Abstract
Animal studies have shown that noxious inputs onto γ-motoneurons can cause an increase in the activity of muscle spindles, and it has been proposed that this causes a fusimotor-driven increase in muscle stiffness that is believed to underlie many chronic pain syndromes. To test whether experimental pain also acts on the fusimotor system in humans, unitary recordings were made from 19 spindle afferents (12 Ia, 7 II) located in the ankle and toe extensors or peronei muscles of awake human subjects. Muscle pain was induced by bolus intramuscular injection of 0.5 ml 5% hypertonic saline into tibialis anterior (TA); skin pain was induced by 0.2 ml injection into the overlying skin. Changes in fusimotor drive to the muscle spindles were inferred from changes in the mean discharge frequency and discharge variability of spindle endings in relaxed muscle. During muscle pain no afferents increased their discharge activity: seven afferents (5 Ia, 2 II) showed a decrease and six (4 Ia, 2 II) afferents were not affected. During skin pain of 13 afferents discharge rate increased in one (Ia) and decreased in two (1 Ia, 1 II). On average, the overall discharge rate decreased during muscle pain by 6.1% (P < 0.05; Wilcoxon), but remained essentially the same during skin pain. There was no detectable correlation between subjective pain level and the small change in discharge rate of muscle spindles. Irrespective of the type of pain, discharge variability parameters were not influenced (P > 0.05; Wilcoxon). We conclude that, contrary to the ‘vicious cycle’ hypothesis, acute activation of muscle or skin nociceptors does not cause a reflex increase in fusimotor drive in humans. Rather, our results are more aligned with the pain adaptation model, based on clinical studies predicting pain-induced reductions of agonist muscle activity.
Chronic musculoskeletal pain syndromes are usually a common group of work-related myalgias. A popular, but not clinically proven hypothesis is referred to as the Johansson/Sojka hypothesis, which suggests that muscle metabolites released by underlying muscle contraction evoke a ‘vicious cycle’, reciprocally aggravating muscle pain and muscle tonus (Johansson & Sojka, 1991). Experiments in anaesthetized animals have demonstrated that group III and IV afferents excite γ-motoneurons and thereby increase the background firing of the muscle spindles (Appelberg et al. 1983b; Thunberg et al. 2002). Muscle spindle excitation may lead to an increased activation level of the homonymous α-motoneurone pool. Consequently, the sustained muscle tone or contraction-induced ischaemia would result in accumulation of metabolites (Johansson & Sojka, 1991). Accordingly, if the production of metabolites is reasonably high to excite nociceptors, or if the nociceptive input from joints (Johansson et al. 1986, 1988a) and ligaments (Johansson et al. 1989a, 1990) also contributes, a process sustaining a ‘vicious cycle’ might be initiated, resulting in chronic muscle pain. In addition, experimental evidence obtained with electrical stimulation suggests that there might be a second positive feedback loop sustained by secondary muscle spindle afferents acting on γ-motoneurones directly, thereby bypassing nociceptive afferents (Appelberg et al. 1983a). It was proposed that, together with other feedback loops, this mechanism may strengthen self-perpetuating vicious cycle reflexes (Wadell et al. 1991).
While γ-motoneurone activation during nociceptive stimulation is well documented in experimental animals to date, clear experimental evidence in humans is lacking. Thus, whether this model should be used to explain mechanisms and develop treatments for chronic muscle pain in healthcare is questionable. Variable results have been obtained when attempting to verify those effects in human subjects by analyses of EMG activity, stretch reflexes and proprioceptive function; however, they all agree that the ‘vicious cycle’ mechanism is very unlikely. Clinically, it is observed that involuntary contractions are seen in patients suffering from pain. However, it is not known whether something other than the muscle pain may cause a spasm, as for example, abdominal rigidity is an indicator of peritoneal inflammation. Painful muscles often do not show any obvious EMG activity, even if subjectively they may feel tense (Simons & Mense, 1998). Rather, a review of clinical studies by Lund et al. (1991) suggests that chronic pain tends to inhibit, not facilitate, activity in painful muscles and their agonists, while there is a small increase in the level of activity at the antagonist. Experimental pain studies in humans failed to evoke increases in EMG which would have any clinical importance (Stohler et al. 1996; Svensson et al. 1998). Moreover, no support for the ‘vicious cycle’ model was provided by Matre et al. (1998), who observed some increase in the stretch reflex but an unchanged H-reflex in human leg muscles. Furthermore, it has been shown that muscle pain has little effect on human ankle proprioception (Matre et al. 2002).
Apart from the fusimotor system, another less explored pathway for pain to modulate muscle spindle activity is via the sympathetic nervous system. Recordings from muscle spindles in animals have demonstrated that electrical stimulation of the cervical sympathetic trunk may exert a net facilitatory (Passatore et al. 1985) or inhibitory (Hellström et al. 2005) effect on muscle spindle activity. Yet direct recordings from human muscle spindle afferents during physiologically evoked increases in muscle sympathetic activity showed no effect (Macefield et al. 2003). In contrast, an excitatory influence on human muscle spindles has been suggested by another study demonstrating facilitation of a stretch reflex in humans (Hjortskov et al. 2005). All previous studies have been designed to focus on either fusimotor or sympathetic influences on muscle spindles separately. A unified model integrating the two mechanims is not available.
To test whether neural mechanisms supporting the ‘vicious cycle’ hypothesis for muscle pain (Johansson & Sojka, 1991) could be observed in humans, we used microneurography to record nerve impulses directly from muscle spindle afferents during experimentally induced deep (muscle) and superficial (skin) pain. From a functional point of view, noxious stimulation to skin and muscle presumably require different reflex strategies. Differences in nociceptive effects originating from muscle and skin have been demonstrated at the effector organ level (Johansson et al. 1989b; Rossi & Decchi, 1997), in the brain (Henderson et al. 2007) and at the perceptual level (Svensson et al. 1997). Unlike studies that have examined the stretch reflex, the H-reflex or proprioceptive acuity we were able to avoid any central influences on α-motoneurones and focus directly on muscle spindles driven by the fusimotor system and, potentially, the sympathetic nervous system. Pain was induced by injection of 5% hypertonic saline solution, as was used in previous animal experiments (Thunberg et al. 2002). Experimental pain induced by hypertonic saline injection is a widely used model of muscle pain (Capra & Ro, 2004) and seems to affect motor control in much the same way as chronic muscle pain (Arendt-Nielsen et al. 1996). Given that the background firing rate of muscle spindles is determined by the degree of stretch of the parent muscle, we used changes in the background firing rate as a measure of static stretch sensitivity of the receptor: in the absence of any change in position or any extrafusal contraction (as evidenced by EMG), an increase or decrease in mean firing rate can be interpreted as a modulation of static stretch sensitivity. In addition, changes in the regularity of muscle spindle firing can be analysed as an indicator reflecting changes in fusimotor modulation (Matthews & Stein, 1969; Burke et al. 1979).
Methods
Data were obtained from 15 healthy subjects, 11 males and 4 females, ranging in age from 18 to 35 years. Each subject provided informed written consent to the procedures, which were approved by the Human Research Ethics Committee of the University of New South Wales and conformed with the principles established in the Declaration of Helsinki. Subjects were seated with the knees flexed ∼15 deg from full extension and the feet held against a rigid foot plate at ∼30 deg from maximal dorsiflexion of the ankle.
Electromyography
Surface EMG was recorded via Ag–AgCl electrodes over the tibialis anterior (TA), extensor digitorum longus (EDL) and peronei muscles to confirm that the muscles were relaxed. The surface electrodes were placed over the muscle belly with an interelectrode distance of 20–25 cm and in optimal positions for detecting EMG from the majority of the muscle's active fibres.
Microneurography
The common peroneal nerve was located at the fibular head by palpation and electrical stimulation via a surface probe. A tungsten microelectrode (Frederick Haer & Co. Inc., Brunswick, ME, USA) was inserted percutaneously into a motor fascicle of the nerve. Fine manipulation of the microelectrode to isolate single afferents was then performed using auditory feedback of the neural activity while providing mechanical stimuli to the pre-tibial muscles and tendons of the lower leg. Unitary recordings were made from muscle spindle afferents active in non-contracting muscles. Neural activity was amplified (gain 1 × 104) and filtered at 0.3–3.0 kHz, 50 Hz notch (ISO-80, World Precision Instruments, USA). All electrophysiological data were recorded on a computer-based data acquisition system (PowerLab 16SP, ADInstruments, Bella Vista, Australia). In addition we monitored the subject's blood pressure using a continuous non-invasive beat-to-beat haemodynamic monitoring system (Finometer, Finapres Medical Systems, Amsterdam, the Netherlands) and heart rate (HR) via standard Ag–AgCl ECG chest electrodes. Respiration was recorded with a strain gauge transducer attached to a strap around the chest (Pneumotrace, UFI, Morro Bay, CA, USA).
Muscle spindle afferent identification
Identification of the muscle spindle afferents was based on the responses to stretch of the muscle along its line of action, palpation and vibration over the muscle tendons and belly and weak voluntary contractions. Single muscle afferents were identified as type I or type II spindle afferents according to criteria previously described (Edin & Vallbo, 1990). Briefly, primary afferents show high dynamic stretch sensitivity, demonstrate an irregular spontaneous or volitionally maintained discharge, and exhibit an off-response at the point of abrupt relaxation following a slow ramping isometric contraction. In contrast, secondary afferents usually exhibit a more regular tonic discharge (Burke et al. 1979; Nordh et al. 1983), decelerate during an unloading contraction and do not exhibit an off-response at the termination of a voluntary ramp-and-hold contraction.
Nociceptive stimuli
Two 23G cannulae were inserted: one ∼1 cm into the belly of the tibialis anterior muscle of the same leg from which the spindle afferent was being recorded, the other subdermally into the overlying skin ∼5 mm away. An intramuscular bolus injection of 5% hypertonic saline (0.5 ml), delivered at a time unknown to the subject, caused sensations of deep, dull and diffuse pain, whereas subdermal injections of 5% hypertonic saline (0.2 ml) caused sensations of localized, sharp burning pain. After an appropriate baseline period was recorded, injections were made at unexpected times and in quasi-random order. Subjective pain level was evaluated on visual-analog scale from 0 to 10, where 0 was described as ‘no pain’ and 10 as ‘the worst pain ever experienced by the subject’. Subjects indicated instantaneous pain level by a calibrated, labelled potentiometer dial. For most of the subjects hypertonic saline injection evoked a distinct pain sensation but in three subjects muscle pain did not reach a sufficiently painful intensity; data obtained in those subjects were analysed and presented in a separate section.
Data analyses
Recorded nerve activity and EMG were exported and saved in Igor Pro 5 format (Wavemetrics, USA). Single spike recognition and analyses of spike frequency and variability were performed using custom software developed using Igor Pro 5. The discharge activity of single muscle spindle afferents were visually scrutinized during the whole time course of the experiment to detect any inconsistencies or abrupt changes in discharge rate. The RMS-filtered EMG signal was inspected visually and total surface area under the EMG curve was calculated and compared with baseline to ensure that muscles remained relaxed. Unless stated otherwise, quantitative analyses were performed on 30 s blocks corresponding to the given phases of the experiment (baseline, peak pain, cessation of pain and complete absence of pain). To evaluate discharge variability over such relatively long periods and to eliminate any effect of slow drifts in discharge rate, we introduced the measure of irregularity index (IRI), which was calculated as the mean of the absolute discharge rate differences measured between every two pairs of consecutive spikes divided by the mean discharge rate.
Statistical tests
Non-parametric statistics were used to test for statistical significance (Siegel & Castellan, 1988). The Mann–Whitney U test was used to test for differences between two independent samples. The Wilcoxon matched-paired signed-ranks test was used to detect the prevailing direction of differences within the population. In this test each afferent was represented by a pair of values representing two different conditions. As a non-parametric measure of correlation, we used Spearman's rank correlation coefficient (rs). In all tests, the level of probability selected as significant was P < 0.05.
Results
Sample
A total of 19 muscle spindle afferents discharging in the absence of any muscle contraction were recorded and identified. Ten muscle spindle afferents innervated extensor digitorum longus (EDL), four supplied the peronei muscles (PER), four the tibialis anterior (TA) and one innervated the extensor hallucis longus (EHL). According to our identification procedure, 12 afferents were classified as Ia-like and 7 as II-like. Group Ia afferents showed a lower mean discharge rate during the control period: 8.38 impulses s−1 in comparison to 12.94 impulses s−1 in group II afferents. The mean coefficient of variation (standard deviation divided by mean discharge rate) was 0.114 for primary endings and 0.051 for secondary endings (cf. irregularity index below).
Pain
The perceived pain typically reached its peak after about 2.5 min irrespective of where it was injected. Muscle pain tended to last a little longer (5.7 min; range 2.6–10 min) than skin pain (4.8 min; range 1.3–13 min); however, those differences were not statistically significant (P > 0.05, Mann–Whitney). Subjective pain intensity ratings on a scale between 0 and 10 varied substantially between subjects. The average pain intensity ratings at the peak were 3.6 for muscle pain and 3.4 for skin pain.
Effect of muscle pain on muscle spindle afferents
Changes in firing pattern during painful stimulation of muscle were examined in 13 muscle spindle afferents discharging in the absence of any muscle contraction. Nine of those were classified as primary Ia-like muscle spindle afferents and four were classified as secondary II-like muscle spindle afferents. Shortly after injection of hypertonic saline into the tibialis anterior muscle subjects reported the rapid onset of cramp-like aching pain, but there was no detectable EMG activity and the muscles remained relaxed, which was a requirement for this experiment. During the induced muscle pain additional recruitment of silent muscle spindle afferents was never observed.
None of the afferents included in the current study showed any sudden changes in the discharge rate in the absence of any muscle contraction. Typically, the discharge rate slowly drifted over the course of the experiment. Discharge rate changes were monitored for every single afferent for the whole duration of the experiment. An example of the behaviour of a spindle primary afferent (Ia) during the control period before hypertonic saline injection, during painful stimulation at the peak of the pain sensation and after the pain had fully subsided is shown in Fig. 1. Despite the strong pain (rated as 6/10), the subject remained relaxed throughout the recording period and the instantaneous frequency was not affected by the noxious stimulation. Visual inspection of single afferent data revealed no evidence of a systematic increase in discharge rate during muscle pain. During peak muscle pain no afferents increased their discharge rate; six (4 Ia, 2 II) were not influenced (change < 5%); whilst seven (5 Ia, 2 II) afferents showed a decrease (Fig. 2A and B). The population average discharge rate decreased from 10.54 to 9.90 impulses s−1. This small decrease (6.1%) was statistically significant (P < 0.05, Wilcoxon). Afferents innervating the TA muscle, in which the injection was made, were no different from the rest: in two the discharge rate decelerated during muscle pain and one remained uninfluenced.
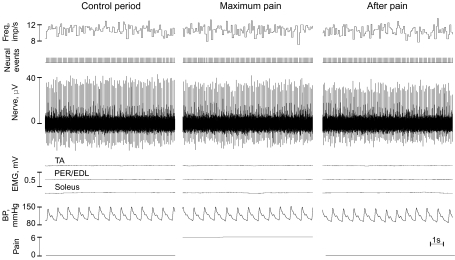
Figure 1. Example of muscle spindle afferent activity recorded from a relaxed leg muscle before, during and after experimentally induced muscle pain.
This sensory unit was classified as Ia muscle spindle afferent innervating a peroneus muscle. Regardless of strong pain (rated 6 on a scale 0–10) instantaneous discharge rate was not affected by nociceptive stimulation; however, during a period when pain fully subsided the discharge rate slightly decelerated (3.6%) and the irregularity index increased (22%). For clarity, the figure shows 10 s intervals from respective periods. Note the absence of surface EMG activity. Blood pressure (BP) was monitored throughout the experiment.
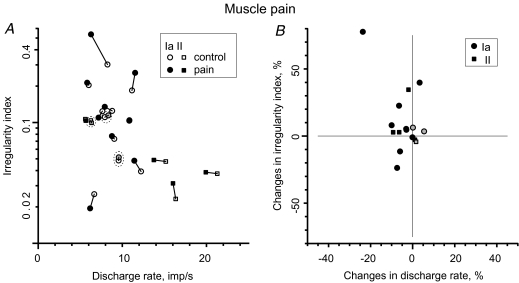
Figure 2. Effect of deep muscle pain on muscle spindle afferent discharge rate and variability.
A, comparison between discharge rate and irregularity index during control period and during painful stimulation. B, changes in the irregularity index as a function of changes in discharge rate. Control and pain periods indicated by open and filled symbols, respectively. Circles represent primary (Ia) muscle spindle afferents and squares represent secondary (II) muscle spindle afferents. Three muscle spindles recorded from subjects who did not feel any pain from hypertonic saline injection are shaded grey instead of black and encircled by the dashed line in A.
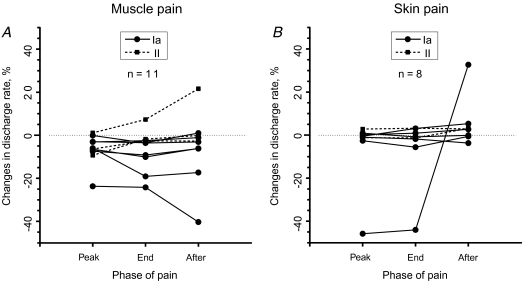
It was shown in animal experiments that activation of the fusimotor system followed the time course of nociceptive stimulation and readily returned to baseline when the noxious input presumably ceased (Thunberg et al. 2002). Thus, if changes in discharge rate detected during painful stimulation in our study were due to nociceptive input then the size of this deviation should correlate with changes in pain level and, when the pain subsides, tend to return to resting levels. Another possibility is that the effect might build up and become stronger over time, irrespective of whether or not the pain had subsided. However, a rank correlation test did not indicate any systematic trend in discharge rate changes during consecutive phases of pain: peak pain, pain recession and complete subsidence (Fig. 3A). This was true for all afferents recorded during all phases (n = 10; rs= 0.15, P > 0.1) and for a subgroup of six afferents in which discharge rate decreased during peak pain (rs= 0.13, P > 0.5). This suggests that the slow drift (see Fig. 3A) in discharge rate observed in some spindle afferents is not necessarily the result of nociceptive stimulation and the perception of pain.
Figure 3. Changes in discharge rate during separate phases of pain.
A, deep muscle pain. B, superficial skin pain. Changes in discharge rate relative to control period are shown for peak pain period (‘Peak’), phase of pain recession (‘End’) and phase after pain completely subsided (‘After’). Single afferents are connected by lines. The circles and continuous lines represent primary (Ia) muscle spindle afferents, while squares and dashed lines represent secondary (II) muscle spindle afferents. Only those afferents which were recorded until pain fully subsided are included in the analyses.
Discharge variability
Discharge variability was quantified and assessed using the ‘irregularity index’ (IRI), which in contrast to the traditionally used ‘coefficient of variation’, primarily reflects irregularity in consecutive interspike intervals and is relatively insensitive to slow drifts in discharge rate. The irregularity index did not change systematically during peak pain (0.104 versus 0.103 median; P > 0.05 Wilcoxon; Fig. 2A). Changes in IRI were small: in seven (4 Ia, 3 II) afferents IRI was virtually unchanged (< 10%), in four (2 Ia, 2 II) afferents IRI increased, and in two (Ia) afferents the index decreased (Fig. 2A and B). The population average was 0.107 before the painful stimulus and 0.132 during peak pain. In population terms, there was no trend for a concomitant increase in discharge variability and increased discharge rate (Fig. 2B), which would be expected from activation of the fusimotor system. There were no obvious differences between primary and secondary endings.
Three muscle spindle afferents (2 Ia, 1 II) were recorded in subjects who did not report appreciable pain from the intramuscular injection of hypertonic saline. Data from these afferents are illustrated in Fig. 2. The measurements corresponding to the peak pain period were obtained during a 150–180 s period after injection of hypertonic saline. Neither discharge rate (< 5%) nor IRI (< 10%) were influenced in these afferents.
Effect of skin pain on muscle spindle afferents
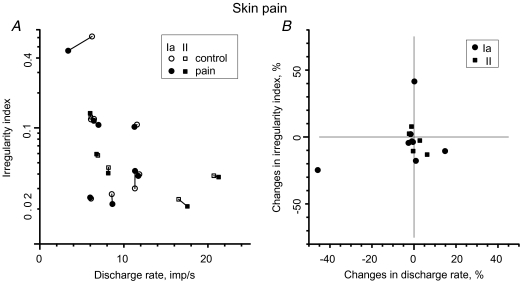
Because the quality of pain differs following subcutaneous injection of hypertonic saline we investigated whether experimentally induced skin pain might influence the discharge rate in 13 muscle spindle afferents discharging in the absence of any muscle contraction. Eight of these were classified as primary-like muscle spindle afferents and five as secondary-like. As with muscle pain, discharge activity increased (slightly) in only two afferents: one primary and one secondary. It remained virtually the same in 10 afferents (< 5% difference) and decreased in one primary afferent. On average, discharge rate showed a negligible decrease, from 9.59 to 9.52 impulses s−1 (0.7%). Discharge variability, as measured by the irregularity index, increased in only one primary afferent; it remained unchanged in seven afferents (< 10% difference) and it decreased in five (3 Ia, 2 II) afferents (Fig. 4A and B). The mean value of the IRI was 0.105 during the control period and 0.093 during peak pain. Statistically, skin pain had no systematic effect on either discharge rate or irregularity index (P > 0.05; Wilcoxon), nor was there any significant time trend corresponding to the different phases of pain (rank correlation test; rs= 0.36, P > 0.05).
Figure 4. Effect of superficial skin pain on muscle spindle afferent discharge rate and variability.
A, comparison between discharge rate and irregularity index during control period and during painful stimulation. B, changes in irregularity index as the function of changes in discharge rate. Control and pain periods indicated by open and filled symbols, respectively. Circles represent primary (Ia) muscle spindle afferents and squares represent secondary (II) muscle spindle afferents.
Discussion
The present study investigated the effects of muscle (deep) and skin (superficial) pain on the activity of muscle spindle afferents innervating relaxed leg muscles in awake human subjects. In particular, we tested the hypothesis derived from animal models that input from muscle nociceptors would activate the fusimotor system and thus increase muscle spindle discharge rate. This mechanism has until now not been proven in humans.
In contrast with animal experiments (Appelberg et al. 1983b; Thunberg et al. 2002), the present study in healthy human volunteers demonstrated neither skin nor muscle nociceptive reflex-driven increases in muscle spindle afferent discharge. In addition, we found no evidence of recruitment of silent muscle spindles – such recruitment would be expected if fusimotor drive was sufficiently high. On the contrary, in the case of muscle pain, muscle spindle discharge rate tended to slightly decelerate. In the following sections we discuss the potential effects γ-motoneurones and the sympathetic nervous system might have on muscle spindle activity.
γ-motor activity
Animal studies have demonstrated that stimulation of muscle nociceptors by intramuscular injection of hypertonic saline in muscle does increase the firing activity of primary and secondary muscle spindle afferents innervating homonymous as well as heteronymous muscles (Thunberg et al. 2002). The mechanism of this effect is attributed to nociceptive activation of group III and IV afferents, which excite γ-motoneurones and thereby increase the discharge rate in Ia and II muscle spindle sensory endings (Appelberg et al. 1983b; Wenngren et al. 1998). In the majority of muscle spindle afferents, modulation of responses has been attributed to the reflex action of static fusimotor drive and in fewer afferents to the action of mixed static and dynamic fusimotor drive (Thunberg et al. 2002). Experiments on anaesthetized cats, conducted in a comparable experimental setup to our current study, demonstrated a substantial nociceptive reflexogenic increase in mean discharge rate (by ∼80%) in afferents innervating homonymous as well as heteronymous muscles (Thunberg et al. 2002).
The current study failed to demonstrate this effect in awake human subjects: there was no net increase in discharge rate during the period of muscle pain and none of the 13 afferents recorded in our study showed an increase in discharge rate, while 7 afferents actually decelerated. In population terms, the net decrease in discharge rate at the peak of muscle and skin pain was only 6.1% and 0.7%, respectively. However, it is not clear whether this decrease was caused by nociceptive modulation of γ-drive, because (i) it is believed that there is little fusimotor activity in relaxed muscles (Gandevia et al. 1986) and (ii) there were no concomitant changes in discharge rate and interspike variability parameters. Furthermore temporal data suggest that fluctuations in discharge rate were not reflexly driven by direct nociceptive input and other factors might have contributed. In cats the effect lasted for about 2 min and the discharge rate gradually subsided to control levels over a period of 6 min. This time course in animals matched very well with the subjective pain experience in humans in our study; however, there was no correlation between the small changes in firing and pain level, nor were there any consistent trends such as returning to control levels or building up over time.
Possible effects mediated by activation of sympathetic nervous activity
The physiological responses to pain are complex, influencing multiple systems including the autonomic nervous system. From our recent study on the effects of muscle and skin pain on the sympathetic nervous system we know that deep muscle pain does cause a sustained increase in muscle sympathetic nerve activity (A. R. Burton, I Birznieks, V.G. Macefield unpublished observations). Influences of the sympathetic nervous system on muscle spindle activity are documented in several reports, but the underlying mechanisms are not well understood. Hellström et al. (2005) argue that the functional effects seen are not merely a consequence of the associated changes in haemodynamics. These authors postulated that altered levels of sympathetic activity, such as those occurring during excessive stress, would be able to interfere with proprioception and as a consequence even increase the risk of developing chronic muscle pain. First of all, there is histological evidence that muscle spindles do receive sympathetic innervation (Barker & Saito, 1981). Direct sympathetic control of the fusimotor fibres is supported by findings that both nuclear chain and bag fibres express α1a-adrenoreceptors (Bombardi et al. 2006). Experiments show that electrical stimulation of the cervical sympathetic trunk may powerfully depress the background mean discharge rate and affect the stretch sensitivity in the majority of Ia muscle spindle afferents innervating the trapezius and splenius muscles in cats. In several muscle spindles, the effect was so strong that they even fell silent (Hellström et al. 2005). However, the strength of the modulation may depend on the experimental animal used, muscle groups investigated, afferent type and stimulation frequency (cf. Roatta et al. 2002; Hellström et al. 2005).
Human research into the effects of the sympathetic nervous system have provided somewhat contradictory results to animal experiments. Recordings from single human muscle spindle afferents by Macefield et al. (2003) failed to find any detectable firing rate modulation in spontaneously active muscle spindle afferents during maximal inspiratory breath-holds, a manoeuvre that causes a powerful and sustained increase in muscle sympathetic nerve activity. Hjortskov et al. (2005) found enhancement of the stretch reflex response during periods of increased sympathetic activity in relaxed human soleus muscles and suggested that the increased stretch reflex may serve the purpose of tuning to a ‘fight-and-flight-reaction’ at the expense of fine motor control. However, there is no consensus on whether proprioceptive acuity is really impaired during states of high sympathetic activity (cf. Noteboom et al. 2001; Matre et al. 2002; Matre & Knardahl, 2003). Furthermore, it has been speculated that, in studies where γ-driven modulation of muscle spindle afferents is indicated (Ribot-Ciscar et al. 2000; Rossi-Durand 2002; Hospod et al. 2007), part of the effect might be sympathetically driven (Hjortskov et al. 2005).
A complex motor control system
Interactions between sympathetic and γ-motor driven mechanisms acting on muscle spindles up to now have not been specifically investigated. Paradoxically, there is no clear answer to whether the potential effect of sympathetic activity is excitatory or inhibitory. There is a possibility that these two systems might act antagonistically. Then, by joining two mechanisms in one model, seemingly contradictory findings might be explained by time- and task-specific changes in the balance between the two systems rather than just by the level of activation in each system separately.
It has been suggested that γ-motoneurones have individualized reflex profiles, meaning that groups of fusimotor neurones and spindle afferents may play specific roles in different motor acts and physiological states. If this is the case, simple population averages may not necessarily be the accurate predictors of reflex action changes. As a rule not all single afferents follow the same trend and opposite effects in different proportions have been observed in response to the same stimuli (see Appelberg et al. 1983b; Johansson et al. 1988b). In another series of studies, a strong nociceptive modulation of single afferents, but an equal balanced proportion of opposite effects in a population, was observed in γ-motoneurones innervating masseter muscles in the rat (Capra et al. 2007).
From a control point of view, the coexistence of two subpopulations with opposite effects is suggestive of a dynamically balanced system integrating several antagonistic inputs. Moreover, the γ-motoneurone responses to given inputs may not be hardwired, but labile, changing depending on the context and integrative state. It also means that the input–output relationship seen in one condition may even reverse if the context or state of the system is altered. Fusimotor reflex reversal has been demonstrated during walking in the cat (Murphy & Hammond, 1997) and this principle of motor control is well known (Lacquaniti et al. 1991; De Serres et al. 1995). This notion would also fit with the suggestion that muscle spindles represent the final step in an integrative network which joins information from muscle, skin and joint receptors (Johansson, 1985). According to this hypothesis, the flow of information is as follows: input from peripheral receptors as well as descending pathways is first integrated in γ-motoneurones, then it is transmitted to muscle spindle receptors where it determines how the muscle spindle should respond in the current overall context. Input from the sympathetic nervous system, reflecting the state of the body, is also integrated, so when input from muscle spindle afferents enters segmental reflex loops it is truly integrative and polymodal. An adaptive peripheral reflex control of the fusimotor system might be the underlying explanation for the wide range of response patterns that have been reported over the years. It means that the range of effects seen is not necessarily inconsistent with the various hypotheses regarding the mechanisms of fusimotor control; rather, these may reflect a spectrum of function and adaptation depending on the context (Murphy, 1999).
Methodological considerations
All of the spindles analysed in the present study were recorded in relaxed muscles, avoiding any unintentional changes in spindle firing consequent to extrafusal muscle fibre contraction – either unloading spindles or initiating concomitant fusimotor activity. The ongoing discharge of muscle spindles analysed in the current study reflected the static stretch of the relaxed receptor-bearing muscle. We did not attempt to test the dynamic sensitivity of muscle spindle afferents as it might introduce unwanted mechanical after-effects in the muscle, adding uncertainty in the case of marginally small changes. Most importantly, our focus on static stretch sensitivity was substantiated by animal studies providing the strongest support to the ‘vicious cycle’ hypothesis, in which static fusimotor drive is affected most (Thunberg et al. 2002; Masri et al. 2005; Hellström et al. 2005).
A small drift in discharge rate was observed during our experiments. Although the ankle joint angle was fixed, it is likely that some subtle unavoidable mechanical changes occurred in the muscle during the experiment.
An important aspect of our experimental protocol was that our subjects received instructions to keep the investigated muscles completely relaxed. We noticed that some subjects might tense up in the upper body during painful stimulation, but they managed to keep the leg muscles relaxed. It may be a possibility that voluntary central control might have counteracted nociceptive activation of α- and γ-motoneurones (see Ludvig et al. 2007).
Functional implications
Chronic musculoskeletal pain syndromes are a common group of, usually, work-related myalgias. According to the Johansson and Sojka hypothesis (Johansson & Sojka, 1991), it has been suggested that chronic muscle pain may develop as a result of a ‘vicious cycle’ reflex activation leading to an increase in muscle tone and exacerbation of pain. To support this hypothesis, first of all, the nociceptive excitation of fusimotor drive must be substantial. While under certain experimental conditions this could be demonstrated in anaesthetized animals, experimental evidence in humans has been lacking (for review see Knutson, 2000). Our current finding firmly contradicts the proposed ‘vicious cycle’ hypothesis. There is a possibility, though, that recruitment of γ-motoneurons might require plastic changes in nociceptive circuits and thus the process might require a much longer time to develop, as indicated by the second phase of EMG increase demonstrated in jaw muscles (Yu et al. 1995). Nevertheless, prolonged experimental pain in animals, induced by the inflammatory agent carrageenan, evoked inhibitory effects (Mense & Skeppar, 1991). Inflammatory mediators, which are readily produced during ischaemic contraction, have been shown to inhibit γ-motoneurons, thus providing an explanation for the weakness and even the atrophy clinically observed in severe chronic cases of muscle damage.
There is serious opposition to the ‘vicious cycle’ mechanism from a clinical standpoint. Simons & Mense (1998) indicated that commonly the painful muscle shows no EMG activity and, if there is, it does not correlate with pain either in the time or intensity domain (see also Ro et al. 2002). Observations using surface EMG electrodes have received some criticism, though; fatigue resistant muscle fibres, which are most likely to be activated by static γ-motoneurons, are preferentially located deeper in a muscle close to the bone. Svensson et al. (1998) did find a slight increase in the intramuscular EMG activity after injection of hypertonic saline but it was not correlated to the intensity or duration of pain. They also concluded that human experimental muscle pain is unable to maintain long-lasting muscle hyperactivity. To have any influence on the blood flow, muscle activity should reach about 30% of maximal voluntary contraction (Simons & Mense, 1998).
Furthermore, Lund et al. (1991) reviewed a wide range of clinical literature and experimental studies and came to the conclusion that chronic pain tends to inhibit, not facilitate, voluntary and reflex contractile activity of a painful muscle or its agonists. They suggest that those effects are beneficial and provide protective adaptation and are definitely not the cause of pain. Our current data add support to this hypothesis.
Conclusions
We conclude that activation of muscle nociceptors does not cause a reflex increase in fusimotor drive (and hence increase in static stretch sensitivity) and urge caution in extending animal data to the clinical setting.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia (grant 350889). I. Birznieks was also supported by the Swedish Medical Research Council.
References
- Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on γ-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. J Physiol. 1983a;335:255–273. doi: 10.1113/jphysiol.1983.sp014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B, Hulliger M, Johansson H, Sojka P. Actions on γ-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. J Physiol. 1983b;335:275–292. doi: 10.1113/jphysiol.1983.sp014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain. 1996;64:231–240. doi: 10.1016/0304-3959(95)00115-8. [DOI] [PubMed] [Google Scholar]
- Barker D, Saito M. Autonomic innervation of receptors and muscle fibres in cat skeletal muscle. Proc R Soc Lond B Biol Sci. 1981;212:317–332. doi: 10.1098/rspb.1981.0042. [DOI] [PubMed] [Google Scholar]
- Bombardi C, Grandis A, Chiocchetti R, Bortolami R, Johansson H, Lucchi ML. Immunohistochemical localization of α1a-adrenoreceptors in muscle spindles of rabbit masseter muscle. Tissue Cell. 2006;38:121–125. doi: 10.1016/j.tice.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Burke D, Skuse NF, Stuart DG. The regularity of muscle spindle discharge in man. J Physiol. 1979;291:277–290. doi: 10.1113/jphysiol.1979.sp012812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra NF, Hisley CK, Masri RM. The influence of pain on masseter spindle afferent discharge. Arch Oral Biol. 2007;52:387–390. doi: 10.1016/j.archoralbio.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra NF, Ro JY. Human and animal experimental models of acute and chronic muscle pain: intramuscular algesic injection. Pain. 2004;110:3–7. doi: 10.1016/j.pain.2004.04.033. [DOI] [PubMed] [Google Scholar]
- De Serres SJ, Yang JF, Patrick SK. Mechanism for reflex reversal during walking in human tibialis anterior muscle revealed by single motor unit recording. J Physiol. 1995;488:249–258. doi: 10.1113/jphysiol.1995.sp020963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin BB, Vallbo AB. Classification of human muscle stretch receptor afferents: a Bayesian approach. J Neurophysiol. 1990;63:1314–1322. doi: 10.1152/jn.1990.63.6.1314. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Miller S, Aniss AM, Burke D. Reflex influences on muscle spindle activity in relaxed human leg muscles. J Neurophysiol. 1986;56:159–170. doi: 10.1152/jn.1986.56.1.159. [DOI] [PubMed] [Google Scholar]
- Hellström F, Roatta S, Thunberg J, Passatore M, Djupsjöbacka M. Responses of muscle spindles in feline dorsal neck muscles to electrical stimulation of the cervical sympathetic nerve. Exp Brain Res. 2005;165:328–342. doi: 10.1007/s00221-005-2309-7. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Bandler R, Gandevia SC, Macefield VG. Distinct forebrain activity patterns during deep versus superficial pain. Pain. 2007;2006:286–296. doi: 10.1016/j.pain.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Hjortskov N, Skotte J, Hye-Knudsen C, Fallentin N. Sympathetic outflow enhances the stretch reflex response in the relaxed soleus muscle in humans. J Appl Physiol. 2005;98:1366–1370. doi: 10.1152/japplphysiol.00955.2004. [DOI] [PubMed] [Google Scholar]
- Hospod V, Aimonetti JM, Roll JP, Ribot-Ciscar E. Changes in human muscle spindle sensitivity during a proprioceptive attention task. J Neurosci. 2007;27:5172–5178. doi: 10.1523/JNEUROSCI.0572-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H. Reflex integration in the γ-motor system. In: Boyd IA, Gladden MH, editors. The Muscle Spindle. New York: Stockton Press; 1985. pp. 297–301. [Google Scholar]
- Johansson H, Lorentzon R, Sjölander P, Sojka P. The anterior cruciate ligament. A sensor acting on the gamma muscle spindle systems of muscles around the knee joint. Neuro-Orthopedics. 1990;9:1–23. [Google Scholar]
- Johansson H, Sjölander P, Sojka P. Actions on γ-motoneurones elicited by electrical stimulation of joint afferent fibres in the hind limb of the cat. J Physiol. 1986;375:137–152. doi: 10.1113/jphysiol.1986.sp016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson H, Sjölander P, Sojka P. Fusimotor effects in triceps surae muscle elicited by natural and electrical stimulation of joint afferents. Neuro-Orthopedics. 1988a;6:67–80. [Google Scholar]
- Johansson H, Sjölander P, Sojka P, Wadell I. Different fusimotor reflexes from the ipsi- and contralateral hind limbs of the cat assessed in the same primary muscle spindle afferents. J Physiol Paris. 1988b;83:281–292. [PubMed] [Google Scholar]
- Johansson H, Sjölander P, Sojka P, Wadell I. Reflex actions on the gamma-muscle spindle systems of muscles acting at the knee joint elicited by stretch of the posterior cruciate ligament. Neuro-Orthopedics. 1989a;8:9–21. [Google Scholar]
- Johansson H, Sjölander P, Sojka P, Wadell I. Effects of electrical and natural stimulation of skin afferents on the gamma-spindle system of the triceps surae muscle. Neurosci Res. 1989b;6:537–555. doi: 10.1016/0168-0102(89)90043-6. [DOI] [PubMed] [Google Scholar]
- Johansson H, Sojka P. Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Med Hypotheses. 1991;35:196–203. doi: 10.1016/0306-9877(91)90233-o. [DOI] [PubMed] [Google Scholar]
- Knutson GA. The role of the γ-motor system in increasing muscle tone and muscle pain syndromes: a review of the Johansson/Sojka hypothesis. J Manipulative Physiol Ther. 2000;23:564–572. doi: 10.1067/mmt.2000.109674. [DOI] [PubMed] [Google Scholar]
- Lacquaniti F, Borghese NA, Carrozzo M. Transient reversal of the stretch reflex in human arm muscles. J Neurophysiol. 1991;66:939–954. doi: 10.1152/jn.1991.66.3.939. [DOI] [PubMed] [Google Scholar]
- Ludvig D, Cathers I, Kearney RE. Voluntary modulation of human stretch reflexes. Exp Brain Res. 2007;183:201–213. doi: 10.1007/s00221-007-1030-0. [DOI] [PubMed] [Google Scholar]
- Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–694. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Sverrisdottir YB, Wallin BG. Resting discharge of human muscle spindles is not modulated by increases in sympathetic drive. J Physiol. 2003;551:1005–1011. doi: 10.1113/jphysiol.2003.040196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri R, Ro JY, Capra N. The effect of experimental muscle pain on the amplitude and velocity sensitivity of jaw closing muscle spindle afferents. Brain Res. 2005;1050:138–147. doi: 10.1016/j.brainres.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Matre D, Arendt-Neilsen L, Knardahl S. Effects of localization and intensity of experimental muscle pain on ankle joint proprioception. Eur J Pain. 2002;6:245–260. doi: 10.1053/eujp.2002.0332. [DOI] [PubMed] [Google Scholar]
- Matre D, Knardahl S. Sympathetic nerve activity does not reduce proprioceptive acuity in humans. Acta Physiol Scand. 2003;178:261–268. doi: 10.1046/j.1365-201X.2003.01122.x. [DOI] [PubMed] [Google Scholar]
- Matre DA, Sinkjaer T, Svensson P, Arendt-Nielsen L. Experimental muscle pain increases the human stretch reflex. Pain. 1998;75:331–339. doi: 10.1016/s0304-3959(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Matthews PB, Stein RB. The regularity of primary and secondary muscle spindle afferent discharges. J Physiol. 1969;202:59–82. doi: 10.1113/jphysiol.1969.sp008795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S, Skeppar P. Discharge behaviour of feline gamma-motoneurones following induction of an artificial myositis. Pain. 1991;46:201–210. doi: 10.1016/0304-3959(91)90077-B. [DOI] [PubMed] [Google Scholar]
- Murphy PR. Adaptive fusimotor reflex control in the decerebrate cat. Brain Res. 1999;821:38–49. doi: 10.1016/s0006-8993(99)01055-0. [DOI] [PubMed] [Google Scholar]
- Murphy PR, Hammond GR. Reversal of fusimotor reflex responses during locomotion in the decerebrate cat. Exp Physiol. 1997;82:837–858. doi: 10.1113/expphysiol.1997.sp004068. [DOI] [PubMed] [Google Scholar]
- Nordh E, Hulliger M, Vallbo AB. The variability of inter-spike intervals of human spindle afferents in relaxed muscles. Brain Res. 1983;271:89–99. doi: 10.1016/0006-8993(83)91367-7. [DOI] [PubMed] [Google Scholar]
- Noteboom JT, Barnholt KR, Enoka RM. Activation of the arousal response and impairment of performance increase with anxiety and stressor intensity. J Appl Physiol. 2001;91:2093–2101. doi: 10.1152/jappl.2001.91.5.2093. [DOI] [PubMed] [Google Scholar]
- Passatore M, Grassi C, Filippi GM. Sympathetically-induced development of tension in jaw muscles: the possible contraction of intrafusal muscle fibres. Pflugers Arch. 1985;405:297–304. doi: 10.1007/BF00595681. [DOI] [PubMed] [Google Scholar]
- Ribot-Ciscar E, Rossi-Durand C, Roll JP. Increased muscle spindle sensitivity to movement during reinforcement manoeuvres in relaxed human subjects. J Physiol. 2000;523:271–282. doi: 10.1111/j.1469-7793.2000.t01-1-00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro JY, Svensson P, Capra N. Effects of experimental muscle pain on electromyographic activity of masticatory muscles in the rat. Muscle Nerve. 2002;25:576–584. doi: 10.1002/mus.10072. [DOI] [PubMed] [Google Scholar]
- Roatta S, Windhorst U, Ljubisavljevic M, Johansson H, Passatore M. Sympathetic modulation of muscle spindle afferent sensitivity to stretch in rabbit jaw closing muscles. J Physiol. 2002;540:237–248. doi: 10.1113/jphysiol.2001.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Decchi B. Changes in Ib heteronymous inhibition to soleus motoneurones during cutaneous and muscle nociceptive stimulation in humans. Brain Res. 1997;774:55–61. doi: 10.1016/s0006-8993(97)81687-3. [DOI] [PubMed] [Google Scholar]
- Rossi-Durand C. The influence of increased muscle spindle sensitivity on Achilles tendon jerk and H-reflex in relaxed human subjects. Somatosens Mot Res. 2002;19:286–295. doi: 10.1080/0899022021000037755. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. 2. New York: McGraw-Hill; 1988. [Google Scholar]
- Simons DG, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998;75:1–17. doi: 10.1016/S0304-3959(97)00102-4. [DOI] [PubMed] [Google Scholar]
- Stohler CS, Zhang X, Lund JP. The effect of experimental jaw muscle pain on postural muscle activity. Pain. 1996;66:215–221. doi: 10.1016/0304-3959(96)03026-6. [DOI] [PubMed] [Google Scholar]
- Svensson P, Beydoun A, Morrow TJ, Casey KL. Human intramuscular and cutaneous pain: psychophysical comparisons. Exp Brain Res. 1997;114:390–392. doi: 10.1007/pl00005648. [DOI] [PubMed] [Google Scholar]
- Svensson P, Graven-Nielsen T, Matre D, Arendt-Nielsen L. Experimental muscle pain does not cause long-lasting increases in resting electromyographic activity. Muscle Nerve. 1998;21:1382–1389. doi: 10.1002/(sici)1097-4598(199811)21:11<1382::aid-mus4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Thunberg J, Ljubisavljevic M, Djupsjöbacka M, Johansson H. Effects on the fusimotor-muscle spindle system induced by intramuscular injections of hypertonic saline. Exp Brain Res. 2002;142:319–326. doi: 10.1007/s00221-001-0941-4. [DOI] [PubMed] [Google Scholar]
- Wadell I, Johansson H, Sjölander P, Sojka P, Djupsjöbacka M, Niechaj A. Fusimotor reflexes influencing secondary muscle spindle afferents from flexor and extensor muscles in the hind limb of the cat. J Physiol Paris. 1991;85:223–234. [PubMed] [Google Scholar]
- Wenngren BI, Pedersen J, Sjölander P, Bergenheim M, Johansson H. Bradykinin and muscle stretch alter contralateral cat neck muscle spindle output. Neurosci Res. 1998;32:119–129. doi: 10.1016/s0168-0102(98)00074-1. [DOI] [PubMed] [Google Scholar]
- Yu XM, Sessle BJ, Vernon H, Hu JW. Effects of inflammatory irritant application to the rat temporomandibular joint on jaw and neck muscle activity. Pain. 1995;60:143–149. doi: 10.1016/0304-3959(94)00104-M. [DOI] [PubMed] [Google Scholar]