Although lactate has been known for almost 80 years to serve as an oxidative energy source in the brain (Ashford & Holmes, 1931), the work of Hill et al. (1924) on skeletal muscle, characterizing the monocarboxylate as a useless by-product of anaerobic exercise, has been, in large part, responsible for lactate's bad reputation both in health and disease. It would not be too far-fetched to presume that the formulators of the glycolytic pathway seven decades ago took into account lactate's reputation in deciding that pyruvate is the end-product of aerobic glycolysis (Schurr, 2006). The pioneering work of Brooks and colleagues, from the mid 1980s on, and the coining of terms such as ‘lactate shuttle’ and ‘muscle lactate extraction and utilization’, have dramatically changed our notions on the roles of lactate in muscular work (Hashimoto & Brooks, 2008). The research of Schurr et al. (1988), Fox et al. (1988) and Pellerin & Magistretti (1994) has ushered in similar conceptual changes on the role of lactate in brain energy metabolism. Consequently, in the brain (and most likely in slow-twitch muscle fibres), lactate should be portrayed (Fig. 1) as the end-product of aerobic glycolysis and the main mitochondrial energy substrate (Schurr & Payne, 2007; Atlante et al. 2007; Hashimoto & Brooks, 2008). In 2004, Pedersen et al. (2004) demonstrated that lactic acid is not responsible for muscle fatigue, destroying a long-standing myth. In this issue of The Journal of Physiology,Larsen et al. (2008) add to the growing number of papers dealing with the brain's eventual utilization of skeletal muscle-produced lactate. The authors describe their attempts to elucidate the mechanism behind the observed exercise-induced reduction in cerebral metabolic ratio (CMR) as measured in healthy human subjects. They no longer use the accepted definition of CMR as O2/glucose, but rather focus instead on the more accurate definition of CMR i.e. O2/(glucose + ½lactate), to make their estimates. Larsen et al. demonstrate the ability of the β2 adrenergic receptor blocker, propranolol, to prevent the exercise-induced reduction in CMR. This is in contrast to the inability of β1 blockade to produce the same effect. Thus, the authors conclude that the β adrenergic system is involved somehow in cerebral energy metabolism, although they are not able yet to explain the mechanism behind this involvement. Nonetheless, the measurements of Larsen et al. clearly show that, as their subjects approached the maximal work load and exhaustion under control conditions, the CMR [O2/(glucose + ½ lactate)] fell from the expected value of approximately 6 to about 3. When the same measurement was done for CMR (O2/glucose), its value did not change significantly throughout the experimental period. Concomitantly, the a–v differences for glucose and oxygen, even at the maximum work load, increased only slightly in comparison to the values at rest. In contrast, the a–v differences for total carbohydrates (CHO) at maximal work load were significantly higher than the values at rest, an increase that could be attributed almost entirely to the significantly higher a–v difference for lactate. One could thus surmise that, at maximal work load, lactate becomes a major energy substrate in the brain as CMR approaches 3, the expected value for the ratio O2/lactate. This ratio is also maintained in the presence of β blockade, signalling that although the subjects reached exhaustion earlier and at a lower work load than during control conditions, lactate was still the main cerebral energy substrate. Thus, just as is the case for muscle fatigue (Pedersen et al. 2004), the perception of exhaustion (‘brain fatigue’) is not due to lactate accumulation.
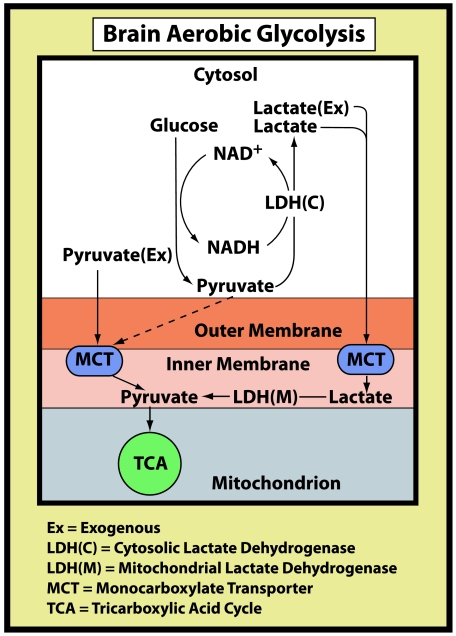
Figure 1. A schematic illustration of the brain glycolytic pathway under aerobic conditions.
A schematic illustration of the brain glycolytic pathway under aerobic conditions in which glucose is broken down to the glycolytic end-product lactate. Lactate, in turn, becomes the mitochondrial substrate, via its conversion to pyruvate, for the tricarboxylic acid cycle. At maximal work load, as measured in the study of Larsen et al. (2008) most of the lactate that is utilized by brain mitochondria is muscular in origin and thus exogenous. Under resting conditions, most of the lactate used by brain mitochondria originates from glucose that is metabolized glycolytically in neurons and glia.
References
- Ashford CA, Holmes EG. Biochem J. 1931;25:2028–2049. doi: 10.1042/bj0252028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlante A, et al. Biochim Biophys Acta. 2007;1767:1285–1299. doi: 10.1016/j.bbabio.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Fox PT, et al. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Brooks GA. Med Sci Sports Exerc. 2008;40:486–494. doi: 10.1249/MSS.0b013e31815fcb04. [DOI] [PubMed] [Google Scholar]
- Hill AV, et al. Proc R Soc Lond B. 1924;97:84–139. [Google Scholar]
- Larsen TS, et al. J Physiol. 2008;586:2807–2815. doi: 10.1113/jphysiol.2008.151449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen TH, et al. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Proc Natl Acad Sci U S A. 1994;91:10624–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A. J Cereb Blood Flow Metab. 2006;26:142–152. doi: 10.1038/sj.jcbfm.9600174. [DOI] [PubMed] [Google Scholar]
- Schurr A, et al. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- Schurr A, Payne RS. Neuroscience. 2007;147:613–619. doi: 10.1016/j.neuroscience.2007.05.002. [DOI] [PubMed] [Google Scholar]