Abstract
The role of cardiac sarcolemmal ATP-sensitive K+ (KATP) channels in the regulation of sinoatrial node (SAN) automaticity is not well defined. Using mice with homozygous knockout (KO) of the Kir6.2 (a pore-forming subunit of cardiac KATP channel) gene, we investigated the pathophysiological role of KATP channels in SAN cells during hypoxia. Langendorff-perfused mouse hearts were exposed to hypoxic and glucose-free conditions (hypoxia). After 5 min of hypoxia, sinus cycle length (CL) was prolonged from 207 ± 10 to 613 ± 84 ms (P < 0.001) in wild-type (WT) hearts. In Kir6.2 KO hearts, CL was slightly prolonged from 198 ± 17 to 265 ± 32 ms. The CL of spontaneous action potentials of WT SAN cells, recorded in the current-clamp mode, was markedly prolonged from 410 ± 56 to 605 ± 108 ms (n = 6, P < 0.05) with a decrease of the slope of the diastolic depolarization (SDD) after the application of the K+ channel opener pinacidil (100 μm). Pinacidil induced a glibenclamide (1 μm)-sensitive outward current, which was recorded in the voltage-clamp mode, only in WT SAN cells. During metabolic inhibition by 2,4-dinitrophenol, CL was prolonged from 292 ± 38 to 585 ± 91 ms (P < 0.05) with a decrease of SDD in WT SAN cells but not in Kir6.2 KO SAN cells. Diastolic Ca2+ concentration, measured by fluo-3 fluorescence, was decreased in WT SAN cells but increased in Kir6.2 KO SAN cells after short-term metabolic inhibition. In conclusion, the present study using Kir6.2 KO mice indicates that, during hypoxia, activation of sarcolemmal KATP channels in SAN cells inhibits SAN automaticity, which is important for the protection of SAN cells.
ATP-sensitive K+ (KATP) channels, originally discovered in cardiomyocytes (Noma, 1983), are present in many tissues and play an important role in various cellular functions (Seino & Miki, 2003). Recent studies have indicated that the cardiac KATP channel is a hetero-octametric complex of four pore-forming subunits (Kir6.2) and four regulatory sulphonylurea receptor (SUR2A) subunits (Seino, 1999). Several studies from our laboratory, using mice with homozygous knockout of the Kir6.2 gene (Kir6.2 KO), have demonstrated that Kir6.2 is essential for the function of KATP channels in atrial and ventricular cells (Suzuki et al. 2001; Saegusa et al. 2005). The activation of KATP channels plays an important role in the action potential shortening but not in the extracellular K+ accumulation during myocardial ischaemia (Suzuki et al. 2002; Saito et al. 2005).
The sinoatrial node (SAN), the location of which was discovered in the mammalian heart a century ago (Keith & Flack, 1907), has spontaneous automaticity generated by the diastolic depolarization. Electrophysiological, pharmacological and genetic investigations have demonstrated that different types of membrane currents are involved in the generation of the action potential and the pacemaker activity (Irisawa et al. 1993; Kodama et al. 1997; Marionneau et al. 2005; Mangoni et al. 2006). A previous electrophysiological study showed that KATP channels exist in the rabbit SAN cells (Han et al. 1996). In addition, a recent study using the RT-PCR technique has indicated that Kir6.2 and SUR2 genes are expressed in mouse SAN tissue (Marionneau et al. 2005). However, functional roles of KATP channels are not fully understood although SAN automaticity is known to be susceptible and vulnerable to metabolic inhibition such as hypoxia (Nishi et al. 1980). The present study was conducted to examine whether the activation of KATP channels regulates the SAN automaticity during hypoxia or metabolic inhibition using isolated hearts and SAN cells of Kir6.2 KO mice. The use of Kir6.2-deficient mice would have an advantage because KATP channel blockers such as glibenclamide have non-specific blocking actions on the other ion channels and transporters (Rosati et al. 1998; Hernandez-Benito et al. 2001; Lee & Lee, 2005).
Methods
Kir6.2-deficient mice
A mouse line deficient in the KATP channels was generated by targeted disruption of the gene coding for Kir6.2, as previously described (Miki et al. 1998). C57BL/6 mice were used as controls because KO animals had been back-crossed to a C57BL/6 strain for five generations. C57BL/6 wild-type (WT) and Kir6.2-deficient (Kir6.2 KO) mice at the age of 8–14 weeks were used in this study. All procedures complied with the Guide for the Care and Use of Laboratory Animals (NIH Pub. no. 85-23, 1996 version) and were approved by the Institutional Animal Care and Use Committee of Chiba University.
Langendorff-perfused hearts
Mice were anaesthetized with urethane (1.5 g kg−1i.p.) and anticoagulated with heparin (100 U kg−1i.v.). Hearts were quickly excised and connected to the Langendorff apparatus by inserting a perfusion cannula into the aorta, as previously described (Suzuki et al. 2001). Retrograde perfusion was maintained at a constant pressure of 80 cmH2O with a modified Tyrode solution containing (mm): 125 NaCl, 4 KCl, 1.8 NaH2PO4, 0.5 MgCl2, 1.8 CaCl2, 5.5 glucose and 25 NaHCO3. The perfusate was equilibrated with 95% O2–5% CO2. The partial pressure of oxygen (PO2) of the oxygenated Tyrode solution, measured by a blood gas analyser (ABL 5500, Radiometer Copenhagen, Denmark), was 862 ± 9 mmHg. After a stabilization period of 15–30 min, hearts were exposed to a substrate-free hypoxic Tyrode solution (hypoxia). It had the same composition as given above, except that it contained no glucose and was gassed with 95% N2–5% CO2, resulting in a PO2 of 36 ± 1 mmHg. The temperature of these solutions was maintained at 36–37°C. After 10 min of hypoxia, the hearts were reoxygenated by changing the perfusate to the normal oxygenated Tyrode solution for 10 min. Right atrial (RA) and right ventricular (RV) electrograms were continuously recorded with monopolar electrodes attached to the walls of RA and RV. A reference electrode was attached to the aorta of the isolated heart. Electrograms were amplified by a bioelectric amplifier (AVB-10, Nihon Kohden, Tokyo, Japan), digitalized with an analog-to-digital converter and stored on a disk of a personal computer with a data acquisition system (PowerLab 4SP, ADInstruments, Castle Hill, Australia). The experimental data were analysed using Chart software (version 5; ADInstruments).
Cell isolation
Single SAN cells were isolated according to the method previously described (Verheijck et al. 1998; Lei et al. 2004). Briefly, isolated hearts were perfused with an oxygenated Hepes-buffered Tyrode solution at 36°C for 5–10 min to wash out the blood and stabilize the sinus rate. After stabilization, each heart was perfused with low enzyme-containing (1–2 U ml−1 Type II collagenase, Worthington Biochemical Corporation, New Jersey, USA) normal Tyrode solution for 5–10 min. The right atrium wall was cut off from the heart, and the SAN region, limited by the crista terminalis, the atrial septum and the orifices of the vena cavae, was anatomically isolated. The excised SAN tissue strip containing the primary pacemaking region was incubated for 5 min in the low-Ca2+ (5 μm) Tyrode solution at 36°C, treated with collagenase (120–160 U ml−1)-containing low-Ca2+ Tyrode solution for 45–60 min, and finally washed with and stored in Kraft-Brühe (KB) medium solution for 1–2 h at 4°C. Single SAN cells were gently isolated from the strip by pipetting KB solution just before experiments. Then the cells were transferred to the recording bath and perfused with the normal Tyrode solution at 36°C.
Composition of solutions
The composition of the Hepes-Tyrode solution was (mm): 143 NaCl, 5.4 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.33 NaH2PO4, 5.5 glucose and 10 Hepes-NaOH buffer (pH 7.4). KB solution contained (mm): 70 KOH, 50 l-glutamic acid, 40 KCl, 20 taurine, 20 KH2PO4, 3 MgCl2, 10 glucose, 1 EGTA and 10 Hepes-KOH buffer (pH 7.4 adjusted with KOH). The pipette solution for ruptured patch experiments contained (mm): 110 KOH, 110 l-aspartic acid, 20 KCl, 1 MgCl2, 1.41 CaCl2, 1 K2-ATP, 1 potassium phosphocreatine, 10 EGTA and 5 Hepes-KOH buffer (pH 7.4 adjusted with KOH), and that for nystatin-perforated patch experiments contained (mm): 110 KOH, 110 l-aspartic acid, 20 KCl, 1 MgCl2, 1 CaCl2, 0.1 EGTA and 5 Hepes-KOH buffer (pH 7.4 adjusted with KOH). Nystatin (Wako Pure Chemicals, Osaka, Japan) was first dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich Japan, Tokyo, Japan) and then added to the pipette solution at a concentration of 300–500 μg ml−1 just prior to use; the final concentration of DMSO was less than 0.1%.
Electrophysiology
Single SAN cells were identified by their characteristic morphology, a long and slender shape with faint sarcomere striations, and spontaneous activity, as previously described (Verheijck et al. 1998; Boyett et al. 2000; Cho et al. 2003). The whole-cell patch-clamp technique was used for the electrical recordings from single SAN cells. A patch-clamp amplifier (CEZ-2400, Nihon Kohden, Tokyo, Japan) was used for current- and voltage-clamp experiments. Pipette electrodes (resistance 2–6 MΩ) were made by using a Narishige pipette puller (PB-7, Narishige Scientific Instruments Laboratory, Tokyo, Japan). After the gigaohm seal between tip and cell membrane was formed, we ruptured the patch membrane to form the whole-cell patch configuration. In the current-clamp mode, spontaneous action potentials were constantly recorded from pacemaker cells. In the voltage-clamp mode, a ramp-pulse protocol was used to record the quasi-steady-state membrane current at the interval of 15 s. The membrane potential was held at −40 mV and depolarized to +50 mV in 1.0 s. It was then repolarized or hyperpolarized to −100 mV in 2.5 s, during which time the change in the membrane current was automatically plotted against the membrane potential. Spontaneous action potentials and whole-cell membrane currents were also recorded by nystatin-perforated patch configuration, as previously described (Sakamoto et al. 1998). After the gigaohm seal, negative pressure was released to await gradual opening of nystatin-induced pores. The experiments were performed at 36°C. The current signals were filtered at 10 kHz and digitalized at 1 or 2 kHz by using an A/D converter (Digidata 1322A, Axon Instruments Inc., Union City, CA, USA). All data were stored on a computer for later analysis with pCLAMP9 software (Axon Instruments).
Cytosolic Ca2+ concentration measurement
The cytosolic Ca2+ concentration ([Ca2+]i) was measured as previously described (Yao et al. 1998; Matsuura et al. 2004). Single SA node cells were incubated in Hepes-Tyrode (loading) solution containing 1 μm fluo-3 acetoxymethyl ester (fluo-3 AM; Molecular Probes, OR, USA) at 36°C in the dark for 30 min. The loading solution was prepared by diluting a 10 μm stock solution, which contained 90% FBS, 10% DMSO and 0.45% Pluronic F127 (Molecular Probes). Fluo-3-loaded SAN cells were perfused with Hepes-buffered Tyrode solution at 36°C. Fluo-3 was excited at 480 nm, and emitted fluorescence was recorded at 520 nm by a photomultiplier tube (AIM-10, InterMedical Co, Aichi, Japan). Although 2,4-dinitrophenol can reportedly cause an increase in flavoprotein autofluorescence excited at 480 nm (Sato et al. 1998), it was confirmed that the autofluorescence did not produce any appreciable effects on the fluo-3 fluorescence because of the very low intensity of the endogenous fluorescence.
Chemicals
Glibenclamide (Sigma-Aldrich Japan) was dissolved in DMSO as a stock solution of 10 mm, and the final concentration of DMSO in the perfusate was less than 0.1%. Pinacidil (Sigma-Aldrich Japan) was dissolved in 0.1 n HCl as a stock solution of 20 mm. 2,4-Dinitrophenol (Wako Pure Chemicals) was directly dissolved in Tyrode solution.
Statistics
All data are presented as mean ± s.e.m. The number of experiments is shown as n. Statistical significance was evaluated by Student's t test for paired or unpaired observations or analysis of variance (ANOVA), where appropriate. Differences with values of P < 0.05 were considered to be statistically significant.
Results
SAN function during hypoxia in isolated hearts
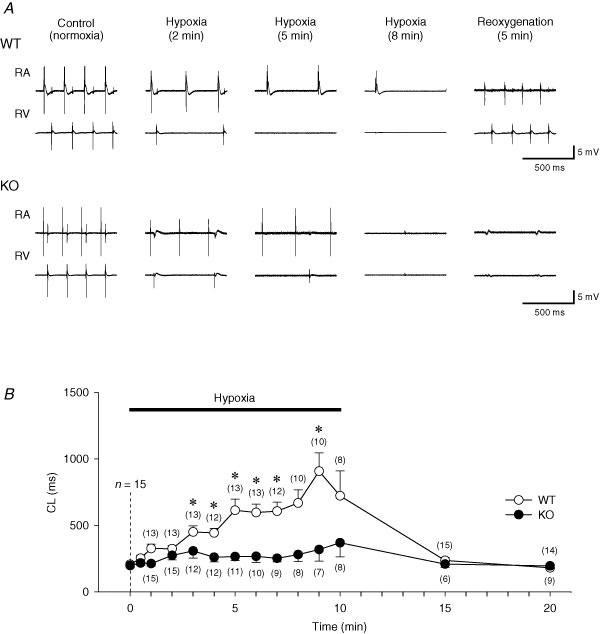
Representative electrograms recorded from RA and RV under control conditions (oxygenated solution), hypoxia (hypoxic and glucose-free solution) and after reoxygenation (reintroduction of control oxygenated solution) are shown in Fig. 1A. In WT hearts, the spontaneous cycle length (CL) of RA electrograms was gradually prolonged during hypoxia and recovered to the control levels after 5 min of reoxygenation. Concomitantly, atrioventricular (AV) block developed during hypoxia and disappeared after reoxygenation in WT hearts. However, in Kir6.2 KO hearts, the prolongation of CL observed during hypoxia was not so marked but atrial electrograms disappeared abruptly. AV block also developed in Kir6.2 KO hearts during hypoxia. RA electrograms did not recover after reoxygenation in most of the Kir6.2 KO hearts.
Figure 1. Effects of hypoxia on sinus cycle length (CL) of wild-type (WT) and Kir6.2-deficient (KO) mouse hearts.
A, representative traces of the electrograms recorded from right atrium (RA) and right ventricle (RV) during normoxia (control), hypoxia (2 and 5 min) and after 5 min of reoxygenation. B, time course changes of the CL during hypoxia and after reoxygenation in WT (n = 15, ○) and Kir6.2 KO (n = 15, •) hearts. The number of hearts from which RA electrograms could be recorded and the CL measured are shown in parentheses. Values are expressed as means ± s.e.m.*P < 0.001 versus 0 min.
Figure 1B summarizes the time course of changes in CL of RA electrograms during hypoxia and reoxygenation in WT and Kir6.2 KO hearts. There was no significant difference in the basal CL between WT (206.5 ± 10.1 ms; n = 15) and Kir6.2 KO hearts (197.5 ± 16.5 ms; n = 15). During hypoxia, CL of RA electrograms in WT hearts was gradually and significantly prolonged to 324.2 ± 23.4 ms at 2 min (n = 13, P < 0.05 versus 0 min) and to 613.2 ± 84.3 ms at 5 min (n = 13, P < 0.001 versus 0 min) of hypoxia although RA electrograms were invisible at these time points in 2 of 15 WT hearts. In Kir6.2 KO hearts, CL was slightly but significantly prolonged to 274.1 ± 34.5 ms (n = 15, P < 0.05 versus 0 min) at 2 min hypoxia. At 5 min of hypoxia RA electrograms were invisible in 4 Kir6.2 KO hearts and CL of RA electrograms in other 11 Kir6.2 KO hearts was insignificantly prolonged to 265.3 ± 31.5 ms (P is not significant (NS) versus 0 min). After 5 min of reoxygenation, CL was almost completely recovered in all WT hearts (236.2 ± 21.9 ms; n = 15, P < 0.05 versus 10 min of hypoxia, NS versus 0 min). However, in only 6 of 15 Kir6.2 KO hearts RA electrograms could be recorded after 5 min of reoxygenation and the CL was 208.5 ± 25.5 ms (n = 6, P = NS versus 10 min of hypoxia).
Effect of KATP channel opener on action potentials of SAN cells
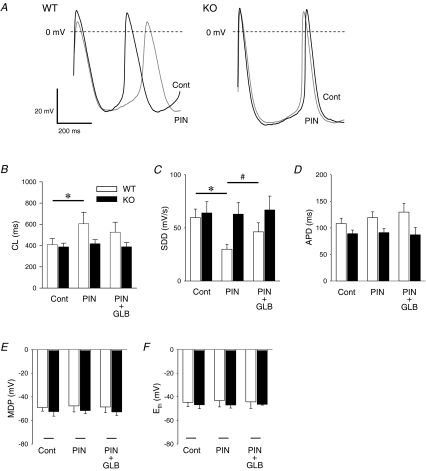
We next examined the effect of pinacidil, a K+ channel opener, on spontaneous pacemaker activity of enzymatically isolated SAN cells in the current-clamp mode of the ruptured patch clamp techniques. Representative recordings of spontaneous action potentials of WT SAN cells before (Cont) and after pinacidil (100 μm)-treatment (PIN) are shown in Fig. 2A. Pinacidil significantly prolonged CL of spontaneous action potentials from 409.7 ± 56.1 to 605.4 ± 108.4 ms (n = 6, P < 0.05) in WT SAN cells, as shown in Fig. 2B. The increasing effect of pinacidil on CL was partially reversed by the addition of glibenclamide (1 μm, PIN + GLB). In contrast, CL of SAN cells of Kir6.2 KO mice was affected by neither pinacidil alone nor co-administration of pinacidil and glibenclamide. Figure 2C summarizes the changes of the slope of the diastolic depolarization (SDD) after administration of these drugs in WT and Kir6.2 KO SAN cells. The decreased spontaneous pacemaker activity in WT SAN cells after pinacidil was accompanied by the decrease of SDD from 59.6 ± 8.1 to 29.9 ± 4.5 mV s−1 (n = 6, P < 0.01). The pinacidil-induced decrease of SDD was antagonized by addition of glibenclamide (46.2 ± 8.5 mV s−1; n = 4, P < 0.05 versus pinacidil alone). However, the change of SDD after pinacidil was not observed in Kir6.2 KO SAN cells.
Figure 2. Effects of pinacidil on the action potential recorded from SAN cells of wild-type (WT) and Kir6.2-deficient (KO) mice.
A, representative traces of action potentials recorded from WT and Kir6.2 KO SAN cells in control conditions (black lines, Cont) and after 100 μm pinacidil (grey lines, PIN). Action potential parameters of WT (open columns) and Kir6.2 KO (filled columns) SAN cells in control conditions, after 100 μm pinacidil (PIN) and after addition of 1 μm glibenclamide (PIN + GLB) are summarized. The cycle length of spontaneous action potentials (CL, B), the slope of the diastolic depolarization (SDD, C), the action potential duration at 80% repolarization (APD80, D), the maximum diastolic potential (MDP, E) and the voltage threshold of the action potential upstroke (Eth, F) are shown. Values are expressed as means ± s.e.m.*P < 0.05 versus Cont, #P < 0.05 versus PIN.
There were also no significant differences in the basal action potential parameters between WT and Kir6.2 KO SAN cells. These drugs produced no significant effects on the other action potential parameters such as the action potential duration at 80% repolarization level (APD80, Fig. 2D), the maximum diastolic potential (MDP, Fig. 2E) and the voltage threshold of the action potential upstroke (Eth, Fig. 2F) in WT as well as Kir6.2 KO SAN cells.
KATP current in SAN cells
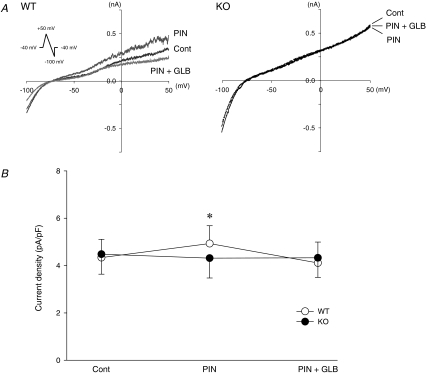
Whole-cell membrane currents were recorded from WT and Kir6.2 KO SAN cells using a ramp-pulse protocol of the ruptured patch clamp. Effects of pinacidil (100 μm) on the quasi-steady-state membrane current were examined in WT and Kir6.2 KO SAN cells. Pinacidil increased an outward current, which was sensitive to 1 μm glibenclamide, in WT SAN cells but not in Kir6.2 KO SAN cells (Fig. 3A). There was no significant difference in the basal value of the outward current density at 0 mV between WT and Kir6.2 KO SAN cells. In WT SAN cells the density of the outward current at 0 mV was increased from 4.3 ± 0.8 to 4.9 ± 0.8 pA pF−1 (n = 7, P < 0.05) after pinacidil and returned to 4.1 ± 0.9 pA pF−1 after addition of glibenclamide (Fig. 3B). In contrast, pinacidil hardly affected the density of the outward current at 0 mV (from 4.5 ± 0.8 to 4.3 ± 0.8 pA pF−1; n = 7, P = NS) in Kir6.2 KO SAN cells (Fig. 3B). Addition of glibenclamide also did not change the outward current. Thus, pinacidil could induce a glibenclamide-sensitive outward current only in WT SAN cells.
Figure 3. Effects of pinacidil on the whole-cell membrane currents in SAN cells of wild-type (WT) and Kir6.2-deficient (KO) mice.
A, representative traces of quasi-steady-state membrane currents elicited by a ramp-pulse protocol (shown in the inset) in control conditions (black, Cont), after 100 μm pinacidil (dark grey, PIN) and after addition of 1 μm glibenclamide (grey, PIN + GLB) are shown. B, PIN-induced current densities at the membrane potential of 0 mV in WT (n = 7, ○) and Kir6.2 KO SAN cells (n = 7, •) in control conditions (Cont), after 100 μm pinacidil (PIN) and after addition of 1 μm glibenclamide (PIN + GLB) are summarized. Values are expressed as means ± s.e.m.*P < 0.05 versus Cont.
Pacemaker activity and membrane currents of SAN cells during metabolic inhibition
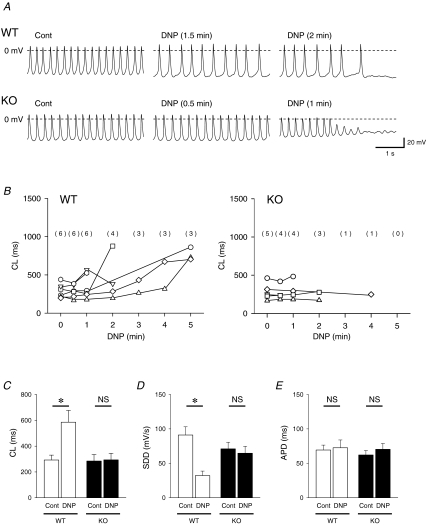
Metabolic inhibition was produced by exposure of WT and Kir6.2 KO SAN cells to the glucose-free and 2,4-dinitrophenol (DNP, 50 μm)-containing Tyrode solution. Figure 4A shows representative traces of spontaneous action potentials recorded from WT and Kir6.2 KO SAN cells by nystatin-perforated patch configuration before (Cont) and after metabolic inhibition (DNP). In Kir6.2 KO SAN cells, MDP was gradually depolarized with the reduction of action potential amplitude during metabolic inhibition. MDP of Kir6.2 KO SAN cells was significantly decreased from −52.6 ± 3.0 mV (Cont) to −44.7 ± 3.6 mV at the time point just prior to the end of spontaneous activity during metabolic inhibition (n = 5, P < 0.05). Such a gradual decrease of MDP during metabolic inhibition was hardly observed in WT SAN cells. MDP of WT SAN cells was changed from −50.2 ± 4.0 mV (Cont) to −50.4 ± 4.3 mV at the time point just prior to the end of spontaneous activity during metabolic inhibition (n = 6, P = NS). Application of DNP markedly decreased SDD in WT SAN cells but not in Kir6.2 KO SAN cells. Changes of CL in WT and Kir6.2 KO SAN cells during metabolic inhibition until disappearance of spontaneous action potentials are summarized in Fig. 4B. The CL of SAN action potentials in WT was significantly prolonged from 292.2 ± 38.2 to 585.4 ± 91.2 ms (n = 6, P < 0.05) just prior to the SAN cell arrest or 5 min after DNP application. However, in Kir6.2 KO SAN cells the CL was changed from 284.6 ± 50.1 to 293.6 ± 51.6 ms (n = 5, P = NS) just prior to the disappearance of action potentials (Fig. 4B and C). Changes of SDD and APD80 after metabolic inhibition in WT and Kir6.2 KO SAN cells are summarized in Fig. 4C. At the time point just prior to the disappearance of action potentials or at 5 min of DNP treatment, SDD was significantly decreased from 91.1 ± 11.9 to 32.2 ± 6.5 mV s−1 (n = 6, P < 0.05) in WT SAN cells (Fig. 4D). However, no significant change of SDD was observed in Kir6.2 KO SAN cells. Metabolic inhibition produced no significant changes in APD80 in WT or Kir6.2 KO SAN cells (Fig. 4E).
Figure 4. Action potential changes of SAN cells of wild-type (WT) and Kir6.2-deficient (KO) mice during metabolic inhibition.
A, representative changes in action potentials recorded from WT and Kir6.2 KO SAN cells in nystatin-perforated patch configuration before (Cont) and after metabolic inhibition. The SAN cells were exposed to a glucose-free, DNP (50 μm 2,4-dinitrophenol)-containing solution. Action potentials before (Cont), after metabolic inhibition and just prior to the end of spontaneous activity are shown. B, time course of the changes in CL of WT and Kir6.2 KO SAN cells. The number of cells with amplitudes more than 50% of control in both groups are indicated in parentheses. C–E, changes in cycle length (CL) of spontaneous action potentials, slope of the diastolic depolarization (SDD), and action potential duration at 80% repolarization (APD) before and after metabolic inhibition (DNP) in WT and Kir6.2 KO SAN cells. Values are expressed as means ± s.e.m.*P < 0.05 versus Cont.
Effects of metabolic inhibition on the membrane currents of WT and Kir6.2 KO SAN cells were also examined using the nystatin-perforated patch clamp method. In WT SAN cells the density at 0 mV of the quasi-steady-state membrane current recorded by a ramp-pulse protocol was significantly increased by 33.6 ± 12.2% of the control (n = 5, P < 0.05) at 2 min after metabolic inhibition. In Kir6.2 KO SAN cells, however, the density at 0 mV of the quasi-steady-state current after 2 min metabolic inhibition was 94.2 ± 3.6% of the control (n = 3, P = NS). Effects of metabolic inhibition on the L-type Ca2+ current (ICa-L) were also examined in WT and Kir6.2 KO SAN cells. The ICa-L was evoked by a 300 ms depolarizing pulse to 0 mV from a holding potential of −40 mV at a frequency of once every 15 s. At 2 min after metabolic inhibition the amplitude of ICa-L, measured as the difference between the peak current and the steady-state current at the end of test pulse, was decreased to 62.3 ± 7.1% (n = 4, P < 0.05) and 66.6 ± 8.7% (n = 4, P < 0.05) of the control in WT and Kir6.2 KO SAN cells, respectively. Thus, after metabolic inhibition the outward current was significantly increased in WT but not in Kir6.2 KO SAN cells although the ICa-L was decreased to a similar extent in both types of SAN cells.
[Ca2+]i changes in SAN cells during metabolic inhibition
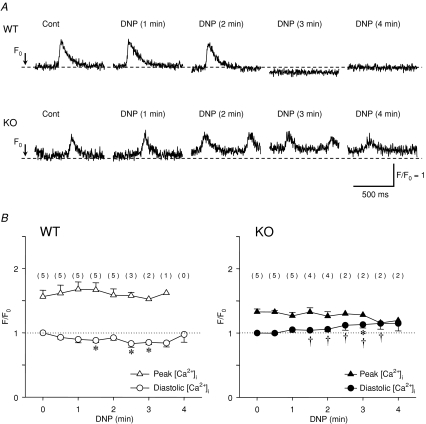
We measured [Ca2+]i by loading SAN cells with fluo-3. A spontaneous Ca2+-transient was observed in both WT and Kir6.2 KO SAN cells under control conditions (Fig. 5A). After the introduction of DNP-containing glucose-free solution (metabolic inhibition) the spontaneous Ca2+ transient of WT SAN cells disappeared and diastolic [Ca2+]i was slightly decreased when spontaneous activity was stopped. However, in Kir6.2 KO SAN cells the spontaneous Ca2+ transient did not quite disappear and the diastolic [Ca2+]i gradually increased with a decreased amplitude of the Ca2+ transient during metabolic inhibition.
Figure 5. Changes in [Ca2+]i during metabolic inhibition in wild-type (WT) and Kir6.2-deficient (KO) SAN cells.
A, representative changes in Ca2+ transient during metabolic inhibition in WT and Kir6.2 KO SAN cells. Note that the diastolic [Ca2+]i in WT SAN cells was slightly decreased after the disappearance of the Ca2+ transient whereas the diastolic [Ca2+]i was gradually increased without the disappearance of the Ca2+ transient in Kir6.2 KO SAN cells. B, time course changes in the peak (triangles) and diastolic [Ca2+]i (circles) during metabolic inhibition in WT (open symbols, n = 5) and Kir6.2 KO SAN cells (filled symbols, n = 5). The numbers of cells in which the Ca2+ transient was detected and the peak [Ca2+]i could be measured are indicated in parentheses. Values are expressed as means ± s.e.m.*P < 0.05 versus 0 min, †P < 0.05 versus WT in diastolic [Ca2+]i values.
Figure 5B summarizes the time course of changes in the peak and diastolic [Ca2+]i during metabolic inhibition. In the control condition the amplitude of the Ca2+ transient was 1.56 ± 0.10 (n = 5) and 1.33 ± 0.05 F/F0 (n = 5) in WT and Kir6.2 KO SAN cells, respectively, and there was no significant difference between these values. In WT SAN cells, diastolic [Ca2+]i (F/F0) was decreased to 88 ± 3% and 83 ± 6% of the control (n = 5) after 1.5 and 2.5 min of metabolic inhibition, respectively. However, in Kir6.2 KO SAN cells diastolic [Ca2+]i was gradually and continuously increased to 104 ± 3% and 115 ± 9% of the control (n = 5) after 1.5 and 2.5 min of metabolic inhibition, respectively. These changes in diastolic [Ca2+]i during metabolic inhibition were significantly different between WT and Kir6.2 KO SAN cells.
Discussion
Using mice with homozygous knockout of the Kir6.2 or Kir6.1 gene, previous studies from our laboratory have indicated that Kir6.2 forms the pore region of ventricular KATP channels whereas Kir6.1 forms that of vascular KATP channels (Suzuki et al. 2001; Miki et al. 2002). In addition, it has been indicated that Kir6.2 is essential for the KATP channel function in mouse atrial cells (Saegusa et al. 2005). Although several studies have indicated the presence of KATP channels in the sinoatrial node (Han et al. 1996; Marionneau et al. 2005), the pathophysiological role is not well defined. We tried to determine the role of SAN cells using Kir6.2-null mice.
In the present study the K+ channel opener pinacidil activated a glibenclamide-sensitive outward current in WT SAN cells but not in Kir6.2 KO SAN cells. Therefore, Kir6.2 forms the pore region of KATP channels of mouse SAN cells, which is similar to KATP channels of atrial and ventricular cells (Suzuki et al. 2001; Saegusa et al. 2005). However, the density of pinacidil-induced outward current in mouse SAN cells (∼0.6 pA pF−1) was much lower than in mouse ventricular cells (∼25 pA pF−1) (Suzuki et al. 2001) and that observed in atrial cells (∼7.5 pA pF−1) during metabolic inhibition (Saegusa et al. 2005). A recent study by Marionneau et al. (2005) has indicated that mRNA expression of Kir6.2 and SUR2 in SAN is lower than that in atrial and ventricular myocardium of the mouse heart. Pinacidil prolonged the cycle length of WT SAN cells by decreasing SDD, which was antagonized by the addition of glibenclamide. However, reduction of spontaneous activity of WT SAN cells after pinacidil was not accompanied by changes of other action potential parameters such as APD and MDP. It was reported that in rabbit SAN cells pinacidil at a concentration of 50 μm hyperpolarized MDP by ∼10 mV and decreased spontaneous pacemaker rate (Han et al. 1996), which is apparently inconsistent with the findings of this study. The different responses to pinacidil between mouse and rabbit SAN cells might be ascribed to different densities of the KATP current in these cells. In this study pinacidil at 100 μm increased the quasi-steady-state outward current at 0 mV by approximately 15% in WT mouse SAN cells (Fig. 3). In our preliminary experiments using rabbit SAN cells pinacidil at 30 and 100 μm increased the quasi-steady-state current at 0 mV by approximately 300% and 600%, respectively, which was antagonized by 1 μm glibenclamide (data not shown).
Metabolic inhibition of 2 min also increased the outward current by about 30% in WT SAN cells but not in Kir6.2 KO SAN cells. However, metabolic inhibition may affect other ionic currents such as L- and T-type Ca2+ current (ICa-L and ICa-T), and hyperpolarization-activated inward current (If), thereby inhibiting SAN automaticity. In fact metabolic inhibition of 2 min decreased ICa-L by 30–40% in both WT and Kir6.2 KO SAN cells. Since the decreases of ICa-L after metabolic inhibition were of similar magnitude in these cells, activation of KATP channels might be responsible for a more marked decrease of SAN automaticity in WT SAN cells.
It was reported that the activation of muscarinic acetylcholine receptor-operated K+ (KACh) current by a low concentration of acetylcholine produced the reduction of spontaneous automaticity which was associated with a decrease in SDD but no change in APD or MDP (DiFrancesco et al. 1989). We also examined the effect of a low concentration (30 nm) of carbachol, a muscarinic agonist, on the pacemaker activity in SAN cells of WT mouse, and found that the carbachol-induced prolongation of CL was associated with only the decrease in SDD (data not shown). Muscarinic receptor stimulation may indirectly inhibit ICa-L and If by decreasing intracellular cAMP and modulate the action potential configuration of SAN tissue in situ. Therefore, similarity of SAN action potential changes during KATP and KACh channel activation may not guarantee the hypothesis. However, it may provide a piece of evidence for the concept that mild activation of inward rectifier K+ channels may inhibit SAN automaticity by decreasing SDD.
When Langendorff-perfused hearts of WT mice were exposed to hypoxic and glucose-free conditions, the CL of RA electrograms, an indirect index of SAN automaticity, was prolonged more markedly compared to the hypoxia-induced CL prolongation observed in Kir6.2 KO hearts. Metabolic inhibition also prolonged the CL of spontaneous action potentials with decrease of SDD in WT SAN cells. The CL prolongation of spontaneous action potentials in WT SAN cells was more marked than that observed in Kir6.2 KO SAN cells during metabolic inhibition. Therefore, activation of sarcolemmal KATP channels might be at least in part involved in the sinus slowing or sinus arrest during hypoxia or metabolic inhibition. However, even in isolated hearts and SAN cells of Kir6.2 KO mice CL of spontaneous automaticity was slightly prolonged during hypoxia or metabolic inhibition. Therefore, changes in other ionic current(s) might also contribute to the decreased SAN automaticity. A decrease in intracellular ATP during metabolic inhibition might result in a decrease in cAMP, thereby decreasing ICa-L, ICa-T and If. It has been reported that these inward currents can be modulated by intracellular cAMP and cAMP-dependent protein kinase A (Kaupp & Seifert, 2001; Qu et al. 2005; Kim et al. 2006). In addition, an increase in [Ca2+]i during metabolic inhibition might lead to decreases of ICa-L and ICa-T. In fact, metabolic inhibition reduced ICa-L to a similar extent in both WT and Kir6.2 KO SAN cells although it activated an outward current in WT but not Kir6.2 KO SAN cells. Therefore, changes in other ionic currents including ICa-L may also be involved in sinus slowing during metabolic inhibition or hyoxia.
Although RA electrograms, probably reflecting SAN automaticity, recovered after reoxygenation in all WT hearts, the recovery was worse in Kir6.2 KO hearts. These results suggest that an increase in [Ca2+]i during hypoxia might be involved in impaired recovery of SAN function in Kir6.2 KO hearts. In WT SAN cells metabolic inhibition suppressed spontaneous firing by decreasing SDD. However, in Kir6.2 KO SAN cells metabolic inhibition produced less marked CL prolongation without a significant change in SDD and slight decrease of MDP. Decrease of spontaneous firing due to activation of KATP channels might decrease Ca2+ influx through the L-type Ca2+ channel in WT SAN cells. Maintenance of MDP resulting from KATP channel activation might keep the efficient Ca2+ extrusion through the Na+–Ca2+ exchange system in WT SAN cells. In Kir6.2 KO SAN cells, a less marked decrease of spontaneous firing and slight decrease in MDP might result in an increase in diastolic [Ca2+]i. An increase in [Ca2+]i during hypoxia might lead to SAN dysfunction. Similarly activation of sarcolemmal KATP channels can prevent hypoxia/reoxygenation-induced Ca2+ overload and improve post-hypoxic cardiac function with reduction in abnormal diastolic Ca2+ homeostasis in ventricular myocardium (Baczkóet al. 2004, 2005). A previous study from our laboratory has indicated that sarcolemmal KATP channels are essential for the protection of the ischaemic myocardium (Suzuki et al. 2002). It has also been reported that an increase in [Ca2+]i can lead to the reduction of gap junction conductance and conduction disturbance during hypoxia (Dhein, 1998). Therefore, sarcolemmal KATP channels are important for the protection of myocardium, the preservation of contractile function and the function of the cardiac conduction system including SAN during metabolic inhibition.
In summary, the present study using Kir6.2-deficient mice has indicated that activation of sarcolemmal KATP channels in SAN cells contributes to the regulation of SAN automaticity during hypoxia. The SAN slowing resulting from the activation of KATP channels may be important for the preservation of SAN function during hypoxia or ischaemia.
Acknowledgments
We are grateful to M. Tamagawa, Y. Reien and I. Sakashita for excellent technical and secretarial assistance. We thank Drs T. Ogura, H. Uemura and T. Kuwaki for valuable suggestions. This study was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan and the K. Watanabe Research Fund.
References
- Baczkó I, Giles WR, Light PE. Pharmacological activation of plasma-membrane KATP channels reduces reoxygenation-induced Ca2+ overload in cardiac myocytes via modulation of the diastolic membrane potential. Br J Pharmacol. 2004;141:1059–1067. doi: 10.1038/sj.bjp.0705702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczkó I, Jones L, McGuigan CF, Manning Fox JE, Gandhi M, Giles WR, Clanachan AS, Light PE. Plasma membrane KATP channel-mediated cardioprotection involves posthypoxic reductions in calcium overload and contractile dysfunction: mechanistic insights into cardioplegia. FASEB J. 2005;19:980–982. doi: 10.1096/fj.04-3008fje. [DOI] [PubMed] [Google Scholar]
- Boyett MR, Honjo H, Kodama I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res. 2000;47:658–687. doi: 10.1016/s0008-6363(00)00135-8. [DOI] [PubMed] [Google Scholar]
- Cho HS, Takano M, Noma A. The electrophysiological properties of spontaneously beating pacemaker cells isolated from mouse sinoatrial node. J Physiol. 2003;550:169–180. doi: 10.1113/jphysiol.2003.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein S. Gap junction channels in the cardiovascular system: pharmacological and physiological modulation. Trends Pharmacol Sci. 1998;19:229–241. doi: 10.1016/s0165-6147(98)01192-4. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D, Ducouret P, Robinson RB. Muscarinic modulation of cardiac rate at low acetylcholine concentrations. Science. 1989;243:669–671. doi: 10.1126/science.2916119. [DOI] [PubMed] [Google Scholar]
- Han X, Light PE, Giles WR, French RJ. Identification and properties of an ATP-sensitive K+ current in rabbit sino-atrial node pacemaker cells. J Physiol. 1996;490:337–350. doi: 10.1113/jphysiol.1996.sp021148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Benito MJ, Macianskiene R, Sipido KR, Flameng W, Mubagwa K. Suppression of transient outward potassium currents in mouse ventricular myocytes by imidazole antimycotics and by glybenclamide. J Pharmacol Exp Ther. 2001;298:598–606. [PubMed] [Google Scholar]
- Irisawa H, Brown HF, Giles W. Cardiac pacemaking in the sinoatrial node. Physiol Rev. 1993;73:197–227. doi: 10.1152/physrev.1993.73.1.197. [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels. Annu Rev Physiol. 2001;63:235–257. doi: 10.1146/annurev.physiol.63.1.235. [DOI] [PubMed] [Google Scholar]
- Keith A, Flack M. The form and nature of the muscular connections between the primary divisions of the vertebrate heart. J Anat Physiol. 1907;41:172–189. [PMC free article] [PubMed] [Google Scholar]
- Kim JA, Park JY, Kang HW, Huh SU, Jeong SW, Lee JH. Augmentation of CaV3.2 T-type calcium channel activity by cAMP-dependent protein kinase A. J Pharmacol Exp Ther. 2006;318:230–237. doi: 10.1124/jpet.106.101402. [DOI] [PubMed] [Google Scholar]
- Kodama I, Nikmaram MR, Boyett MR, Suzuki R, Honjo H, Owen JM. Regional differences in the role of the Ca2+ and Na+ currents in pacemaker activity in the sinoatrial node. Am J Physiol Heart Circ Physiol. 1997;272:H2793–H2806. doi: 10.1152/ajpheart.1997.272.6.H2793. [DOI] [PubMed] [Google Scholar]
- Lee SY, Lee CO. Inhibition of Na+-K+ pump and L-type Ca2+ channel by glibenclamide in guinea pig ventricular myocytes. J Pharmacol Exp Ther. 2005;312:61–68. doi: 10.1124/jpet.104.074369. [DOI] [PubMed] [Google Scholar]
- Lei M, Jones SA, Liu J, Lancaster MK, Fung SS, Dobrzynski H, Camelliti P, Maier SK, Noble D, Boyett MR. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J Physiol. 2004;559:835–848. doi: 10.1113/jphysiol.2004.068643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, Escande D, Charpentier F, Nargeot J, Lory P. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/a1G T-type calcium channels. Circ Res. 2006;98:1422–1430. doi: 10.1161/01.RES.0000225862.14314.49. [DOI] [PubMed] [Google Scholar]
- Marionneau C, Couette B, Liu J, Li H, Mangoni ME, Nargeot J, Lei M, Escande D, Demolombe S. Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol. 2005;562:223–234. doi: 10.1113/jphysiol.2004.074047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–11391. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- Nishi K, Yoshikawa Y, Sugahara K, Morioka T. Changes in electrical activity and ultrastructure of sinoatrial nodal cells of the rabbit's heart exposed to hypoxic solution. Circ Res. 1980;46:201–213. doi: 10.1161/01.res.46.2.201. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Qu Y, Baroudi G, Yue Y, El-Sherif N, Boutjdir M. Localization and modulation of α1D (Cav1.3) L-type Ca channel by protein kinase A. Am J Physiol Heart Circ Physiol. 2005;288:H2123–H2130. doi: 10.1152/ajpheart.01023.2004. [DOI] [PubMed] [Google Scholar]
- Rosati B, Rocchetti M, Zaza A, Wanke E. Sulfonylureas blockade of neural and cardiac HERG channels. FEBS Lett. 1998;440:125–130. doi: 10.1016/s0014-5793(98)01444-6. [DOI] [PubMed] [Google Scholar]
- Saegusa N, Sato T, Saito T, Tamagawa M, Komuro I, Nakaya H. Kir6.2-deficient mice are susceptible to stimulated ANP secretion: KATP channel acts as a negative feedback mechanism? Cardiovasc Res. 2005;67:60–68. doi: 10.1016/j.cardiores.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Saito T, Sato T, Miki T, Seino S, Nakaya H. Role of ATP-sensitive K+ channels in electrophysiological alterations during myocardial ischemia: a study using Kir6.2-null mice. Am J Physiol Heart Circ Physiol. 2005;288:H352–H357. doi: 10.1152/ajpheart.00695.2004. [DOI] [PubMed] [Google Scholar]
- Sakamoto N, Uemura H, Hara Y, Saito T, Masuda Y, Nakaya H. Bradykinin B2-receptor-mediated modulation of membrane currents in guinea-pig cardiomyocytes. Br J Pharmacol. 1998;125:283–292. doi: 10.1038/sj.bjp.0702060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, O'Rourke B, Marban E. Modulation of mitochondrial ATP-dependent K+ channels by protein kinase C. Circ Res. 1998;83:110–114. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marbán E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheijck EE, Wessels A, van Ginneken AC, Bourier J, Markman MW, Vermeulen JL, de Bakker JM, Lamers WH, Opthof T, Bouman LN. Distribution of atrial and nodal cells within the rabbit sinoatrial node: models of sinoatrial transition. Circulation. 1998;97:1623–1631. doi: 10.1161/01.cir.97.16.1623. [DOI] [PubMed] [Google Scholar]
- Yao A, Su Z, Nonaka A, Zubair I, Spitzer KW, Bridge JH, Muelheims G, Ross J, Jr, Barry WH. Abnormal myocyte Ca2+ homeostasis in rabbits with pacing-induced heart failure. Am J Physiol Heart Circ Physiol. 1998;274:H1441–H1448. doi: 10.1152/ajpheart.1998.275.4.H1441. [DOI] [PubMed] [Google Scholar]