Abstract
Purpose
To compare two laser photocoagulation techniques for treatment of diabetic macular edema (DME): modified-ETDRS direct/grid photocoagulation (mETDRS) and a, potentially milder, but potentially more extensive, mild macular grid (MMG) laser technique in which small mild burns are placed throughout the macula, whether or not edema is present, and microaneurysms are not treated directly.
Methods
263 subjects (mean age 59 years) with previously untreated DME were randomly assigned to receive laser photocoagulation by mETDRS (N=162 eyes) or MMG (N=161 eyes) technique. Visual acuity, fundus photographs and OCT measurements were obtained at baseline and after 3.5, 8, and 12 months. Treatment was repeated if DME persisted.
Main Outcome Measure
Change in OCT measures at 12-months follow up.
Results
From baseline to 12 months, among eyes with baseline central subfield thickness ≥ 250 microns, central subfield thickening decreased by an average of 88 microns in the mETDRS group and decreased by 49 microns in the MMG group (adjusted mean difference: 33 microns, 95% confidence interval 5 to 61 microns, P=0.02). Weighted inner zone thickening by OCT decreased by 42 and 28 microns, respectively (adjusted mean difference: 14 microns, 95% confidence interval 1 to 27 microns, P=0.04), maximum retinal thickening (maximum of the central and four inner subfields) decreased by 66 and 39 microns, respectively (adjusted mean difference: 27 microns, 95% confidence interval 6 to 47 microns, P=0.01), and retinal volume decreased by 0.8 and 0.4 mm3, respectively (adjusted mean difference: 0.3 mm3, 95% confidence interval 0.02 to 0.53 mm3, P=0.03). At 12 months, the mean change in visual acuity was 0 letters in the mETDRS group and 2 letters worse in the MMG group (adjusted mean difference: 2 letters, 95% confidence interval −0.5 to 5 letters, P=0.10).
Conclusions
At 12 months after treatment, the MMG technique is less effective at reducing OCT measured retinal thickening than the more extensively evaluated current mETDRS laser photocoagulation approach. However, the visual acuity outcome with both approaches is not substantially different. Given these findings a larger long-term trial of the MMG technique is not justified.
Application to Clinical Practice
Modified ETDRS focal photocoagulation should continue as a standard approach for treating diabetic macular edema.
Introduction
The Early Treatment Diabetic Retinopathy Study (ETDRS) demonstrated that focal (direct/grid) laser photocoagulation reduces moderate vision loss from diabetic macular edema (DME) by 50% or more. The effectiveness of focal laser treatment may be due in part to closure of leaky microaneurysms, but the specific mechanisms by which focal photocoagulation reduces DME is not known. Histopathological studies show changes located in the retina and retinal pigment epithelium (RPE).1, 2 Some investigators have hypothesized that with reduced retinal tissue following photocoagulation, autoregulation results in decreased retinal blood flow with lower fluid flow resulting in decreased edema.3, 4 Others have suggested that the reduced retinal blood flow is due to improved oxygenation following photocoagulation.4 Biochemical and physiological studies suggest that resolution of the edema may also result from changes in the biochemical processes within the retinal pigment epithelium (RPE).5–11 The effectiveness of grid treatment alone (without focal treatment of microaneurysms) supports an indirect effect of retinal photocoagulation on macular edema.11–14
Although effective, ETDRS protocol photocoagulation may require placement of burns close to the center of the macula. Over time, laser burns may develop into areas of progressive RPE and retinal atrophy that become larger than the original laser spot size and encroach upon fixation. Photocoagulation for DME may be associated with loss of central vision, central scotomas, and decreased color vision.15–16 In an attempt to reduce these adverse effects, many retinal specialists now treat using burns that are lighter and less intense than originally specified in the ETDRS (modified-ETDRS technique, mETDRS).16–19
An alternative approach (mild macular grid, MMG) is the application of mild, widely spaced burns throughout the macula, avoiding the foveal region. By design, some burns could be placed in clinically normal retina if the entire retina was not abnormally thickened, including areas within the macula that are relatively distant from the area of thickening. The lighter burns applied to the macula are theoretically less likely to result in thermal injury to the overlying retina and less likely to break Bruch’s membrane. The widespread application also might lead to improved oxygenation, development of healthier RPE, and overall physiologic improvement of the entire macula. This improvement might lead to both resolution of edema and prevention of edema recurrence. Burns placed in unthickened retina might help normalize the numerous cellular changes in the retina induced by diabetes by the time DME develops. Effects on retinal areas distant from the laser burn, as proposed for MMG, have been demonstrated in photocoagulation used for proliferative retinopathy where regression of neovascularization occurs distant from the laser burns.
The current study, comparing the MMG technique with the mETDRS technique in eyes with previously untreated DME, was performed to determine whether the MMG technique might provide enough added benefit over the mETDRS approach to warrant conducting a larger, longer-term trial. This study was the first initiated by the Diabetic Retinopathy Clinical Research Network (DRCR.net), which is funded by the National Eye Institute of the National Institutes of Health, U.S. Department of Health and Human Services to support a multi-center clinical trial research network dedicated to investigating diabetic retinopathy. Thus, an additional purpose of the study was to certify the network sites and standardize procedures to be used in subsequent studies.
Methods
The protocol and HIPAA-compliant informed consent forms were approved by multiple institutional review boards. Each subject gave written informed consent to participate in the study. Study oversight was provided by an independent data and safety monitoring committee. The study is listed on www.clinicaltrials.gov, under identifier NCT00071773.
Study Population
To be eligible for the study, a participant had to be at least 18 years old with type 1 or type 2 diabetes, have no history of renal failure requiring dialysis or renal transplant, and have one or both eyes meeting the following criteria: (1) best corrected electronic-ETDRS visual acuity letter score ≥ 19 (approximately 20/400 or better)20, (2) definite retinal thickening due to previously untreated DME (and not primarily due to vitreoretinal interface disease as determined by investigator clinical exam) within 500 microns of the macular center on clinical examination, (3) retinal thickness measured on Optical Coherence Tomography (OCT) ≥ 250 microns in the central subfield or ≥ 300 microns in at least one of the four inner subfields, and (4) no prior laser or other treatment for DME. Eyes were not eligible that had retinal thickening from epiretinal membranes or vitreomacular traction (as determined by the investigator), needed or had received panretinal scatter photocoagulation within the prior 4 months, YAG capsulotomy within the prior 2 months, or major ocular surgery including cataract extraction within the prior 6 months. A subject could have two study eyes in the trial only if both were eligible at the time of study entry.
Synopsis of Study Design
After informed consent was obtained and eligibility confirmed, the randomly-assigned laser treatment for each study eye (MMG or mETDRS) was obtained from the DRCR.net website. For participants with one study eye, randomization was stratified according to the presence/absence of unthickened subfields on OCT (to assure balance between treatment groups), with the randomization of eyes having at least one unthickened subfield also stratified by clinical center. For participants with two study eyes, one eye was randomly assigned to receive one treatment technique and the other eye received the other treatment technique. Visual acuity testers were masked to treatment assignment. Investigators by the nature of the study were not masked.
After the initial laser treatment, follow-up visits were performed at 3.5 months (± 2 weeks), 8 months (± 4 weeks), and 12 months (± 4 weeks). Testing at each visit included measurement of best corrected electronic-ETDRS20 acuity and OCT-measured retinal thickness by certified evaluators. Macular laser photocoagulation was repeated if DME persisted and such treatment was warranted in the opinion of the investigator according to the guidelines presented below. The primary study outcome was change in OCT measures at 12-months follow up. Change in visual acuity was evaluated as a secondary outcome.
Treatment Protocols
The two treatment techniques are detailed in Table 1. They differ in the intensity, density and location of the laser burns. MMG burns are lighter and more diffuse in nature and are distributed throughout the macula in both areas of thickened and unthickened retina. Microaneurysms are not directly photocoagulated. In contrast, mETDRS direct/grid photocoagulation is comprised of treating only areas of thickened retina (and areas of retinal nonperfusion) and leaking microaneurysms. Although microaneurysms are directly treated, the treatment has been modified from the original ETDRS protocol to not require a treatment-induced change in microaneurysm color. The other primary modification to the original ETDRS protocol is that the laser burns are less intense (gray) and smaller (50 microns). These changes were instituted to reflect the approach currently in use in the US by most treating ophthalmologists, based on a survey of DRCR.net investigators. The treatment sessions for both techniques generally were completed in a single sitting.
Table 1.
Modified-ETDRS (mETDRS) and the Mild Macular Grid (MMG) Laser Photocoagulation Techniques
| Burn Characteristic | Direct/Grid Photocoagulation (Modified-ETDRS technique) | Mild Macular Grid Photocoagulation Technique |
|---|---|---|
| Direct Treatment | Directly treat all leaking microaneurysms in areas of retinal thickening between 500 and3000 microns from the center of the macula (but not within 500 microns of disc) | Not applicable |
| Change in MA Color with Direct Treatment | Not required, but at least a mild gray-white burn should be evident beneath all microaneurysms | Not applicable |
| Burn Size for Direct Treatment | 50 microns | Not applicable |
| Burn Duration for Direct Treatment | 0.05 to 0.1 sec | Not applicable |
| Grid Treatment | Applied to all areas with diffuse leakage or nonperfusion within area described below for treatment | Applied to entire area described below for treatment (including unthickened retina) |
| Area Considered for Grid Treatment | 500 to 3000 microns superiorly, nasally and inferiorly from center of macula 500 to 3500 microns temporally from macular center
No burns are placed within 500 microns of disc |
500 to 3000 microns superiorly, nasally and inferiorly from center of macula
500 to 3500 microns temporally from macular center No burns are placed within 500 microns of the disc |
| Burn Size for Grid Treatment | 50 microns | 50 microns |
| Burn Duration for Grid Treatment | 0.05 to 0.1 sec | 0.05 to 0.1 sec |
| Burn Intensity for Grid Treatment | Barely visible (light gray) | Barely visible (light gray) |
| Burn Separation for Grid Treatment | 2 visible burn widths apart | 200 to 300 total burns evenly distributed over the treatment area outlined above (approx. 2 to 3 burn widths apart) |
| Wavelength (Grid and Focal Treatment) | Green to yellow wavelengths | Green to yellow wavelengths |
At each follow-up study visit, the investigator assessed whether persistent, recurrent or new DME was present that warranted additional photocoagulation. In general, retreatment was to be administered unless the DME had resolved or there was substantial improvement in the DME in the opinion of the investigator (e.g., > 50% decrease in total macular thickened area or > 50% decrease in retinal thickening by OCT in central or inner subfields with previous retinal thickening). For the modified-ETDRS group, retreatment consisted of the same modified-ETDRS treatment technique. For the MMG group, the first retreatment used the same MMG technique limited to only the area of retinal thickening. If required, a second retreatment used the modified-ETDRS technique (which allows focal treatment of leaking microaneurysms in the area of retinal thickening).
Examination Procedures
At baseline and at each follow-up visit, visual acuity was measured by a certified evaluator using the electronic-ETDRS procedure20 following a standardized refraction. OCT and ETDRS-protocol fundus photographs (7-fields at baseline and 12 months, and 3-field at 3.5 and 8 months) were obtained by certified personnel. Adverse events related to treatment were recorded on electronic case report forms completed at each study visit. OCTs, fundus photographs, and fluorescein angiograms were sent to the Fundus Photograph Reading Center at the University of Wisconsin-Madison for masked grading.
OCT images were obtained on each eye following pupil dilation by a certified operator using the OCT3 throughout the study in 246 subjects. Scans were 6 mm in length and included the 6 radial line pattern (fast macular scan option with OCT3) for quantitative measures and the cross hair pattern (6–12 to 9–3 o’clock) for qualitative assessment of retinal morphology. For 17 study participants, the OCT2 was initially utilized, but scans were eventually obtained using the OCT3 during the course of the study for 14 of these subjects (OCT2 was used throughout the study for 3 subjects). Scans were sent to the Reading Center where they were visually inspected. For 19% of the 323 baseline scans and 13% of the 872 follow-up scans, the automated thickness measurements were judged to be inaccurate and center point thickness was manually determined and used to impute a value for the central subfield using a regression equation (since the correlation of the two measures is 0.99).
Four principal quantitative OCT outcomes were examined: retinal thickness in the central subfield, weighted inner zone thickness, maximum retinal thickening within the inner zone, and retinal volume. Inner zone thickness was averaged across the central subfield and the four inner subfields according to retinal area to calculate a weighted inner zone thickness; the larger inner subfields were each given a weight of 8/9 disc areas and the smaller central subfield was given a weight of 4/9 disc areas. Retinal thickening was defined as the observed thickness minus the mean normal thickness in each subfield (normative data based on unpublished data provided by Carl Zeiss Meditec from a study of 260 nondiabetic eyes with a normal macula in which the following mean thicknesses were determined: central subfield = 202 ± 22 microns, inner temporal = 267 ± 17 microns, inner superior = 269 ± 16 microns, inner nasal = 267 ± 17 microns, and inner inferior = 271 ± 16 microns). Maximum retinal thickening was the largest value of thickening among the central and each of the 4 inner subfields. Retinal volume was the automated value taken directly from the OCT scan, representing a weighted average of the central, inner and outer subfields multiplied by the area of the grid, expressed in mm3. Retinal morphology was assessed from OCT images for cystoid abnormalities (five-level grading scale), central subretinal fluid (three-level grading scale) and vitreoretinal interface abnormalities (three-level grading scale).
The grading method for color fundus photographs was the same as the method used in the ETDRS,21 except that areas of retinal thickening and hard exudates (in color photographs) were estimated as continuous variables (in disc area units, DA) rather than on ordinal scales. In order to assess change in area of retinal thickening, change in the square root of the area, which represents the average diameter of the area in disc diameters (DD), was used. A change of ≥ 0.6 DD was considered substantial, e.g., from 6 DA (2.4 DD) to 3.4 DA (1.8 DD) or from 2 DA (1.4 DD) to 0.6 DA (0.8 DD). Due to the difficulty in assessing very small areas of thickening, and to avoid placing undue weight on disappearance of small areas, the square root of any area < 0.17 DA (including 0) was set to 0.4 DD.
Statistical Methods
The primary outcome was change in retinal thickening in the central subfield on OCT. Change in visual acuity was a secondary outcome. The study was designed to have a minimum sample size of 200 subjects with 50 sites each enrolling 4 subjects. With a sample size of 200 eyes and assuming no more than 10% loss to follow up and a 2-sided alpha of 0.05, the study was designed to have 90% power to detect a minimum difference between groups of 50 microns in the reduction of central retinal thickening, assuming that the common standard deviation for the change from baseline was 100 microns. With the actual sample size of 213 eyes that had baseline central subfield thickness ≥ 250 microns and 12-month follow up, and using the observed common standard deviation of 96 microns as a better approximation of the population value than the 100 microns used in the prestudy power calculation, statistical power was 90% to detect a difference in change in OCT central subfield thickening between groups of 43 microns and 80% to detect a difference of 37 microns. For the visual acuity analysis that included 284 eyes with baseline and 12-month data, power was 90% to detect a difference in visual acuity between groups of 4.3 letters and 80% to detect a difference of 3.7 letters.
Continuous OCT and visual acuity outcome measures were assessed using repeated measures least squares regression models adjusted for baseline values and accounting for the correlated data from subjects with two study eyes. Adjusted mean differences and confidence intervals were determined from these models. Similarly, repeated measures generalized estimating equations (GEE) models adjusting for baseline values were used for binary outcomes. When outliers were truncated at ± 3 standard deviations from the mean, there was little effect on the results (data not shown). Analyses using the last observation carried forward (LOCF) imputation method gave similar results (data not shown). Eyes were deemed to be within the normal range if central and inner subfield values were all within 2 standard deviations of the mean values obtained from normal eyes. Analyses were also conducted on the subset of subjects with two symmetrically-involved study eyes, defined as having an inter-eye difference of less than 100 microns in central retinal thickening and less than 15 letters in visual acuity.
Results
Between July 2003 and October 2004, 263 subjects (mean age 59±11 years; 40% women) were enrolled at 79 sites in 30 states. There were 323 study eyes with DME that were randomly assigned to either mETDRS treatment (N=162) or to MMG treatment (N=161). Mean visual acuity in study eyes was 20/32 (74±13 letters) and mean OCT central subfield retinal thickness was 340±123 microns. Nineteen percent of eyes had a central subfield thickness < 250 microns but were eligible based on having at least one of the four inner subfields with thickness ≥ 300 microns. The baseline characteristics of the two groups were similar (Table 2).
Table 2.
Baseline Demographics and Clinical Characteristics According to Treatment Group
| mETDRS Group (N=162 eyes)* | MMG Group (N=161 eyes)* | |
|---|---|---|
| Subject Characteristics | ||
|
| ||
| Gender: Women - N(%) | 61(38%) | 69(43%) |
| Age (yrs) - Mean ± SD | 58 ± 11 | 59 ± 11 |
| Race – N(%) | ||
| White | 102(63%) | 103(64%) |
| African-American | 29(18%) | 31(19%) |
| Hispanic or Latino | 17(10%) | 13(8%) |
| Asian | 8(5%) | 7(4%) |
| Other | 6(4%) | 7(4%) |
| Diabetes Type - N(%) | ||
| Type 1 | 12(7%) | 9(6%) |
| Type 2 | 150(93%) | 152(94%) |
| Duration of Diabetes (years) - Mean±SD | 14±9 | 13±8 |
| HbA1c (%) - Mean±SD | 8.2±1.9 | 8.2±2.1 |
| Ocular Characteristics | ||
|
| ||
| E-ETDRS20 Visual Acuity (letter score) - N(%) | ||
| ≥ 84: 20/20 or better | 43(27%) | 32(20%) |
| 83–69: 20/25 to 20/40 | 76(47%) | 91(57%) |
| 68–49: 20/50 to 20/100 | 36(22%) | 27(17%) |
| 48–34: 20/125 to 20/200 | 7(4%) | 7(4%) |
| 33–19: 20/250–20/400 | 0(0%) | 4(2%) |
| Mean±SD – letters | 74±12 | 73±14 |
| OCT | ||
| Central Subfield Thickness (microns) Mean±SD | 335±128 | 346±118 |
| Maximum retinal thickening of central and inner subfields (microns, see text) Mean±SD | 148±122 | 163±111 |
| Number of eyes with at least 1 unthickened subfield† - N(%) | 97(60%) | 89(55%) |
| Fundus Photography | ||
| Retinopathy Severity21‡ - N(%) | ||
| Mild NPDR (level 35) | 25(15%) | 17(11%) |
| Moderate NPDR (level 43) | 21(13%) | 22(14%) |
| Moderately severe NPDR (level 47) | 79(49%) | 76(47%) |
| Severe NPDR (level 53) | 16(10%) | 29(18%) |
| Mild PDR (level 61) | 10(6%) | 9(6%) |
| Moderate PDR (level 65) | 5(3%) | 1(1%) |
| High Risk PDR (levels 71 and 75) | 0(0%) | 0(0%) |
| Clinically Significant Macular Edema23‡ - N(%) | ||
| None | 12(7%) | 10(6%) |
| Questionable | 3(2%) | 2(1%) |
| Zone of retinal thickening ≥ 1 disc area, within 1 disc diameter from center. | 6(4%) | 3(2%) |
| Retinal thickening or adjacent hard exudates ≤ 500μ from center but no thickening in center | 18(11%) | 17(11%) |
| Retinal thickening or adjacent hard exudates ≤ 500μ from center with questionable thickening in center | 19(12%) | 17(11%) |
| Retinal thickening or adjacent hard exudates ≤ 500μ from center with definite thickening in center | 99(61%) | 109(68%) |
| Area of Hard Exudates (within the Grid) | ||
| Mean ± SD - Disc Area | 0.1 ± 0.2 | 0.1 ± 0.2 |
| Area of Retinal Thickening (in the Inner Zone) | ||
| Mean ± SD - Disc Area | 1.7 ± 1.3 | 1.9 ± 1.3 |
| Area of Retinal Thickening (within the Grid) | ||
| Mean ± SD - Disc Area | 4.1 ± 3.4 | 5.0 ± 3.7 |
Subjects with two study eyes are included in both groups
Based on clinician assessment of the OCT
Nongradable for up to 3% of eyes per group
Missing data for OCT and photographic measurements of up to 6 eyes per group
Follow Up
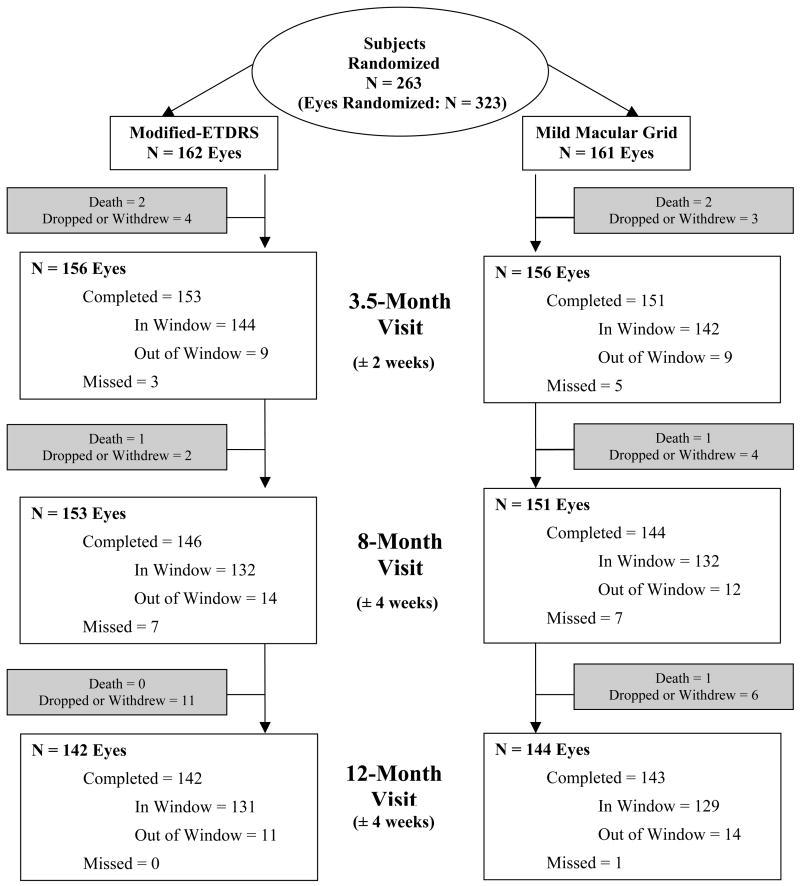
Figure 1 outlines the 12-month follow up for all eyes, which was completed for 142 (88%) of the 162 eyes in the mETDRS group and 143 (89%) of the 161 eyes in the MMG group. There were 4 participants who died during the study, representing 3 study eyes in the mETDRS group and 4 in the MMG group. Twenty-two participants were lost to follow up or withdrew, representing 17 and 13 study eyes from the mETDRS and MMG groups, respectively. The mean change in HbA1c between baseline and 12 months was similar in each group (−0.1% in the mETDRS group and −0.2% in the MMG group).
Figure 1.
Flow chart showing visit completion and reasons for study discontinuation in the two treatment groups.
Laser and Other Treatments Received for DME
All eyes received the randomization-assigned treatment regimen at baseline, except for two eyes of two subjects both of whom had two study eyes and did not return for treatment of the second eye (neither subject had any follow up).
The proportion of eyes that were retreated during the 12 months of follow up was similar in the two groups (P=0.35): in the mETDRS and MMG groups, respectively, 59 (42%) and 47 (33%) had no additional laser treatment, 47 (33%) and 58 (41%) were retreated once, and 36 (25%) and 38 (27%) were retreated twice.
Prior to the 12-month visit, DME therapy other than laser was received in 2 eyes assigned to mETDRS (vitrectomy in one and peribulbar triamcinolone acetonide in the other).
Effect of Treatment on Retinal Thickening
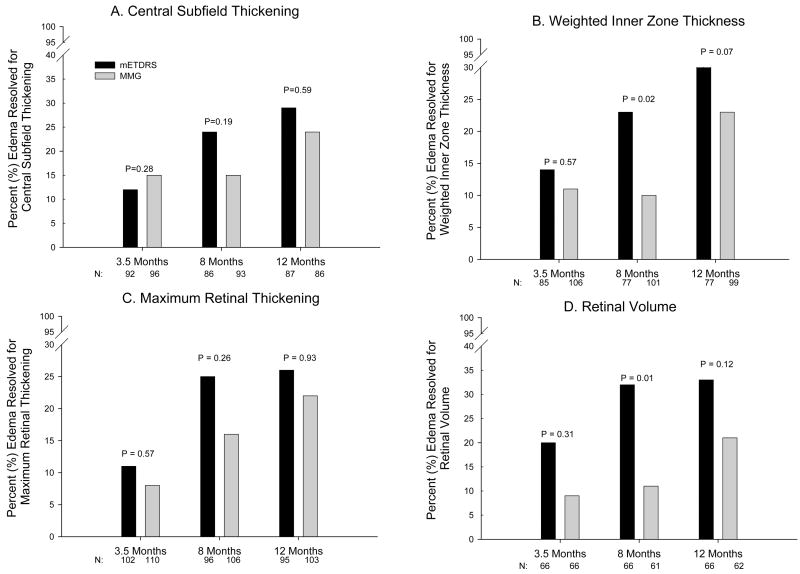
Central subfield thickening, weighted inner zone thickening, maximum retinal thickening and retinal volume all decreased over the 12-month period in both treatment groups (P<0.002 for each measure in both groups comparing baseline and 12 months). The reduction in retinal thickening was slightly more evident in the mETDRS group than in the MMG group (Table 3). There also was a trend for resolution of DME by each of these measures that was slightly more frequent in the mETDRS group (Figure 2). From baseline to 12 months, among eyes with baseline central subfield thickness ≥ 250 microns, central subfield thickening decreased by an average of 88 microns in the mETDRS group and decreased by 49 microns in the MMG group (adjusted mean difference: 33 microns, 95% confidence interval 5 to 61 microns, P=0.02). Weighted inner zone thickening decreased by 42 and 28 microns respectively (adjusted mean difference: 14 microns, 95% confidence interval 1 to 27 microns, P=0.04), maximum retinal thickening decreased by an average of 66 microns in the mETDRS group and 39 microns in the MMG group (adjusted mean difference: 27 microns, 95% confidence interval 6 to 47 microns, P=0.01), and retinal volume decreased by 0.8 and 0.4 mm3, respectively (adjusted mean difference: 0.3 mm3, 95% confidence interval 0.02 to 0.53 mm3, P=0.03). Among eyes with central and all four inner subfield thickness measurements at 12 months, the central subfield and all four inner subfields were all within the normal range in 29 (23%) of eyes in the mETDRS group and 21 (17%) in the MMG group (P=0.67). The treatment group differences were larger with greater retinal thickening at baseline (Table 4).
Table 3.
A Comparison of Retinal Thickening in the Two Treatment Groups at 3.5, 8, and 12 Months
| OCT Measurement in all eyes | |||||
|---|---|---|---|---|---|
| mETDRS Group | MMG Group | P-Value* | |||
| N | mean ± SD | N | mean ± SD | ||
| Central Subfield Thickness† | |||||
| Baseline | 121 | 371 ± 128 | 128 | 374 ± 113 | -- |
| 3.5 months | 114 | 323 ± 90 | 118 | 355 ± 129 | 0.04 |
| 8 months | 105 | 310 ± 98 | 113 | 339 ± 121 | 0.11 |
| 12 months‡ | 106 | 290 ± 81 | 107 | 324 ± 134 | 0.02 |
| Weighted Inner Zone Thickness | |||||
| Baseline | 151 | 341 ± 80 | 153 | 350 ± 76 | -- |
| 3.5 months | 133 | 320 ± 56 | 138 | 339 ± 80 | 0.03 |
| 8 months | 125 | 306 ± 48 | 131 | 327 ± 69 | 0.01 |
| 12 months‡ | 123 | 302 ± 50 | 127 | 317 ± 74 | 0.04 |
| Maximum Retinal Thickening§ | |||||
| Baseline | 160 | 148 ± 122 | 157 | 163 ± 111 | -- |
| 3.5 months | 147 | 116 ± 90 | 145 | 144 ± 121 | 0.08 |
| 8 months | 139 | 100 ± 89 | 139 | 131 ± 117 | 0.08 |
| 12 months‡ | 139 | 87 ± 78 | 134 | 120 ± 126 | 0.01 |
| Retinal Volume | |||||
| Baseline | 136 | 8.5 ± 1.6 | 121 | 8.7 ± 1.5 | -- |
| 3.5 months | 106 | 8.0 ± 1.2 | 95 | 8.5 ± 1.6 | 0.01 |
| 8 months | 105 | 7.9 ± 1.1 | 90 | 8.1 ± 1.4 | 0.19 |
| 12 months‡ | 107 | 7.7 ± 1.0 | 91 | 8.1 ± 1.5 | 0.03 |
P-value obtained using repeated measures least squares regression models adjusting for baseline values and accounting for the correlated data from subjects with 2 study eyes
Central subfield thickness-includes only eyes with central subfield thickness ≥ 250 microns at baseline
P-values for log transformed 12-month data: Central subfield thickness P=0.05, weighted inner zone thickness P=0.06, maximum retinal thickening P=0.46 and retinal volume P=0.04
Maximum of central and 4 inner subfields
Figure 2.
Resolution of edema in the two treatment groups at 3.5, 8, and 12 months for OCT measurements: A. Central subfield thickening: only includes eyes with at least 275 microns of thickness at baseline; resolution defined as central subfield < 250 microns and at least a 50 micron decrease. B. Weighted inner zone thickness: only includes eyes with at least 300 microns of thickness at baseline; resolution defined as < 285 microns and at least a 50 micron decrease. C. Maximum retinal thickening in the central subfield, or any inner subfield: only includes eyes with at least 75 microns of thickening at baseline; resolution defined as < 50 microns of thickening and at least a 50 micron decrease from baseline. D. Retinal volume: only includes eyes with volume at least 7.82 mm3 (3 standard deviations greater than normal) at baseline; resolution defined as thickening within 2 standard deviations of normal in each subfield and volume decreased at least 0.5 mm3.
N refers to the number of eyes with edema on the measure at baseline and with a gradable OCT at the visit. Dark bars are mETDRS group and lighter bars are MMG group.
P-values obtained using repeated measures least squares regression models adjusting for baseline values and accounting for the correlated data from subjects with two study eyes.
Table 4.
Changes in Retinal Thickening from Baseline to 12 Months in the Two Treatment Groups Stratified by Degree of Thickening at Baseline
| mETDRS Group | MMG Group | |||
|---|---|---|---|---|
| N | mean ± SD | N | mean ± SD | |
| Change in Central Subfield Thickening at 12 Months* | ||||
| Total | 106 | −88 ± 138 | 107 | −49 ± 122 |
| Baseline Central Subfield Thickness* | ||||
| 250–349 microns | 62 | −22 ± 62 | 53 | −7 ± 79 |
| 350–449 microns | 22 | −75 ± 84 | 32 | −74 ± 100 |
| ≥ 450 microns | 22 | −285 ± 155 | 22 | −112 ± 189 |
| P-value for interaction† | <0.001‡ / 0.04§ | |||
| Change in Weighted Inner Zone Thickening at 12 Months | ||||
| Total | 123 | −42 ± 83 | 127 | −28 ± 67 |
| Baseline Weighted Inner Zone Thickness | ||||
| 250–349 microns | 88 | −14 ± 42 | 81 | −12 ± 52 |
| 350–449 microns | 22 | −55 ± 60 | 36 | −49 ± 56 |
| ≥ 450 microns | 13 | −208 ± 120 | 9 | −92 ± 146 |
| P-value for interaction† | <0.001‡ / 0.04§ | |||
| Change in Maximum Retinal Thickening at 12 Months|| | ||||
| Total | 139 | −66 ± 123 | 134 | −39 ± 114 |
| Baseline Maximum Retinal Thickening | ||||
| 0 – 99 microns | 63 | −4 ± 53 | 49 | +17 ± 86 |
| 100 – 199 microns | 38 | −51 ± 65 | 43 | −55 ± 73 |
| ≥ 200 microns | 38 | −184 ± 162 | 42 | −89 ± 147 |
| P-value for interaction† | <0.001‡ / 0.24§ | |||
| Change in Retinal Volume at 12 Months | ||||
| Total | 107 | −0.8 ± 1.4 | 91 | −0.4 ± 1.3 |
| Baseline Retinal Volume | ||||
| < 8 mm3 | 54 | −0.2 ± 0.5 | 33 | −0.4 ± 0.4 |
| 8 – < 9 mm3 | 29 | −0.6 ± 0.8 | 29 | 0.2 ± 1.5 |
| ≥ 9 mm3 | 24 | −2.3 ± 2.1 | 29 | −1.1 ± 1.4 |
| P-value for interaction† | 0.03‡ / 0.38§ | |||
Central subfield thickening includes only eyes with central subfield thickness = 250 microns at baseline
Statistical test for whether treatment group difference varies according to baseline OCT measurement. P-value obtained using repeated measures least squares regression models adjusting for baseline values and accounting for the correlated data from subjects with 2 study eyes.
P-value based on the absolute change from baseline to follow up
P-value based on the log of the ratio of follow up to baseline
Maximum of central and 4 inner subfields
Note one eye in the MMG group had baseline Weighted Inner Zone thickness < 250 microns
On fundus photographs, at 12 months, the average diameter of the area of DME had decreased by 0.2 DD in the mETDRS group and by 0.08 DD in the MMG group (P=0.07). DME had decreased by = 0.6 DD in 41 (31%) and 28 (22%) of eyes in the mETDRS and MMG groups, respectively (P=0.03), and had increased by this amount in 22 (17%) and 26 (20%), respectively (P=0.26).
Effect of Treatment on Visual Acuity
At 12 months, the mean change in visual acuity was 0 letters in the mETDRS group and 2 letters worse in the MMG group (adjusted mean difference: 2 letters, 95% confidence interval −0.5 to 5 letters, P=0.10, Table 5). Ten (7%) and 7 (5%) of eyes were improved by 15 or more letters from baseline in the two groups, respectively (P=0.24), and 10 (7%) and 14 (10%) were worse by 15 or more letters (P=0.37).
Table 5.
Change in Visual Acuity According to Treatment Group
| 3.5 Months | 8 Months | 12 Months | ||||
|---|---|---|---|---|---|---|
| Change in Visual Acuity from Baseline | METDRS Group | MMG Group | METDRS Group | MMG Group | METDRS Group | MMG Group |
| Overall* | N=152 | N=150 | N=144 | N=144 | N=142 | N=142 |
| Mean±SD (Letters) | 0±13 | −1±12 | −1±12 | −2±10 | 0±11 | −2±11 |
| P-value† | 0.10 | 0.46 | 0.10 | |||
| Distribution of VA change - N(%) | ||||||
| ≥ 15 letter improvement | 13(9%) | 7(5%) | 11(8%) | 4(3%) | 10(7%) | 7(5%) |
| 14–10 letter improvement | 8(5%) | 9(6%) | 8(6%) | 8(6%) | 11(8%) | 8(6%) |
| 9–5 letter improvement | 24(16%) | 19(13%) | 19(13%) | 20(14%) | 24(17%) | 23(16%) |
| Same ± 4 letters | 79(52%) | 71(47%) | 72(50%) | 63(44%) | 69(49%) | 60(42%) |
| 5–9 letters worse | 14(9%) | 23(15%) | 16(11%) | 34(24%) | 12(8%) | 17(12%) |
| 10–14 letters worse | 7(5%) | 12(8%) | 6(4%) | 6(4%) | 6(4%) | 13(9%) |
| ≥ 15 letters worse | 7(5%) | 9(6%) | 12(8%) | 9(6%) | 10(7%) | 14(10%) |
| Baseline Acuity ≥ 84 letters (≥ 20/20) | N=41 | N=28 | N=41 | N=29 | N=42 | N=26 |
| Mean±SD (Letters) | −2±5 | −2±8 | −4±9 | −3±5 | −3±7 | −2±7 |
| Distribution of VA change - N(%) | ||||||
| ≥10 letter improvement | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) | 0(0%) |
| 9–5 letter improvement | 3(7%) | 4(14%) | 2(5%) | 2(7%) | 4(10%) | 4(15%) |
| Same ± 4 letters | 29(71%) | 16(57%) | 29(71%) | 13(45%) | 26(62%) | 15(58%) |
| 5–9 letters worse | 6(15%) | 4(14%) | 4(10%) | 12(41%) | 9(21%) | 3(12%) |
| 10–14 letters worse | 1(2%) | 2(7%) | 2(5%) | 2(7%) | 1(2%) | 3(12%) |
| ≥ 15 letters worse | 2(5%) | 2(7%) | 4(10%) | 0(0%) | 2(5%) | 1(4%) |
| Baseline Acuity 69–83 letters (20/40 – < 20/20) | N=71 | N=86 | N=65 | N=83 | N=65 | N=85 |
| Mean±SD (Letters) | −2±15 | −3±12 | −2±12 | −2±10 | −2±12 | −3±10 |
| Distribution of VA change – N(%) | ||||||
| ≥ 15 letter improvement | 1(1%) | 0(0%) | 1(2%) | 1(1%) | 0(0%) | 2(2%) |
| 14–10 letter improvement | 2(3%) | 3(3%) | 4(6%) | 5(6%) | 4(6%) | 5(6%) |
| 9–5 letter improvement | 17(24%) | 11(13%) | 10(15%) | 14(17%) | 15(23%) | 11(13%) |
| Same ± 4 letters | 38(54%) | 44(51%) | 31(48%) | 33(40%) | 32(49%) | 38(45%) |
| 5–9 letters worse | 7(10%) | 15(17%) | 10(15%) | 20(24%) | 3(5%) | 11(13%) |
| 10–14 letters worse | 3(4%) | 8(9%) | 3(5%) | 4(5%) | 5(8%) | 10(12%) |
| ≥ 15 letters worse | 3(4%) | 5(6%) | 6(9%) | 6(7%) | 6(9%) | 8(9%) |
| Baseline Acuity 19–68 letters (< 20/40 – > 20/400) | N=40 | N=36 | N=38 | N=32 | N=35 | N=31 |
| Mean±SD (Letters) | +6±13 | +4±14 | +6±12 | +1±12 | +7±10 | 1±17 |
| Distribution of VA change - N(%) | ||||||
| ≥ 15 letter improvement | 12(30%) | 7(19%) | 10(26%) | 3(9%) | 10(29%) | 5(16%) |
| 14–10 letter improvement | 6(15%) | 6(17%) | 4(11%) | 3(9%) | 7(20%) | 3(10%) |
| 9–5 letter improvement | 4(10%) | 4(11%) | 7(18%) | 4(13%) | 5(14%) | 8(26%) |
| Same ± 4 letters | 12(30%) | 11(31%) | 12(32%) | 17(53%) | 11(31%) | 7(23%) |
| 5–9 letters worse | 1(3%) | 4(11%) | 2(5%) | 2(6%) | 0(0%) | 3(10%) |
| 10–14 letters worse | 3(8%) | 2(6%) | 1(3%) | 0(0%) | 0(0%) | 0(0%) |
| ≥ 15 letters worse | 2(5%) | 2(6%) | 2(5%) | 3(9%) | 2(6%) | 5(16%) |
Positive changes represent an improvement in visual acuity
P-value obtained using repeated measures least squares regression models adjusting for baseline visual acuity and central subfield values and accounting for the correlated data from subjects with 2 study eyes
Results for Subjects with Two Study Eyes
In an analysis of the 34 subjects with two study eyes and relatively symmetrical DME at baseline, defined as having an inter-eye difference of less than 100 microns in central retinal thickening and less than 15 letters in visual acuity, there were no significant differences between mETDRS and MMG groups in the mean change in central retinal thickening (P=0.91) or in the change in visual acuity (P=0.13) from baseline to 12 months.
Adverse Effects
The only major treatment-related adverse event reported was a neurosensory detachment following the initial laser treatment in the MMG group, which resolved after 1 month. In this case, baseline visual acuity was 20/25 (80 letters) and 12-month acuity was 20/50 (67 letters). Review of the post-treatment photographs did not suggest that the photocoagulation treatment was heavier than the photographic standard for this treatment.
Discussion
At present, despite the enthusiasm for evaluation of several novel treatments for DME including intravitreal therapies for DME (e.g., corticosteroids, and anti-VEGF drugs), laser photocoagulation remains the current standard of care and the only treatment with proven efficacy in a large-scale clinical trial for this condition. This DRCRnet trial was designed to compare two laser techniques for previously untreated DME. One technique was the most commonly used approach in current clinical practice among DRCRnet investigators, a modification of the original technique used in the ETDRS (mETDRS). The other approach has theoretical advantages in which laser is given in a grid without specifically treating microaneurysms (MMG). After 12 months of follow up, there was no indication that the eyes treated with MMG had a better outcome than those receiving mETDRS treatment, and in fact eyes in the mETDRS group experienced a slightly greater reduction in retinal thickening and a trend towards a slightly better visual acuity outcome. Although a decrease in retinal thickening was observed with both treatments, this trial did not include an untreated control group, thus preventing determination of how the observed changes in retinal thickening would have differed from the natural history of untreated DME. Unexpected adverse effects of treatment were minimal.
The primary outcome measure of the study was change in retinal thickening as a surrogate for longer-term change in visual acuity. In phase 2 studies, surrogate outcome measures are often used to determine whether there is sufficient evidence of treatment effect to proceed to a longer-term, more costly phase 3 study with visual acuity as the primary outcome. The trial had a sample size that was sufficiently large so that it is unlikely that a true benefit of MMG over the mETDRS approach in reducing retinal thickening after 12 months went undetected. Furthermore, since the results slightly favored the mETDRS approach, it is even more unlikely that a true meaningful beneficial effect on retinal thickening of MMG over the mETDRS treatment after 12 months was missed. While the correlation between retinal thickening and visual acuity is only moderate,23 the study had greater than 90% statistical power to detect at least a one line difference in mean acuity between groups. Thus, it is unlikely that a meaningful beneficial effect of MMG treatment on visual acuity after 12-months was missed. The treatment groups were generally well balanced with regard to baseline factors. Retinal thickening was slightly greater in the MMG group but this was controlled for in the analyses and did not confound the results. Bias is unlikely to have prevented detection of a true benefit of MMG, because both OCT and fundus photographs were used to document macular edema and both measures were graded at a reading center masked to treatment group. Visual acuity was measured using a computerized system that reduces technician bias. The large number of investigators could have increased the variation in treatment technique compared with a smaller number of surgeons but this is not a likely explanation for the lack of difference between groups. It also seems unlikely that a relative benefit of MMG compared with mETDRS would have been seen with longer follow up since there was no suggestion of benefit after 12 months.
In conclusion, despite potential theoretical advantages, after 12 months of follow up the MMG laser technique is less effective in reducing OCT measure retinal thickening than the mETDRS technique frequently used in current clinical practice. However, the visual acuity outcomes with both approaches are not substantially different. Thus, this study does not provide data to suggest that a larger long-term trial of the MMG technique is likely to show substantial clinical benefit over the current mETDRS approach.
Acknowledgments
Supported through a cooperative agreement from the National Eye Institute EY14231, EY14269, EY14229
Writing Committee: Lead Authors: Donald S. Fong (lead author), Samara F. Strauber. Additional Writing Committee Members: Lloyd Paul Aiello, Roy W. Beck, David G. Callanan, Ronald P. Danis, Matthew D. Davis, Stephen S. Feman, Frederick Ferris, Scott M. Friedman, Charles A. Garcia, Adam R. Glassman, Dennis P. Han, Darma Ie, Craig Kollman, Andreas K. Lauer, Franco M. Recchia, Sharon D. Solomon
The Diabetic Retinopathy Clinical Research Network
Clinical Sites that Participated in this Protocol
Sites are listed in order by number of subjects randomized into the study. The number of subjects randomized is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Investigator, (C) for Coordinator, (V) for Visual Acuity Tester, and (P) for Photographer.
Baltimore, MD - Wilmer Eye Institute at Johns Hopkins (7): Sharon D. Solomon (I); Susan B. Bressler (I); Daniel Finkelstein (I); Peter L. Gehlbach (I); Quan Dong Nguyen (I); Jennifer U. Sung (I); Ingrid Zimmer-Galler (I); Warren Doll (C); Deborah Donohue (V); Robert Jurao (V); Siobhan E. Sheehan (V); Judith Belt (P); Boston, MA - Joslin Diabetes Center (5): George S. Sharuk (I); Paul G. Arrigg (I); Deborah K. Schlossman (I); Sabera T. Shah (I); Ann Kopple (C); Margaret E. Stockman (C); Leila Bestourous (V); Richard M. Calderon (V); Jerry D. Cavallerano (V); Tak Chau (V); Robert W. Cavicchi (P); James Strong (P); Columbia, SC - Palmetto Retina Center (5): John A. Wells (I); W. Lloyd Clark (I); Ruth T. Bearden (C); Mallie M. Taylor (C); Robbin Spivey (V); Mark A. Evans (P); Marsha L. Stone (P); Hershey, PA - Penn State College of Medicine (5): Thomas W. Gardner (I); Kimberly A. Neely (I); Susan M. Chobanoff (C); Mary L. Frawley (V); Kathleen C. Ringenbach (V); Ernesto Rodriguez (V); Timothy J. Bennett (P); Aiea, HI - The Retina Center at Pali Momi (4): Gregg T. Kokame (I); Jacqueline Shen (C); Sheila M. Chamian (C); Andrew Yuen (V, P); Arlington, TX -Texas Retina Associates (4): David G. Callanan (I); Wayne A. Solley (I); Glenda Janay Elmore (C); Bob Boleman (P); Jodi Creighton (V); Keith Gray (P); Austin, TX - Austin Retina Associates (4): Jose A. Martinez (I); James W. Dooner (I); Clio Armitage Harper (I); Chris A. Montesclaros (C); Carrie E. Odean (C); Ian Cadena (P); Richard A. Sabo (P); Austin, TX - Brian B. Berger, M.D. (4): Brian B. Berger (I); Margaret Rodriguez (C); Jeni Rathman (C); Bobbi Gallia (V); Ben Ostrander (P); Baltimore, MD - Elman Retina Group, P.A. (4): Michael J. Elman (I); Robert Z. Raden (I); Michelle D. Sloan (C); JoAnn Starr (C); Dena Salfer-Firestone (V); Terri Cain (P); Peter Sotirakos (P); Bethesda, MD - National Eye Institute/National Institutes of Health (4): Emily Y. Chew (I); Hanna Rodriguez Coleman (I); Ruby Lerner (C); Gregory L. Short (V); Denise Cunningham (P); Guy E. Foster (P); Ernest M. Kuehl (P); Marilois Palmer (P); Charlotte, NC - Charlotte Eye, Ear, Nose and Throat Assoc., PA (4): David J. Browning (I); Andrew N. Antoszyk (I); Jennifer V. Helms (C); Angela K. Price (C); Lisa B. Chatari (V); Heather L. Murphy (V); Robert M. Bowen (P); Brian Lutman (P); Michael D. McOwen (P); Columbia, SC - Carolina Retina Center (4): Jeffrey G. Gross (I); Barron C. Fishburne (I); Peggy W. Cummings (C); Jennifer C. Enlow (C); Regina A. Gabriel (V); Heidi K. Lovit (V); Randall L. Price (P); Dallas, TX - Texas Retina Associates (4): Gary E. Fish (I); Jean Arnwine (C); Sally Arceneaux (V); Brenda Sanchez (V); Hank Aguado (P); Penny Ellenich (P); Keith Gray (P); Kimberly Cummings (P); Diana Jaramillo (P); Denver, CO - Denver Health Medical Center (4): Antonio P. Ciardella (I); Graciela C. Gallardo (C); Dorothy L. Thomas (C); Rosemary C. Rhodes (V); Colleen J. Smith (V); Janelle Dane Zapata (V); Debbie M. Brown (P); Detroit, MI - Henry Ford Health System, Dept of Ophthalmology and Eye Care Services (4): Paul Andrew Edwards (I); Janet Murphy (C); Sheila M. Rock (C); James P. Bryant (V); Barbara Millsap (V); George Ponka (V); Mark Croswell (P); Lisa M. Schillace (P); Tracy A. Troszak (P); Detroit, MI - Kresge Eye Institute (4): Gary W. Abrams (I); Dean Eliott (I); Raymond Iezzi (I); Patrick L. Murphy (I); James E. Puklin (I); Laura L. Schulz (C); Susan Loomis (C); Cheryl Milanovic (C); Jeannine M. Gartner (V); Vicki R. Krzeminski (V); Kyohei Abe (P); Kenneth W. Christopherson (P); Zlatan Sadikovic (P); Dublin, OH - Retinal Consultants, Inc. (4): Frederick H. Davidorf (I); Robert B. Chambers (I); Louis J. Chorich, III (I); Jill D. Milliron (C); Chhanda G. Chaudhuri (V); Jerilyn G. Perry (V); Michael J. Keating (P); Scott J. Savage (P); Fort Myers, FL - Retina Consultants of Southwest Florida (4): Glenn Wing (I); A. Thomas Ghuman (I); Paul A. Raskauskas (I); Joseph P. Walker (I); Cheryl Kiesel (C); Danielle Dyshanowitz (V); Eileen Knips (P); Dixie L. McGuire (P); Ft. Lauderdale, FL - Retina Vitreous Consultants (4): Ronald J. Glatzer (I); W. Scott Thompson (I); Jaclyn A. Lopez (C); Alicia A. Tardif (C); Janet Benton-Murray (V); Michelle Earl (P); Galveston, TX - University of Texas Medical Branch, Dept of Ophthalmology and Visual Sciences (4): Helen K. Li (I); Susan K. Busch (C); Wiline Jean (C); Adol Esquivel (P); John Horna (P); Zbigniew Krason (P); Grand Rapids, MI - Associated Retinal Consultants (4): Thomas M. Aaberg, Jr. (I); Sandy Kronlein (C); Verla M. Zuiderveen (C); Debra Markus (V); Joan M. Videtich (V); Sandra Lewis (P); Houston, TX - Charles A. Garcia, P.A and Associates (4): Charles A. Garcia (I); John McCrary (I); Penelope Villeda (C); Elizabeth Garibay (C); Otila Martinez (C); Daniel Banda (V); Juan P. Montoya (V); Ben Livas (P); Angela Ramirez (P); Shadi Qassim Al-Khatib (V); Houston, TX - Retina and Vitreous of Texas (4): H. Michael Lambert (I); Roberto Diaz-Rohena (I); Joseph A. Khawly (I); Arthur W. Willis (I); Susan K. Busch (C); Mikki O’Neal (C); Debbie Fredrickson (V); Maritza F. Gorrin (V); Joseph A. Morales (P); Houston, TX - Vitreoretinal Consultants (4): David M. Brown (I); Rosa Y. Kim (I); Tien P. Wong (I); Rebecca De La Garza (C); Amanda Faszholz (V); Eric N. Kegley (P); Karin A. Mutz (V); Indianapolis, IN - Midwest Eye Institute (4): Raj K. Maturi (I); Thomas A. Ciulla (I); John T. Minturn (I); Donna Agugliaro (C); Laura A. Bleau (C); Stephanie J. Morrow (V); Denise L. Samaniego (V); Thomas Steele (P); Lakeland, FL -Central Florida Retina Institute (4): Scott M. Friedman (I); Steve Carlton (C); Vickie D. Bassford (V); Damanda A. Fagan (V); Virginia Gregory (V); Lawrenceville, NJ -Delaware Valley Retina Associates (4): Darma Ie (I); Susan Lilienfield (C); Morgan Harper (V); Lydia Polanco (V); Linda McCall (P); Louisville, CO - Eldorado Retina Associates, P.C. (4): Mary Lansing (I); Lauren B. Hatch (C); Jeanne Ross (V); Kimberly A. Alexander (P); Ramona Smith (P); Madison, WI - University of Wisconsin-Madison, Dept of Ophthalmology/Retina Service (4): Justin Gottlieb (I); Barbara A. Blodi (I); T. Michael Nork (I); Kathryn F. Burke (C); Shelly R. Olson (V); Alyson J. Pohlman (V); Erika D. Soderling (V); Barbara H. Soderling (V); Gene E. Knutson (P); Denise A. Krolnik (P); John C. Peterson (P); Minneapolis, MN - University of Minnesota (4): Timothy W. Olsen (I); Sally Cook (C); Ann M. Holleschau (C); Pamela K. Patterson (V); Dave Philiph (V); Sabrina M. Rolfer (V); Mark J. Cohen (P); Pat Stanaitis Harvey (P); Nashville, TN - Vanderbilt University Medical Center (4): Franco M. Recchia (I); Anita Agarwal (I); Sandy Owings (C); Genise G. Mofield (V); Tony Adkins (P); Cynthia C. Recchia (P); Oklahoma City, OK - Dean A. McGee Eye Institute (4): Ronald M. Kingsley (I); Robert E. Leonard (I); Lisa M. Ogilbee (C); Misty D. Youngberg (C); Sara L. M. Ceresa (V); Connie J. Dwiggins (V); Russ Burris (P); William R. Richmond (P); Paducah, KY - Paducah Retinal Center (4): Carl W. Baker (I); Tracey M. Caldwell (C); Lynnette F. Lambert (V); Dawn D. Smith (P); Peabody, MA - Lahey Clinic, Inc./The Eye Institute (4): Jeffrey L. Marx (I); Fleming D. Wertz (I); Avon P. Stewart (C); Steve M. Kelly (C); Patti-Ann L. Morse (C); Michael R. Johnson (V); Tracy Scrivano (V); Ellen L. Casazza (P); Richard Selter (P); Philadelphia, PA - University of Pennsylvania Scheie Eye Institute (4): Alexander J. Brucker (I); Robert A. Stoltz (I); Joan C. DuPont (C); Sheri Drossner (C); Tanya Metelitsina (V); Tomas S. Aleman (P); Cheryl Devine (P); William Nyberg (P); Laurel Weeney (P); Elizabeth A. Windsor (P); Portland, OR - Casey Eye Institute (4): Andreas K. Lauer (I); Christina J. Flaxel (I); Shelley A. Hanel (C); Susan K. Nolte (V); Jessica M. Gaultney (P); Chris S. Howell (P); Ellen F. Redenbo (P); Peter N. Steinkamp (P); Patrick R. Wallace (P); Salt Lake City, UT - Rocky Mountain Retina Consultants (4): Roy A. Goodart (I); David W. Faber (I); Hollie J. Murphy (C); Donna Knight (P); Richard W. Osguthorpe (P); San Francisco, CA - West Coast Retina Medical Group, Inc. (4): J. Michael Jumper (I); Arthur D. Fu (I); Robert N. Johnson (I); H. Richard McDonald (I); Margaret Stolarczuk (C); Brandi Teske (C); Silvia C. Linares (V); Rona Lyn Esquejo (P); Sean T. Grout (P); Sarah Huggans (P); Jeremy Miller (P); Sarasota, FL - Sarasota Retina Institute (4): Keye L. Wong (I); John H. Niffenegger (I); Christine Holland (C); Karen Hagin (V); Hasseema R. Shelton (V); Rosa Miller (P); Charlotte Rodman (P); Mark Sneath (P); Seattle, WA - University of Washington Medical Center (4): James L. Kinyoun (I); Susan A. Rath (C); Patricia K. Ernst (V); Betty S. Lawrence (V); Juli Pettingill (V); Brad C. Clifton (P); James D. Leslie (P); Chuck Stephens (P); Slingerlands, NY - The New Lions Eye Institute, Retina Consultants, PLLC (4): Paul M. Beer (I); Naomi Falk (I); Eugenia Olmeda (C); Denise Garza (C); Robert Davis (P); Joe Fischer (P); St. Louis, MO - St. Louis University Eye Institute (4): Stephen S. Feman (I); Levent Akduman (I); Kevin L. Anderson (C); Patrick Burke (V); Thomas I. Porter (V); Joshua S. Anderson (P); Christopher J. Kleber (P); Syracuse, NY - Retina-Vitreous Surgeons of Central New York, PC (4): G. Robert Hampton (I); Samuel C. Spalding (I); Cindy J. Grinnell (C); Lynn M. Kwasniewski (V); Jeanne L. Burke (P); Lynn A. Capone (P); Peter B. Hay (P); Mark E. Zalewski (P); Tampa, FL - International Eye Center (4): Don John Perez Ortiz (I); Madelyn Alvarez (C); Rita L. Johnson (C); Sandra E. Montoya (C); Ross Jarrett (P); Tampa, FL - University of South Florida (4): P. Reed Pavan (I); Burton Goldstein (I); Sue Sherouse (C); Amy L. Kimball (C); Sonya Edison (V); Wyatt Saxon (P); Winston-Salem, NC - Wake Forest University Eye Center (4): Craig Michael Greven (I); Nicholas Engelbrecht (I); M. Madison Slusher (I); Joan Fish (C); Frances Ledbetter (C); David T. Miller (P); Marshall Tyler (P); Augusta, GA - Medical College of Georgia (3): Julian Nussbaum (I); Dennis M. Marcus (I); Judy Ann Johnson (C); June Benson (C); Sandra A. Grubisa (C); Ashley J. Goodwin (V); Judith Hendrickson (V); Mike Stanley (P); Beachwood, OH - Retina Associates of Cleveland, Inc. (3): Lawrence J. Singerman (I); David G. Miller (I); Diane E. Weiss (C); Maureen Cunningham (V); Kimberly A. Dubois (V); Vivian Tanner (V); John C. DuBois (P); Gregg A. Greanoff (P); Chapel Hill, NC - University of North Carolina, Dept of Ophthalmology (3): Mary Elizabeth R. Hartnett (I); Travis A. Meredith (I); Cassandra M. Barnhart (C); Carrie D. Vallar (V); Teresa Hawks (P); Kelly D. Shields (P); Charlotte, NC - Horizon Eye Care, PA (3): Miriam E. Ridley (I); Frederick H. D. Weidman (I); Mara-Leigh Schafer (C); Amy A. Brogdon (V); Crystal L. Tingle (V); Jennifer T. Lummis (P); David C. Peterson (P); Grand Rapids, MI -Vitreo-Retinal Associates (3): Frank W. Garber (I); Landine K. Litts (C); Christine E. Feehan (V); Brenda K. Kilbourne (V); Angela D. Listerman (V); Donald E. Kuitula (P); Sue Weatherbee (P); Greenbelt, MD - The Retina Group of Washington (3): William B. Phillips, II (I); Richard Garfinkel (I); Manfred A. Von Fricken (I); Joulia C. Haziminas (C); Heather A. McManus (C); Mike Flory (P); Honolulu, HI - Retina Associates of Hawaii, Inc. (3): John H. Drouilhet (I); Susan Pelke (C); Deborah Nobler (P); Kingsport, TN - Southeastern Retina Associates, PC (3): Howard L. Cummings (I); D. Allan Couch (I); Gail Darnell (C); Deanna Jo Long (C); Stacy Carpenter (V); Rachel Mallard (V); Julie P. Berry (P); Melissa Sturgill (P); Los Angeles, CA - Doheny Eye Institute (3): Jennifer I. Lim (I); Christina J. Flaxel (I); Margaret Padilla (C); Jesus M. Garcia (V); Frances Walonker (V); Len S. Richine (P); Lori Levin (P); Minneapolis, MN - Retina Center, PA (3): Abdhish R. Bhavsar (I); Tanya M. Pierce (C); Shelly A. Ellard (C); William B. Carli (V); Melinda Spike-Kivel (V); Jef Jodell (P); Carmen W. Chan (P); Laura Taylor-Reetz (P); Rochester, NY - University of Rochester (3): David Allen DiLoreto (I); Mina M. Chung (I); Nancy Fedick (C); Dorothea Castillo (V); Terrance Schaefer (V); William S. Fischer (P); Julie Howell (P); Rockford, IL - Northern Illinois Retina, Ltd. (3): Susan M. Fowell (I); James P. Watson (C); Nancy L. Mercurio (C); Maureen L. Cain (P); Jacquie S. Button (P); Chris M. Gomez (P); Santa Ana, CA - Southern California Permanente Medical Group (3): Keith J. Pince (I); Paula Chase (C); Marie O. Haas (V); Bruce A. Moore (V); Abilene, TX - West Texas Retina Consultants P.A. (2): Sunil S. Patel (I); S. Young Lee (I); Brandi L. Dunn (C); Kristen L. Garcia (V); Gwyn R. Nafe (V); Birmingham, AL - Retina Consultants of Alabama (2): John O. Mason (I); Tracy L. Emond (C); Tonya Davis (V); Denise Iovino (P); Buddy Skellie (P); Chicago, IL - Rush University Medical Center (2): Mathew W. MacCumber (I); Pauline T. Merrill (I); Sarah J. Levine (C); Nisha D. Sheth (C); Sarice R. Smith (C); Bruce I. Gaynes (V); Pamela Hulvey (P); Frank Morini (P); Loreen Pappas (P); Durham, NC - Duke University Eye Center (2): Srilaxmi Bearelly (I); Michael J. Cooney (I); Glenn J. Jaffe (I); Brooks W. McCuen (I); Malcolm Anderson (C); Neeru Sarin (V); Russell E. Burns (P); Gregory C. Hoffmeyer (P); Jeffrey M. Napoli (P); Irvine, CA - University of California, Irvine (2): Baruch D. Kuppermann (I); Jeff Grijalva (C); Rosie Magallon (V); Bret Trump (P); Lexington, KY - University of Kentucky (2): Andrew Pearson (I); Michele Reg (C); Susie Craig (V); Philip Moss (V); Toni Scoggins (V); Phyllis Gillespie (P); Michael Hanson (P); Loma Linda, CA -Loma Linda University Health Care, Department of Ophthalmology (2): Joseph T. Fan (I); Michael E. Rauser (I); Arun K. Chakrabarty (C); William H. Kiernan (V); Gene Saldana (P); Los Angeles, CA - Jules Stein Eye Institute (2): Steven D. Schwartz (I); Christine R. Gonzales (I); Anurag Gupta (I); Rosaleen Ostrick (C); Melissa Chun (V); Jennie Kageyama (V); Bita Shokouh (V); David L. Le Beck (P); Mirella Tetreault (P); Joel Moral (P); Milwaukee, WI - Medical College of Wisconsin (2): Judy E. Kim (I); Dennis P. Han (I); Troy S. Drescher (C); Christine Y. Lange (C); Kelly Reiter (C); Vicki Barwick (V); Joseph R. Beringer (P); Richmond, VA - Richmond Retinal Associates/Virginia Eye Institute (2): George Sanborn (I); Byron S. Ladd (I); Melissa Vaughan (C); Robin M. Driver (V); Karen E. Sullivan (V); Megan E. Bicknell (P); Michael Palczynski (P); Mark E. Zalewski (P); Royal Oak, MI - Associated Retinal Consultants, PC (2): Michael T. Trese (I); Alan J. Ruby (I); Mary Zajechowski (C); Beth L. Mitchell (C); Cindy Huckabone (V); Patricia Streasick (P); Lynette D. Szydlowski (P); Santa Barbara, CA - California Retina Consultants (2): Dante J. Pieramici (I); Tamara A. Norton (C); Elizabeth A. Robbins (C); Kelly Avery (V); Liz Tramel (V); Karen Boyer (P); Matthew Giust (P); Melissa Kruzel (P); St. Louis, MO -Barnes Retina Institute (2): Rajendra S. Apte (I); Ginny S. Nobel (C); Lynda K. Boyd (V); Carolyn L. Walters (V); Matt L. Raeber (P); Jarrod Wehmeier (P); Towson, MD -Retina Specialists (2): Raymond N. Sjaarda (I); John T. Thompson (I); Maryanth Constantine (C); Janette L. Herron (V); Marcia A. Sentz (V); Leslie G. Russel (P); John L. Davis (V); Ventura, CA - Miramar Eye Specialist Medical Group (2): Joel M. Corwin (I); Donald A. Frambach (I); Lisa Jue (C); Claudia P. Acosta (C); Mark A. Brunette (V); Michael Feldman (P); Andrea M. Ferrari (P); Chicago, IL - Northwestern Medical Faculty Foundation (1): Alice T. Lyon (I); Jeevan R. Mathura (I); Lori Kaminski (C); Annie Munana (C); Jonathan Shankle (P); Portland, OR - Retina Northwest, PC (1): Mark A. Peters (I); Colin Ma (I); Stephen Hobbs (C); Patricia A. Bartholomew (C); Katie J. Reichenberger (C); Marcia Kopfer (V); Milt Johnson (P); Joe Logan (P); Harry Wohlsein (P); Providence, RI - Retina Consultants (1): Robert H. Janigian (I); Emiliya German (C); Erika Banalewicz (V); Sandra Henriques (V); Mark Hamel (P); Washington, DC - The George Washington University, Department of Ophthalmology (1): B. Eric Jones (I); Ronald J. Olszowy (C); Abdul Habib (V); Bertrand P. Miskell (P); Columbia, SC - Columbia Eye Clinic, PA (0): W. Lloyd Clark (I); Ruth T. Bearden (C); Mary P. Thompson (V); Marsha L. Stone (P)
DRCR.net Coordinating Center – Tampa, FL: Roy W. Beck (Executive Director), Kimberly E. McLeod (DRCR.net Associate Director), Kelly A. Blackmer, Brian B. Dale, Adam R. Glassman, Nicola B. Hill, Paula A. Johnson, Craig Kollman, Brenda L. Loggins, Ana C. Perez, Apryl C. Quillen, Cynthia R. Stockdale, Samara F. Strauber
DRCR.net Chairman’s Office – Boston, MA: Neil M. Bressler – Baltimore, MD (Network Chair), Lloyd P. Aiello – Boston, MA (Network Chair 2002 – 2005), Kia Graves
Fundus Photograph Reading Center – Madison, WI: Matthew D. Davis (Director Emeritus), Ronald P. Danis (Director), Larry Hubbard (Associate Director), James Reimers (Lead Color Photography Evaluator), Pamela Vargo (Lead Photographer), Ericka Lambert (Digital Imaging Specialist), Dawn Myers (Lead OCT Evaluator), Julee Elledge (Lead Angiography Evaluator)
Data and Safety Monitoring Committee: John Connett (Chair), Harry W. Flynn, Jr., Robert N. Frank, Saul Genuth, Lee Jampol, Jeanette Resnick, Stephen Wisniewski
National Eye Institute: Päivi H. Miskala, Donald F. Everett (2002 – 2004)
DRCR.net Executive Committee: Lloyd P. Aiello (Chair 2002 – 2005), Roy W. Beck, Neil M. Bressler (Chair), David M. Brown, David J. Browning, Ronald P. Danis, Matthew D. Davis, Michael J. Elman, Frederick L. Ferris, Adam R. Glassman, Kimberly E. McLeod, Päivi H. Miskala
Laser Photocoagulation Study Steering Committee: Lloyd P. Aiello, Roy W. Beck, Neil M. Bressler, Alexander J. Brucker, Steve Carlton, Emily Y. Chew, Ronald P. Danis, Frederick L. Ferris, Donald S. Fong (Protocol Chair), Adam Glassman, Jeffrey G. Gross, Julia A. Haller, Helen K. Li, Kimberly McLeod, Päivi H. Miskala
References
- 1.Tso MOM, Wallow IHL, Elgin S. Experimental photocoagulation of the human retina. I. Correlation of physical, clinical, and pathologic data. Arch Ophthalmol. 1977;95:1035–40. doi: 10.1001/archopht.1977.04450060121012. [DOI] [PubMed] [Google Scholar]
- 2.Apple DJ, Goldberg MF, Wyhinny G. Histopathology and ultrastructure of the argon laser lesion in human retinal and choroidal vasculatures. Am J Ophthalmol. 1973;75:595–609. doi: 10.1016/0002-9394(73)90812-x. [DOI] [PubMed] [Google Scholar]
- 3.Wilson DJ, Finkelstein D, Quigley HA, Green WR. Macular grid photocoagulation. An experimental study on the primate retina. Arch Ophthalmol. 1988;106:100–5. doi: 10.1001/archopht.1988.01060130106038. [DOI] [PubMed] [Google Scholar]
- 4.Arnarsson A, Stefansson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2000;41:877–9. [PubMed] [Google Scholar]
- 5.Ogata N, Tombran-Tink J, Jo N, Mrazek D, Matsumura M. Upregulation of pigment epithelium-derived factor after laser photocoagulation. Am J Ophthalmol. 2001;132:427–9. doi: 10.1016/s0002-9394(01)01021-2. [DOI] [PubMed] [Google Scholar]
- 6.Ogata N, Ando A, Uyama M, Matsumura M. Expression of cytokines and transcription factors in photocoagulated human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol. 2001;239:87–95. doi: 10.1007/s004170000235. [DOI] [PubMed] [Google Scholar]
- 7.Shinoda K, Ishida S, Kawashima S, et al. Clinical factors related to the aqueous levels of vascular endothelial growth factor and hepatocyte growth factor in proliferative diabetic retinopathy. Curr Eye Res. 2000;21:655–61. [PubMed] [Google Scholar]
- 8.Spranger J, Hammes HP, Preissner KT, Schatz H, Pfeiffer AFH. Release of the angiogenesis inhibitor angiostatin in patients with proliferative diabetic retinopathy: association with retinal photocoagulation. Diabetologia. 2000;43:1404–7. doi: 10.1007/s001250051546. [DOI] [PubMed] [Google Scholar]
- 9.Xiao M, McLeod D, Cranley J, Williams G, Boulton M. Growth factor staining patterns in the pig retina following retinal laser photocoagulation. Br J Ophthalmol. 1999;83:728–36. doi: 10.1136/bjo.83.6.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akduman L, Olk RJ. Subthreshold (invisible) modified grid diode laser photocoagulation in diffuse diabetic macular edema (DDME) Ophthalmic Surg Lasers. 1999;30:706–14. [PubMed] [Google Scholar]
- 11.Olk RJ. Modified grid argon (blue-green) laser photocoagulation for diffuse diabetic macular edema. Ophthalmology. 1986;93:938–50. doi: 10.1016/s0161-6420(86)33638-8. [DOI] [PubMed] [Google Scholar]
- 12.Olk RJ. Argon green (514 nm) versus krypton red (647 nm) modified grid laser photocoagulation for diffuse diabetic macular edema. Ophthalmology. 1990;97:1101–13. doi: 10.1016/s0161-6420(90)32449-1. [DOI] [PubMed] [Google Scholar]
- 13.Striph GG, Hart WM, Olk RJ. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology. 1988;95:1673–9. doi: 10.1016/s0161-6420(88)32957-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee CM, Olk RJ. Modified grid laser photocoagulation for diffuse diabetic macular edema. Long-term visual results. Ophthalmology. 1991;98:1594–1602. doi: 10.1016/s0161-6420(91)32082-7. [DOI] [PubMed] [Google Scholar]
- 15.Schatz H, Madeira D, McDonald HR, Johnson RN. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109:1549–51. doi: 10.1001/archopht.1991.01080110085041. [DOI] [PubMed] [Google Scholar]
- 16.Roider J. Laser treatment of retinal diseases by subthreshold laser effects. Semin Ophthalmol. 1999;14:19–26. doi: 10.3109/08820539909056059. [DOI] [PubMed] [Google Scholar]
- 17.Mainster MA, White TJ, Tips JH, Wilson PW. Retinal-temperature increases produced by intense light sources. J Opt Soc Adm. 1970;60:264–70. doi: 10.1364/josa.60.000264. [DOI] [PubMed] [Google Scholar]
- 18.Stanga PE, Reck AC, Hamilton AMP. Micropulse laser in the treatment of diabetic macular edema. Semin Ophthalmol. 1999;14:210–3. doi: 10.3109/08820539909069539. [DOI] [PubMed] [Google Scholar]
- 19.Friberg TR. Subthreshold (invisible) modified grid diode laser photocoagulation and diffuse diabetic macular edema (DDME) Ophthalmic Surg Lasers. 1999;30:705. [PubMed] [Google Scholar]
- 20.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 21.Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs - an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 22.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–33. [PubMed] [Google Scholar]
- 23.Diabetic Retinopathy Clinical Research Network. The relationship between OCT-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. doi: 10.1016/j.ophtha.2006.06.052. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]