-00017. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, Bushnell MC, Farrar JT, Galer BS, Haythornthwaite JA, Hewitt DJ, Loeser JD, Max MB, Saltarelli M, Schmader KE, Stein C, Thompson D, Turk DC, Wallace MS, Watkins LR, Weinstein SM. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- Ersek M, Turner J, Kemp C. Association of catastrophizing and physical disability in older, community-dwelling adults with persistent pain. Gerontologist. 2004a;44:367. [Google Scholar]
- Ersek M, Turner JA, Cain KC, Kemp CA. Chronic pain self-management for older adults: a randomized controlled trial [ISRCTN11899548] BMC Geriatr. 2004b;4:7. doi: 10.1186/1471-2318-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersek M, Turner JA, McCurry SM, Gibbons L, Kraybill BM. Efficacy of a self-management group intervention for elderly persons with chronic pain. Clin J Pain. 2003;19:156–167. doi: 10.1097/00002508-200305000-00003. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Geisser ME, Robinson ME, Keefe FJ, Weiner ML. Catastrophizing, depression and the sensory, affective and evaluative aspects of chronic pain. Pain. 1994;59:79–83. doi: 10.1016/0304-3959(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez VM, Stewart A, Ritter PL, Lorig K. Translation and validation of arthritis outcome measures into Spanish. Arthritis Rheum. 1995;38:1429–1446. doi: 10.1002/art.1780381010. [DOI] [PubMed] [Google Scholar]
- Hadjistavropoulos HD, MacLeod FK, Asmundson GJ. Validation of the Chronic Pain Coping Inventory. Pain. 1999;80:471–481. doi: 10.1016/S0304-3959(98)00224-3. [DOI] [PubMed] [Google Scholar]
- Hunkeler EM, Katon W, Tang L, Williams JW, Jr, Kroenke K, Lin EH, Harpole LH, Arean P, Levine S, Grypma LM, Hargreaves WA, Unutzer J. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. BMJ. 2006;332:259–63. doi: 10.1136/bmj.38683.710255.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MP, Strom SE, Turner JA, Romano JM. Validity of the Sickness Impact Profile Roland scale as a measure of dysfunction in chronic pain patients. Pain. 1992;50:157–162. doi: 10.1016/0304-3959(92)90156-6. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol. 2001;69:655–662. doi: 10.1037//0022-006x.69.4.655. [DOI] [PubMed] [Google Scholar]
- Jensen MP, Turner JA, Romano JM, Strom SE. The Chronic Pain Coping Inventory: development and preliminary validation. Pain. 1995;60:203–216. doi: 10.1016/0304-3959(94)00118-X. [DOI] [PubMed] [Google Scholar]
- Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Caldwell DS, Queen K, Gil KM, Martinez S, Crisson JE, Ogden W, Nunley J. Osteoarthritic knee pain: a behavioral analysis. Pain. 1987a;28:309–321. doi: 10.1016/0304-3959(87)90066-2. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Caldwell DS, Queen KT, Gil KM, Martinez S, Crisson JE, Ogden W, Nunley J. Pain coping strategies in osteoarthritis patients. J Consult Clin Psychol. 1987b;55:208–212. doi: 10.1037//0022-006x.55.2.208. [DOI] [PubMed] [Google Scholar]
- Keefe FJ, Salley AN, Jr, Lefebvre JC. Coping with pain: conceptual concerns and future directions. Pain. 1992;51:131–134. doi: 10.1016/0304-3959(92)90253-8. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- LeFort SM, Gray-Donald K, Rowat KM, Jeans ME. Randomized controlled trial of a community-based psychoeducation program for the self-management of chronic pain. Pain. 1998;74:297–306. doi: 10.1016/s0304-3959(97)00190-5. [DOI] [PubMed] [Google Scholar]
- Lin EH, Katon W, Von Korff M, Tang L, Williams JW, Jr, Kroenke K, Hunkeler E, Harpole L, Hegel M, Arean P, Hoffing M, Della Penna R, Langston C, Unützer J IMPACT Investigators. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290:2428–9. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- Logsdon RG, Teri L. Depression in Alzheimer’s disease patients: caregivers as surrogate reporters. J Am Geriatr Soc. 1995;43:150–155. doi: 10.1111/j.1532-5415.1995.tb06380.x. [DOI] [PubMed] [Google Scholar]
- Lorig K, Gonzalez VM, Laurent DD, Morgan L, Laris BA. Arthritis self-management program variations: three studies. Arthritis Care Res. 1998;11:448–454. doi: 10.1002/art.1790110604. [DOI] [PubMed] [Google Scholar]
- Lorig K, Holman H. Arthritis self-management studies: a twelve-year review. Health Educ Q. 1993;20:17–28. doi: 10.1177/109019819302000104. [DOI] [PubMed] [Google Scholar]
- Lorig K, Lubeck D, Kraines RG, Seleznick M, Holman HR. Outcomes of self-help education for patients with arthritis. Arthritis Rheum. 1985;28:680–685. doi: 10.1002/art.1780280612. [DOI] [PubMed] [Google Scholar]
- Lorig K, Stewart A, Ritter PL, Gonzalez VM, Laurent D, Lynch J. Outcome measures for health education and other health care interventions. Thousand Oaks, CA: Sage Publications; 1996. [Google Scholar]
- Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26:1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- Moore JE, Von Korff M, Cherkin D, Saunders K, Lorig K. A randomized trial of a cognitive-behavioral program for enhancing back pain self care in a primary care setting. Pain. 2000;88:145–153. doi: 10.1016/S0304-3959(00)00314-6. [DOI] [PubMed] [Google Scholar]
- Montorio I, Izal M. The Geriatric Depression Scale: A review of its development and utility. Inter Psychogeriatr. 1996;8:103–112. doi: 10.1017/s1041610296002505. [DOI] [PubMed] [Google Scholar]
- Mossey JM, Gallagher RM. The longitudinal occurrence and impact of comorbid chronic pain and chronic depression over two years in continuing care retirement community residents. Pain Med. 2004;5:335–348. doi: 10.1111/j.1526-4637.2004.04041.x. [DOI] [PubMed] [Google Scholar]
- Nielson WR, Jensen MP, Hill ML. An activity pacing scale for the chronic pain coping inventory: development in a sample of patients with fibromyalgia syndrome. Pain. 2001;89:111–115. doi: 10.1016/s0304-3959(00)00351-1. [DOI] [PubMed] [Google Scholar]
- Oishi SM, Shoai R, Katon W, Callahan C, Unutzer J, Arean P, Callahan C, Della Penna R, Harpole L, Hegel M, Noel PH, Hoffing M, Hunkeler EM, Katon W, Levine S, Lin EH, Oddone E, Oishi S, Unutzer J, Williams J IMPACT Investigators. Impacting late life depression: integrating a depression intervention into primary care. Psychiatr Q. 2003;74:75–89. doi: 10.1023/a:1021197807029. [DOI] [PubMed] [Google Scholar]
- Olin JT, Schneider LS, Eaton EM, Zemansky MF, Pollock VE. The Geriatric Depression Scale and the Beck Depression Inventory as screening instruments in an older adult outpatient population. Psychol Assess. 1992;4:190–192. [Google Scholar]
- Roland M, Fairbank J. The Roland-Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25:3115–3124. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- Roland M, Morris R. A study of the natural history of back pain. Part I: development of a reliable and sensitive measure of disability in low-back pain. Spine. 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- Smith BH, Elliott AM. Active self-management of chronic pain in the community. Pain. 2005;113:249–250. doi: 10.1016/j.pain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Solomon DH, Warsi A, Brown-Stevenson T, Farrell M, Gauthier S, Mikels D, Lee TH. Does self-management education benefit all populations with arthritis? A randomized controlled trial in a primary care physician network. J Rheumatol. 2002;29:362–368. [PubMed] [Google Scholar]
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Turner JA, Ersek M, Kemp CA. Self-efficacy for managing pain is associated with disability, depression, and pain coping among retirement community residents with chronic pain. J Pain. 2005;6:471–479. doi: 10.1016/j.jpain.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Turner JA, Jensen MP, Romano JM. Do beliefs, coping, and catastrophizing independently predict functioning in patients with chronic pain? Pain. 2000;85:115–125. doi: 10.1016/s0304-3959(99)00259-6. [DOI] [PubMed] [Google Scholar]
- Underwood MR, Barnett AG, Vickers MR. Evaluation of two time-specific back pain outcome measures. Spine. 1999;24:1104–1112. doi: 10.1097/00007632-199906010-00010. [DOI] [PubMed] [Google Scholar]
- Unutzer J, Katon W, Callahan CM, Williams JW, Jr, Hunkeler E, Harpole L, Hoffing M, Della Penna RD, Noel PH, Lin EH, Arean PA, Hegel MT, Tang L, Belin TR, Oishi S, Langston C IMPACT Investigators. Improving Mood-Promoting Access to Collaborative Treatment. Collaborative care management of late-life depression in the primary care setting: a randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Moore JE, Lorig K, Cherkin DC, Saunders K, Gonzalez VM, Laurent D, Rutter C, Comite F. A randomized trial of a lay person-led self-management group intervention for back pain patients in primary care. Spine. 1998;23:2608–2615. doi: 10.1097/00007632-199812010-00016. [DOI] [PubMed] [Google Scholar]
- Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med. 2004;164:1641–1649. doi: 10.1001/archinte.164.15.1641. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]