Abstract
Intense exercise decreases the cerebral metabolic ratio of oxygen to carbohydrates [O2/(glucose + ½lactate)], but whether this ratio is influenced by adrenergic stimulation is not known. In eight males, incremental cycle ergometry increased arterial lactate to 15.3 ± 4.2 mm (mean ± s.d.) and the arterial–jugular venous (a–v) difference from −0.02 ± 0.03 mm at rest to 1.0 ± 0.5 mm (P < 0.05). The a–v difference for glucose increased from 0.7 ± 0.3 to 0.9 ± 0.1 mm (P < 0.05) at exhaustion and the cerebral metabolic ratio decreased from 5.5 ± 1.4 to 3.0 ± 0.3 (P < 0.01). Administration of a non-selective β-adrenergic (β1 + β2) receptor antagonist (propranolol) reduced heart rate (69 ± 8 to 58 ± 6 beats min−1) and exercise capacity (239 ± 42 to 209 ± 31 W; P < 0.05) with arterial lactate reaching 9.4 ± 3.6 mm. During exercise with propranolol, the increase in a–v lactate difference (to 0.5 ± 0.5 mm; P < 0.05) was attenuated and the a–v glucose difference and the cerebral metabolic ratio remained at levels similar to those at rest. Together with the previous finding that the cerebral metabolic ratio is unaffected during exercise with administration of the β1-receptor antagonist metropolol, the present results suggest that the cerebral metabolic ratio decreases in response to a β2-receptor mechanism.
Cerebral metabolism is considered to be aerobic indicating that six molecules of O2 are used for oxidation of one molecule of glucose, but during cerebral activation the ratio between O2 and glucose uptake (O2-to-glucose index; OGI) is reduced as more glucose than O2 is taken up by the brain (Fox et al. 1988; Madsen et al. 1995). However, glucose is not the only carbohydrate of interest for the brain. During exercise as the arterio-jugular venous lactate difference (a–v diff) is enlarged, the cerebral lactate uptake increases with its arterial concentration (Ide et al. 1999; Dalsgaard et al. 2004c), and lactate does not accumulate in the cerebrospinal fluid or within the brain tissue (Dalsgaard et al. 2004b). Thus, during maximal exercise with an increase in arterial lactate to about 20 mm, the cerebral metabolic ratio (CMR; O2/(glucose + ½lactate)) is lowered to 1.7 (Volianitis et al. 2008).
Maximal exercise also involves intense activation of the sympathetic nervous system that appears to regulate cerebral uptake of carbohydrates in the rat (Mylecharane & Raper, 1973). Catecholamines affect glucose homeostasis as both glucose and lactate increase in blood following activation of β-adrenergic receptors. Conversely, during brain activation in the rat, CMR is affected by the non-selective β-adrenergic blocking agent propranolol when it is administered before exposure to intense sensory stimulation (Schmalbruch et al. 2002). Since the CMR only decreases when exercise becomes physically and mentally challenging, administration of a β-adrenergic blocking agent known to reduce exercise tolerance should lead to an early decrease of CMR and the β1-adrenergic antagonist metropolol provokes a decrease in CMR when exercise becomes demanding at a low workload (Dalsgaard et al. 2004a). Taken together, this finding and that by Schmalbruch et al. (2002) led to the hypothesis that the decrease in CMR is mediated by a β2-adrenergic mechanism and that propranolol attenuates the decrease during exhaustive exercise in humans.
In the present study, healthy subjects were included in a cross-over design for evaluation of the effect of the non-selective β-receptor antagonist propranolol on cerebral metabolism as determined by measuring the effect on the a–v diff for O2, lactate and glucose during incremental cycling to exhaustion.
Methods
Eight healthy males (age 27 ± 6 years, height 182 ± 4 cm and weight 76 ± 7 kg; mean ± s.d.) participated in the study after written informed consent as approved by the local ethics committee (KF 01-305335). After an overnight fast, the subjects arrived at the laboratory and strenuous physical activity was not allowed 24 h prior to the experiment. The subjects were placed slightly head-down and, under local anaesthesia (lidocain, 2%) and guided by ultrasound (Lamperti et al. 2007), a catheter (1.6 mm; 14 gauge; ES-04706, Arrow International, PA, USA) was inserted retrograde in the right internal jugular vein and advanced to the bulb of the vein. A second catheter (1.1 mm; 20 G) was inserted in the brachial artery of the non-dominant arm.
After 1 h of rest, the subjects were seated with the upper body elevated ∼60 deg on a modified Krogh cycle ergometer (Galbo et al. 1987) with the feet fastened to the pedals. Separated by 1 h of recovery, the subjects performed two incremental exercise bouts to exhaustion. The subjects started cycling for 5 min at a light intensity (30 W) and, thereafter, the workload was increased by ∼60 W every fifth minute until exhaustion, defined as when the subject was unable to maintain a pedalling rate of 60 revs min−1 despite verbal encouragement. The subjects were allowed to recover for 1 h to normalize CMR (Dalsgaard et al. 2002). Immediately before the second bout of exercise, propranolol (0.15 mg kg−1; Ben Venue Laboratories, Inc., Bedford, OH, USA) was intravenously administered until heart rate (HR) was reduced ∼10 beats min−1 and cycling began at the same intensity as in the first control bout. During exercise, propranolol (0.02 mg kg−1) was supplemented at the third workload (180 W) because plasma catecholamines increase exponentially with workload (Kjaer et al. 1987). At the termination of each workload, the level of perceived exertion (RPE) was rated according to a visual scale (Borg, 1975).
To indicate changes in cerebral blood flow (CBF), mean flow velocity (V̇mean) of the proximal segment of the left middle cerebral artery (MCA) was located by transcranial Doppler sonography through the posterior temporal ultrasound window (Multidop X, DWL, Sipplingen, Germany). Once the optimal signal-to-noise ratio was obtained, the probe was mounted on a headband and acoustic coupling was secured by adhesive ultrasonic gel (Tensive, Parker Laboratories, Orange, NJ, USA). The MCA V̇mean was calculated from the integral of the maximal frequency Doppler shifts over one heart beat, assuming a constant vessel diameter (Bradac et al. 1976; Serrador et al. 2000). Continuous-wave near-infrared spectroscopy (NIRS; INVOS, Somantics, Troy, MI, USA) determined tissue O2 saturation by differentiating the absorption spectra of, and hence concentration changes in, deoxygenated and oxygenated haemoglobin (Madsen & Secher, 1999). With a NIRS probe placed on the forehead, haemoglobin O2 saturation in vessels of the frontal lobe from the right hemisphere was determined. Mean arterial pressure (MAP) was measured through a transducer (Edwards Life Sciences, Irving, CA, USA) at the level of the heart, connected to a monitor (Dialogue-2000 IBC-Danica Electronic, Denmark), and sampled at 100 Hz (DI-720, Dataq, OH, USA) for later analysis with HR and cardiac output (CO) assessed from the pressure curve (Bogert & van Lieshout, 2005).
Arterial and venous blood samples were obtained in pre-heparinized syringes from the arterial and venous catheters at rest, in the last minute of each workload, and 15 min into the recovery after exercise. Samples were placed in ice-cooled water and the concentrations of glucose and lactate as well as blood gas variables were analysed using an ABL 725 apparatus (Radiometer, Copenhagen, Denmark). Based on the assumption that the cerebral uptake of pyruvate during exercise is of an order of magnitude smaller than that of lactate (Rasmussen et al. 2006), the OGI and CMR were calculated and both OGI and CMR considered to be independent of CBF (Dalsgaard, 2006). The use of intravenous β-adrenergic blockade may affect regional blood concentrations of electrolytes which, in turn, may impact development of fatigue (Unsworth et al. 1998). Thus, arterial and jugular venous blood concentrations of Na+ and K+ were provided by the ABL 725 apparatus (Radiometer), and blood concentrations of Mg2+ were determined using a Modular Analytics P-modul (Roche Diagnositcs, Hvidovre, Denmark).
Statistics
Variables are presented as means ± s.d. Power calculations revealed that to detect an expected reduction in the CMR of 2.7 ± 1.3 (mean reduction ± s.d.) from the usual resting value of 5.7 to 3.0, a sample size of n = 8 is sufficient (alpha level < 0.01 and statistical power > 90%). The data conformed to a normal distribution and a two-way repeated measures ANOVA was used to evaluate differences between workloads and between treatments (control versusβ 1 + 2 blockade). In the case when a statistical significant deviation was detected, a post hoc Bonferroni analysis was applied for multiple comparisons. P < 0.05 was considered statistically significant.
Results
Control exercise
At rest the cardiovascular variables (Table 1) and the arterial and internal jugular venous concentrations of glucose, lactate and O2 (Tables 2 and 3) were within normal limits. The subjects became exhausted at a workload of 239 ± 42 W and the MCA V̇mean increased 27 ± 17% above the resting value. Immediately after cessation of exercise, the MCA V̇mean decreased and reached its pre-exercise value after 15 min of recovery. The NIRS-determined frontal lobe oxygenation did not change significantly in response to exercise.
Table 1.
Cardiovascular variables, MCA V̇mean and  during exercise with and without β 1 + 2 adrenergic blockade
during exercise with and without β 1 + 2 adrenergic blockade
| Exercise | |||||||
|---|---|---|---|---|---|---|---|
| Rest | 60 W | 120 W | 180 W | 210 W | 240 W | Recovery | |
| MAP (mmHg) | |||||||
| Control | 87 ± 8 | 101 ± 6* | 109 ± 6* | 114 ± 4* | 117 ± 6* | 121 ± 6* | 95 ± 12 |
| Propranolol | 86 ± 6 | 92 ± 6*† | 108 ± 6* | 110 ± 8* | 111 ± 6*† | — | 90 ± 6 |
| HR (beats min−1) | |||||||
| Control | 69 ± 8 | 92 ± 12* | 128 ± 10* | 160 ± 16* | 178 ± 16* | 189 ± 14* | 96 ± 16* |
| Propranolol | 58 ± 6† | 82 ± 12* | 116 ± 6*† | 125 ± 10*† | 135 ± 8*† | — | 74 ± 8*† |
| CO (l min−1) | |||||||
| Control | 6.2 ± 1.3 | — | — | — | 19.9 ± 4.0* | 20.8 ± 3.9* | 6.9 ± 1.3 |
| Propranolol | 6.0 ± 1.2 | — | — | — | 16.6 ± 3.6*† | — | 6.4 ± 1.4 |
| MCA V̇mean (cm s−1) | |||||||
| Control | 48 ± 8 | 55 ± 10 | 58 ± 10 | 61 ± 10* | 61 ± 8* | 61 ± 8* | 48 ± 6 |
| Propranolol | 44 ± 8 | 47 ± 8 | 49 ± 8 | 52 ± 10 | 52 ± 8† | — | 45 ± 10 |
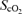 (%) (%) | |||||||
| Control | 73.8 ± 9.3 | 73.5 ± 7.3 | 74.8 ± 7.5 | 76.3 ± 7.6 | 72.7 ± 8.9 | 69.8 ± 11.6 | 75.7 ± 9.3 |
| Propranolol | 71.8 ± 7.1 | 69.1 ± 7.6 | 67.6 ± 7.0 | 72.0 ± 9.1 | 64.3 ± 11.3* | — | 76.1 ± 10.7 |
| RPE | |||||||
| Control | — | 8 (7–11) | 13 (11–15)* | 16 (15–17)* | 18 (18–19)* | 20 (20)* | — |
| Propranolol | — | 13 (12–14)* | 16 (15–18)*† | 18 (17–19)*† | 20 (19–20)*† | — | — |
Cardiovascular variables, frontal lobe oxygenation 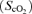 by near-infrared spectroscopy, and rating of perceived exertion (RPE) during exercise with and without non-selective β-blockade. MAP, mean arterial pressure; HR, heart rate; CO, cardiac output; MCA V̇mean, middle cerebral artery mean flow velocity.
by near-infrared spectroscopy, and rating of perceived exertion (RPE) during exercise with and without non-selective β-blockade. MAP, mean arterial pressure; HR, heart rate; CO, cardiac output; MCA V̇mean, middle cerebral artery mean flow velocity.
Different from rest (P < 0.05);
different from control exercise (P < 0.05). Values are means ± s.d., with RPE presented as median and range.
Table 2.
Blood gas variables during exercise with and without β 1 + 2 adrenergic blockade
| Exercise | ||||||||
|---|---|---|---|---|---|---|---|---|
| Rest | 60 W | 120 W | 180 W | 210 W | 240 W | Recovery | ||
| CO2 (mm) | ||||||||
| Control | a | 9.0 ± 0.3 | 8.9 ± 0.6 | 9.2 ± 0.6 | 9.4 ± 0.8 | 9.6 ± 0.8* | 9.7 ± 0.8* | 8.9 ± 0.6 |
| v | 5.5 ± 0.6 | 6.1 ± 0.6 | 6.2 ± 0.6 | 6.1 ± 0.8 | 5.4 ± 1.1 | 5.4 ± 0.8 | 5.2 ± 0.8 | |
| Propranolol | a | 8.9 ± 0.6 | 8.9 ± 0.6 | 9.2 ± 0.8 | 9.5 ± 0.6* | 9.8 ± 0.6* | — | 8.8 ± 0.6 |
| v | 5.5 ± 0.8 | 5.8 ± 0.6 | 5.6 ± 0.6 | 5.5 ± 0.8 | 5.5 ± 0.8 | — | 5.3 ± 0.8 | |
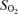 (%) (%) | ||||||||
| Control | a | 98.3 ± 0.6 | 98 ± 0.8 | 97.5 ± 0.6 | 97.1 ± 0.8 | 96.9 ± 0.6 | 96.8 ± 1.1* | 96.7 ± 1.7* |
| v | 66.0 ± 4.5 | 65.7 ± 2.8 | 65.8 ± 3.9 | 58.6 ± 7.4 | 57.4 ± 7.4* | 53.3 ± 8.5* | 55.6 ± 5.7* | |
| Propranolol | a | 98.0 ± 0.8 | 97.7 ± 0.8 | 97.7 ± 0.8 | 97.5 ± 0.6 | 97.6 ± 1.1 | — | 97.2 ± 1.7 |
| v | 60.5 ± 6.8 | 63.2 ± 5.1 | 60.8 ± 4.5 | 56.7 ± 7.6 | 51.2 ± 8.2* | — | 59.4 ± 7.6 | |
| Hb (mm) | ||||||||
| Control | a | 8.7 ± 0.6 | 9.0 ± 0.6* | 9.4 ± 0.6* | 9.6 ± 0.8* | 9.9 ± 0.8* | 10.0 ± 0.8* | 9.2 ± 0.6* |
| v | 8.8 ± 0.6 | 9.1 ± 0.6 | 9.4 ± 0.6 | 9.8 ± 0.8 | 9.3 ± 1.7 | 10.0 ± 0.6 | 9.2 ± 0.6 | |
| Propranolol | a | 8.8 ± 0.8 | 9.0 ± 0.6* | 9.3 ± 0.6* | 9.7 ± 0.6* | 9.7 ± 0.6* | — | 9.0 ± 0.6* |
| v | 8.8 ± 0.6 | 9.1 ± 0.6 | 9.4 ± 0.6* | 9.8 ± 0.6* | 9.8 ± 0.6* | — | 9.1 ± 0.6* | |
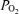 (kPa) (kPa) | ||||||||
| Control | a | 13.4 ± 0.8 | 13.2 ± 0.8 | 12.7 ± 0.8 | 13.1 ± 1.7 | 13.1 ± 1.1 | 13.3 ± 2.3 | 12.6 ± 1.7 |
| v | 4.9 ± 0.3 | 4.9 ± 0.3 | 5.0 ± 0.3 | 4.7 ± 0.3 | 4.5 ± 0.3 | 4.5 ± 0.3* | 4.4 ± 0.3* | |
| Propranolol | a | 13.1 ± 1.3 | 12.6 ± 0.8 | 12.6 ± 1.1 | 12.7 ± 0.6 | 13.0 ± 0.8 | — | 12.2 ± 1.9 |
| v | 4.5 ± 0.6 | 4.7 ± 0.6 | 4.6 ± 0.3 | 4.5 ± 0.6 | 4.1 ± 0.6 | — | 4.6 ± 0.6 | |
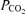 (kPa) (kPa) | ||||||||
| Control | a | 5.5 ± 0.3 | 5.6 ± 0.3 | 5.5 ± 0.3 | 5.0 ± 0.6* | 4.4 ± 0.6* | 3.9 ± 0.3* | 4.7 ± 0.6* |
| v | 7.0 ± 0.3 | 7.1 ± 0.3 | 6.9 ± 0.3 | 6.5 ± 0.6* | 6.3 ± 0.8* | 6.3 ± 0.3* | 6.5 ± 0.3 | |
| Propranolol | a | 5.5 ± 0.6 | 5.6 ± 0.3 | 5.5 ± 0.3 | 4.1 ± 0.3* | 4.6 ± 0.6* | — | 5.0 ± 0.6 |
| v | 7.1 ± 0.3 | 7.1 ± 0.3 | 7.1 ± 0.3 | 6.8 ± 0.3 | 6.6 ± 0.3* | — | 6.7 ± 0.3* | |
Blood gas variables during exercise with and without non-selective β-blockade. CO2, CO2 concentration; 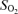 , O2 saturation; Hb, haemoglobin concentration;
, O2 saturation; Hb, haemoglobin concentration; 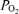 , O2 partial pressure;
, O2 partial pressure;  , carbon dioxide partial pressure.
, carbon dioxide partial pressure.
Different from rest (P < 0.05);
different from control exercise (P < 0.05). Values are means ± s.d.
Table 3.
Blood metabolites and electrolytes during exercise with and without β 1 + 2 adrenergic blockade
| Exercise | ||||||||
|---|---|---|---|---|---|---|---|---|
| Rest | 60 W | 120 W | 180 W | 210 W | 240 W | Recovery | ||
| Glucose (mm) | ||||||||
| Control | a | 6.1 ± 0.6 | 5.6 ± 0.6 | 5.3 ± 0.6* | 5.2 ± 0.6* | 5.0 ± 0.8* | 5.2 ± 0.8* | 6.2 ± 1.1 |
| v | 5.4 ± 0.6 | 5.0 ± 0.6 | 4.7 ± 0.6 | 4.5 ± 0.6* | 4.3 ± 0.8* | 4.3 ± 0.8* | 5.6 ± 1.1 | |
| Propranolol | a | 5.5 ± 0.6† | 5.2 ± 0.6 | 4.7 ± 0.6*† | 4.5 ± 0.6*† | 4.5 ± 1.1* | — | 5.4 ± 0.6 |
| v | 4.8 ± 0.6† | 4.6 ± 0.6 | 4.1 ± 0.6*† | 3.9 ± 0.6* | 3.8 ± 0.8* | — | 4.8 ± 0.6 | |
| Lactate (mm) | ||||||||
| Control | a | 0.9 ± 0.3 | 1.3 ± 0.6 | 2.8 ± 1.3 | 6.5 ± 2.5* | 11.4 ± 3.7* | 15.3 ± 4.2* | 8.0 ± 5.1* |
| v | 0.9 ± 0.3 | 1.3 ± 0.6 | 2.7 ± 1.1 | 6.2 ± 2.6* | 10.5 ± 3.7* | 14.3 ± 3.9* | 7.7 ± 4.3* | |
| Propranolol | a | 1.1 ± 0.3 | 1.4 ± 0.6 | 3.0 ± 1.5 | 6.3 ± 2.8* | 9.4 ± 3.6* | — | 4.8 ± 3.4*† |
| v | 1.2 ± 0.3 | 1.3 ± 0.6 | 3.1 ± 1.5 | 5.9 ± 2.6* | 8.9 ± 3.8* | — | 4.6 ± 3.1* | |
| Arterial pH | ||||||||
| Control | 7.42 ± 0.03 | 7.41 ± 0.03 | 7.41 ± 0.03 | 7.38 ± 0.03 | 7.35 ± 0.03* | 7.29 ± 0.05* | 7.33 ± 0.05* | |
| Propranolol | 7.41 ± 0.03 | 7.41 ± 0.03 | 7.40 ± 0.03 | 7.39 ± 0.02 | 7.37 ± 0.05 | — | 7.37 ± 0.05* | |
| Na+ (mm) | ||||||||
| Control | a | 137.9 ± 2.0 | 138.4 ± 1.7 | 139.8 ± 2.3* | 141.3 ± 2.3* | 142.9 ± 2.0* | 144.8 ± 2.0* | 139.3 ± 1.7 |
| v | 138.9 ± 2.3 | 139.1 ± 1.4 | 140.7 ± 2.3* | 142.4 ± 2.0* | 144.0 ± 2.0* | 145.8 ± 2.0* | 140.3 ± 1.7 | |
| Propranolol | a | 138.2 ± 2.0 | 139.1 ± 1.7 | 140.5 ± 1.7* | 142.2 ± 1.7* | 142.8 ± 2.8* | — | 138.7 ± 1.4 |
| v | 138.7 ± 2.0 | 140.2 ± 1.7 | 141.3 ± 1.7* | 142.8 ± 1.7* | 143.6 ± 2.5* | — | 140.0 ± 1.4 | |
| K+ (mm) | ||||||||
| Control | a | 3.8 ± 0.3 | 4.3 ± 0.3* | 4.6 ± 0.3* | 4.9 ± 0.3* | 5.3 ± 0.3* | 5.8 ± 0.3* | 3.7 ± 0.3 |
| v | 3.9 ± 0.3 | 4.3 ± 0.3 | 4.7 ± 0.6* | 5.0 ± 0.3* | 5.4 ± 0.6* | 5.8 ± 0.3* | 3.8 ± 0.3 | |
| Propranolol | a | 3.9 ± 0.3 | 4.4 ± 0.3* | 4.7 ± 0.3* | 5.2 ± 0.3* | 5.6 ± 0.3*† | — | 4.0 ± 0.6 |
| v | 4.0 ± 0.3 | 4.4 ± 0.3* | 4.9 ± 0.3* | 5.3 ± 0.3* | 5.9 ± 0.6*† | — | 4.1 ± 0.3 | |
| Mg2+ (mm) | ||||||||
| Control | a | 0.78 ± 0.03 | — | — | — | — | 0.77 ± 0.08 | — |
| v | 0.78 ± 0.06 | — | — | — | — | 0.76 ± 0.06 | — | |
| Propranolol | a | 0.78 ± 0.11 | — | — | — | 0.79 ± 0.06 | — | — |
| v | 0.77 ± 0.17 | — | — | — | 0.78 ± 0.06 | — | — | |
Blood metabolites and electrolytes during exercise with and without non-selective β-blockade.
Different from rest (P < 0.05);
different from control exercise (P < 0.05). Values are mean ± s.d.
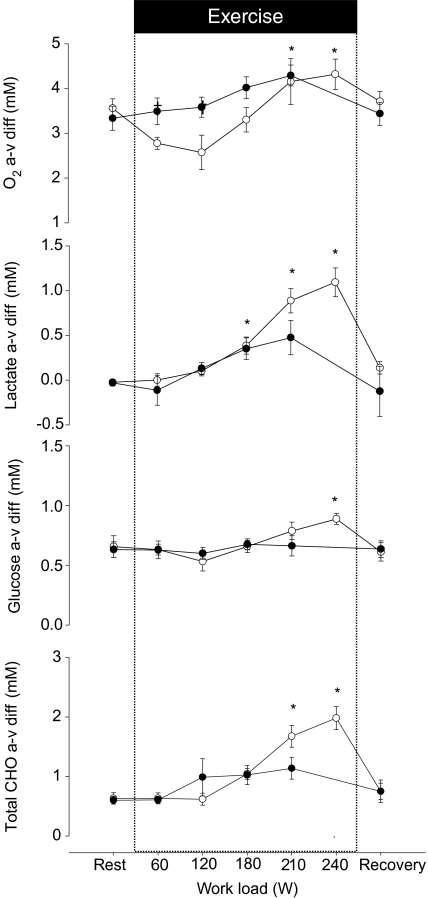
During exercise the arterial glucose concentration decreased (P < 0.05), but it recovered to the resting level early after exercise when the subjects were exhausted. The arterial lactate concentration reached a peak value (P < 0.05) and the arterial O2 content was increased at exhaustion. Arterial O2 tension 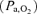 did not change during exercise, but the venous O2 tension
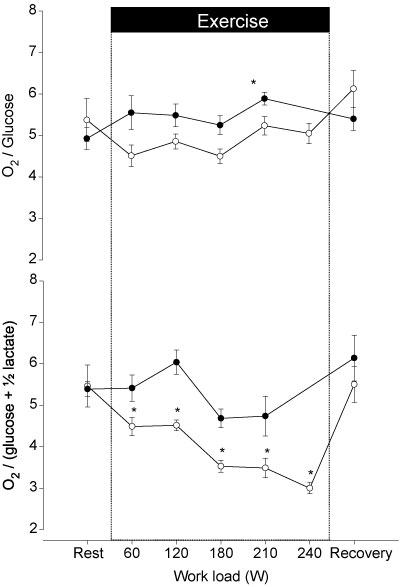
did not change during exercise, but the venous O2 tension 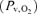 decreased at exhaustion (P < 0.05). Furthermore, the arterial and venous O2 saturation decreased (P < 0.05) and at exhaustion the a–v diff for glucose, lactate and O2 were increased (Fig. 1). The CMR was 5.5 ± 1.4 at rest and decreased to 3.0 ± 0.3 (P < 0.05) at exhaustion (Fig. 2), while the OGI remained stable.
decreased at exhaustion (P < 0.05). Furthermore, the arterial and venous O2 saturation decreased (P < 0.05) and at exhaustion the a–v diff for glucose, lactate and O2 were increased (Fig. 1). The CMR was 5.5 ± 1.4 at rest and decreased to 3.0 ± 0.3 (P < 0.05) at exhaustion (Fig. 2), while the OGI remained stable.
Figure 1. Arterial–internal jugular venous differences of O2, glucose, lactate and (glucose + ½lactate) across the brain in response to incremental exercise with (•) and without (○) administration of propranolol.
During control exercise the subjects became exhausted at a work load of 239 W, but administration of propranolol reduced exercise capacity, with exhaustion appearing at 209 W. Values are mean ± s.e.m.*Different from rest (P < 0.05); †different from control exercise (P < 0.05).
Figure 2. The arterial–internal jugular venous difference for O2/glucose (O2-to-glucose index) and for [O2/(glucose + ½lactate)] (cerebral metabolic ratio) during control exercise (○) and during exercise with propranolol (•).
Values are mean ± s.e.m.*Different from rest (P < 0.05); †different from control exercise (P < 0.05).
The arterial and venous [Na+] and [K+] increased during control exercise but there was no significant change in the a–v diff at any workload. The arterial and venous [Mg2+] were within normal limits at rest and no significant changes were observed during exercise (Table 3).
β 1 + 2 blockade
Administration of propranolol reduced resting HR by ∼10 beats min−1 leaving MAP and CO unaffected, and during exercise, the increase in HR, MAP and CO was attenuated. At rest MCA V̇mean was similar compared to before the control experiment and at exhaustion the increase was attenuated (18 ± 22%; P = 0.07). Frontal lobe oxygenation decreased at exhaustion (P < 0.05) but it was not significantly lower than during control exercise. With propranolol, the exercise capacity was reduced to 209 ± 31 W (P < 0.05), corresponding to a decrease of ∼8% and the RPE was higher at comparable workloads.
At rest, the arterial glucose concentration was lower compared to the control experiment (Table 3) and decreased during exercise. At exhaustion, the arterial glucose concentration was similar compared to the first bout. Immediately before administration of propranolol, the arterial lactate concentration was at the pre-exercise level and it increased during exercise (P < 0.05). The a–v diff for glucose did not change significantly but that for lactate did increase (Fig. 1). The arterial O2 content increased during exercise with propranolol but both 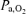 and
and 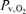 remained unchanged. As opposed to control exercise arterial O2 saturation (SaO2) did not change but internal jugular venous O2 saturation (SvO2) decreased at exhaustion. The increase in the a–v diff for O2 was similar to that observed during control exercise; however, the a–v diff for O2 was higher with β 1 + 2 blockade at the lowest workloads. After 1 h of recovery, CMR had reached a similar value (5.4 ± 0.5) as prior to the control exercise (Fig. 2) and CMR was not significantly changed with a value of 4.7 ± 1.4 at exhaustion and thereby higher than during control exercise at all comparable workloads. Also, the OGI increased at exhaustion and was higher during exercise with β 1 + 2 blockade.
remained unchanged. As opposed to control exercise arterial O2 saturation (SaO2) did not change but internal jugular venous O2 saturation (SvO2) decreased at exhaustion. The increase in the a–v diff for O2 was similar to that observed during control exercise; however, the a–v diff for O2 was higher with β 1 + 2 blockade at the lowest workloads. After 1 h of recovery, CMR had reached a similar value (5.4 ± 0.5) as prior to the control exercise (Fig. 2) and CMR was not significantly changed with a value of 4.7 ± 1.4 at exhaustion and thereby higher than during control exercise at all comparable workloads. Also, the OGI increased at exhaustion and was higher during exercise with β 1 + 2 blockade.
The arterial and venous [Na+] and [K+] increased during exercise with β 1 + 2 blockade and there was no significant change in the a–v diff at any workload or between control exercise and exercise with β 1 + 2 blockade. However, the arterial and venous concentrations of K+ were higher during exercise with β 1 + 2 blockade at 209 W. The arterial and venous concentrations of Mg2+ were not changed by exercise or β 1 + 2 blockade.
Discussion
This study demonstrates that during exercise in humans, the CMR is reduced by ∼50% when subjects become exhausted and that administration of a non-selective β-adrenergic antagonist attenuates this decrease in CMR. The effect of β-blockade was achieved with an increase in perceived exertion as compared to the control trial and CMR decreases when exercise becomes mentally and physically challenging regardless of its duration (Dalsgaard, 2006). Thus, with the results from animal studies and the lack of effect of metropolol taken into consideration, the data suggest that stimulation of β2-adrenergic receptors contribute to regulation of cerebral glucose and lactate uptake during exercise in humans.
The mechanisms behind the exercise-induced reduction of the CMR are unknown and it seems not accounted for by hormones, cytokines and metabolites (Dalsgaard, 2006). Instead, focus is on the biochemical reactions that fuel neuronal activity. The ‘glycogen shunt hypothesis’ assumes that intense neuronal activity causes CMR to decrease by intermittent glycogen synthesis and breakdown and predicts a nadir for the CMR of 3 (Shulman et al. 2001), which is the value reported during physical exhaustion involving a large muscle mass (e.g. Gonzalez-Alonso et al. 2004) although maximal ergometer rowing reduces CMR to 1.7 (Volianitis et al. 2008). Increased glycogen breakdown would spare glucose taken up by the brain if glucose is primarily coming from glycogen and, thus, increase CMR. On the other hand, increased glycogen synthesis favours an increased glucose uptake driving CMR below 6 suggesting accumulation and/or efflux of lactate from the brain. During exercise, however, the brain takes up lactate according to the arterial concentration (Ide et al. 1999) and it appears to be the cerebral uptake of lactate that determines the reduction in CMR as lactate is transported over the blood–brain barrier by the monocarboxylate transporter (MCT) and shuttled to neurons for aerobic oxidation (Pellerin & Magistretti, 1994). Furthermore, brain lactate uptake depends on the concentration gradient between blood and the extracellular fluid and with a marked increase in the arterial lactate concentration, the CMR should decrease below 3 as shown when ergometer rowing raises lactate to above 20 mm (Volianitis et al. 2008). The higher cerebral lactate uptake observed during control exercise may reflect a higher peak lactate concentration at exhaustion, but factors other than cerebral lactate uptake need to be considered as prolonged exercise in hyperthermia elicits a decrease in CMR with no significant increase in the arterial lactate concentration (Nybo et al. 2003). In addition, CMR decreases in the visual cortex in response to intense visual stimulation in a positron emission tomography scanner (Fox et al. 1988) and for the brain as a whole while performing a mental task (Madsen et al. 1995). Thus, it is only when lactate is available that changes in brain metabolism during activation are played out with lactate as the main substrate. In other words, lactate may replace glucose for metabolism of the activated brain as suggested by Kemppainen et al. (2005).
The cerebral uptake of glucose could be affected by β-adrenergic antagonists as these substances are rapidly transported through the blood–brain barrier (Olesen et al. 1978). In humans, administration of the selective β1-blocker metropolol has no effect on CMR during exhaustive exercise (Dalsgaard et al. 2004a), suggesting that the actions of propranolol are mediated via β2-receptors which are present in the brain (Sutin & Minneman, 1985). This is further substantiated by the finding that administration of propranolol prevents a decrease in CMR during brain activation in the rat (Schmalbruch et al. 2002). As noradrenalin stimulates glycolysis in astrocytes (Magistretti et al. 1981), the effect of propranolol on the CMR could be mediated through inhibition of the release and uptake of noradrenalin from adrenergic nerves (Mylecharane & Raper, 1973). At least some experimental data support the idea that propranolol inhibits glycolysis in astrocytes (Estler & Ammon, 1966; Fray et al. 1996). The ability of the adrenergic system to affect brain metabolism is substantiated by the finding that noradrenalin accumulates in the cerebrospinal fluid following strenuous exercise (Dalsgaard et al. 2004b). Glycogen serves both as a store for glucose and is integrated in cerebral metabolism (Madsen et al. 1999), and replenishment of the astrocytes' glycogen stores provides a driving force to increase the uptake of glucose. Propranolol-induced inhibition of glycolysis during exercise, consequently, leads to a smaller driving force for the cerebral uptake of glucose and contributes to its unchanged uptake during exercise.
Rather than focusing on the glucose and lactate uptake as two separate mechanisms for CMR, the total uptake of carbohydrates is of interest, and cerebral uptake of carbohydrates (glucose + ½lactate) tended to be higher during control exercise (P = 0.06; Fig. 1). Plasma catecholamines are elevated during exercise after administration of propranolol (Schneider et al. 1995) and with noradrenalin released from adrenergic nerves, the expression of the specific MCT2 increases (Pierre et al. 2003). Although speculative, the propranolol-induced inhibition of noradrenalin release and uptake by adrenergic nerves could lead to a larger extracellular lactate pool within the brain, and create a less optimal gradient for lactate transport across the blood–brain barrier.
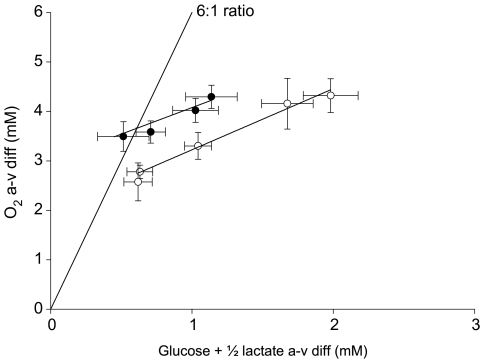
The third factor that influences the CMR is the cerebral O2 uptake and during the two lowest comparable workloads, the a–v diff O2 was higher during exercise with β 1 + 2 blockade. With the a–v diff O2 plotted against the a–v diff (glucose + ½lactate), the deviation from the 6: 1 ratio is illustrated (Fig. 3). Administration of propranolol does not change the slope of the curve but it is shifted upwards, meaning that for a given a–v diff (glucose + ½lactate), the a–v diff O2 is 1 point higher compared to control exercise (P < 0.05). It is suggested that propranolol has no effect on cerebral O2 consumption (Madsen et al. 1990) or it may even be reduced as demonstrated in anaesthetized baboons (MacKenzie et al. 1976). However, during exercise in humans, the present study finds that propranolol increases the a–v diff for O2 supporting the idea that CBF is reduced with the attenuated increase in CO.
Figure 3. The ratio between the arterial–internal jugular venous difference for O2 and the arterial–internal jugular venous difference for glucose + ½lactate.
The theoretical ratio of 6: 1 is illustrated. Control exercise (○) causes the ratio to deviate from the expected ratio of 6: 1 as the uptake of carbohydrates increases more than that of O2. During exercise with β-blockade (•), the curve is shifted parallel but upward (P < 0.05) demonstrating that for a given a–v diff for glucose + ½lactate, the a–v diff for O2 is higher during exercise with β-blockade. Values are mean ± s.e.m.*Different from rest (P < 0.05); †different from control exercise (P < 0.05).
We did not measure cerebral O2 consumption, but administration of propranolol attenuated the cardiovascular responses to exercise (Pawelczyk et al. 1992; Dalsgaard et al. 2004a) and as MCA V̇mean correlates to CO (Ogoh et al. 2005), changes in CO explain the lower MCA V̇mean with propranolol. In addition, enhanced sympathetic activation during exercise with propranolol may reduce MCA V̇mean (Ide et al. 2000) and while the Na+,K+-ATPase is influenced by adrenergic activity (Clausen & Flatman, 1977), blood concentrations of electrolytes were not affected.
Limitations
An objection to the present observations made on the effect of propranolol on the CMR during exercise is the order of exercise protocols because administration of β 1 + 2 blockade followed control exercise after 1 h of recovery. However, the CMR had recovered after control exercise and similar results have been obtained in a study with a similar design (Dalsgaard et al. 2004a). Furthermore, the second exercise bout seemed to be more strenuous for the subjects, in that β 1 + 2 adrenergic blockade reduced exercise capacity leading to higher expression of RPE at comparable workloads. As the decrease in the CMR is observed only when exercise becomes mentally and physically demanding regardless of its duration (Nybo et al. 2003; Gonzalez-Alonso et al. 2004), it was expected that the ratio would have decreased further during exercise with β 1 + 2 blockade. Another objection to the finding that administration of propranolol reduces the increase in MCA V̇mean during exercise is that the use of TCD measures blood flow velocity. However, during exercise, MCA V̇mean increases in parallel with the inflow of the internal carotid artery (Hellstrom et al. 1996), with the ‘initial slope index’ of the 133xenon clearance-determined CBF that is considered to represent the average value and about half of the increase in grey matter flow as reported by the F1 of the 133xenon determined CBF (Jørgensen et al. 1992a,b). Furthermore, the diameter of the large cerebral arteries does not change significantly during exercise, and regulation of CBF takes place in the smaller arteries (Giller et al. 1993). On this basis the use of TCD to track changes in CBF seems justified (Secher et al. 2008), but the main conclusion of this study relates to CMR, which is independent of CBF.
This study demonstrates that administration of a non-selective β blockade affects cerebral metabolism by preventing the normal decrease of the cerebral metabolic ratio during exhaustive exercise in humans. The mechanisms appear to be mediated via β2 receptors as the β1 selective agent metropolol has no effect on CMR during exercise. The mechanisms responsible for the findings in this study cannot be answered but use of stable isotopes would provide more detailed information regarding brain glucose and lactate metabolism during exercise. These findings provide a novel insight into regulation of cerebral metabolism during activation and target the intriguing question as to when cerebral metabolic balance is re-established following activation.
Acknowledgments
The study was funded by a grant from the Danish Research Agency (the Strategic Programme for Young Scientists) no. 2117–05-0095, and by the Faculty of Health Sciences, University of Copenhagen.
References
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol. 2005;90:437–446. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- Borg G. Simple rating for estimation of perceived exertion. In: Borg G, editor. Physical Work and Effort. New York: Pergamon; 1975. pp. 39–46. [Google Scholar]
- Bradac GB, Simon RS, Heidsieck CH. Angiographically verified transient alteration of intercranial arteries and veins in dependence of different CO2 tension. Neuroradiol. 1976;10:257–262. doi: 10.1007/BF00327574. [DOI] [PubMed] [Google Scholar]
- Clausen T, Flatman JA. The effect of catecholamines on Na–K transport and membrane potential in rat soleus muscle. J Physiol. 1977;270:383–414. doi: 10.1113/jphysiol.1977.sp011958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard MK. Fuelling cerebral activity in exercising man. J Cereb Blood Flow Metab. 2006;26:731–750. doi: 10.1038/sj.jcbfm.9600256. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Ide K, Cai Y, Quistorff B, Secher NH. The intent to exercise influences the cerebral O2/carbohydrate uptake ratio in humans. J Physiol. 2002;540:681–689. doi: 10.1113/jphysiol.2001.013062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard MK, Ogoh S, Dawson EA, Yoshiga CC, Quistorff B, Secher NH. Cerebral carbohydrate cost of physical exertion in humans. Am J Physiol Regul Integr Comp Physiol. 2004a;287:R534–R540. doi: 10.1152/ajpregu.00256.2004. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Ott P, Dela F, Juul A, Pedersen BK, Warberg J, Fahrenkrug J, Secher NH. The CSF and arterial to internal jugular venous hormonal differences during exercise in humans. Exp Physiol. 2004b;89:271–277. doi: 10.1113/expphysiol.2003.026922. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Volianitis S, Yoshiga CC, Dawson EA, Secher NH. Cerebral metabolism during upper and lower body exercise. J Appl Physiol. 2004c;97:1733–1739. doi: 10.1152/japplphysiol.00450.2004. [DOI] [PubMed] [Google Scholar]
- Estler CJ, Ammon HP. The influence of the beta-sympathicolytic agent propranolol on glycogenolysis and glycolysis in muscle, brain and liver of white mice. Biochem Pharmacol. 1966;15:2031–2035. doi: 10.1016/0006-2952(66)90231-0. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Fray AE, Forsyth RJ, Boutelle MG, Fillenz M. The mechanisms controlling physiologically stimulated changes in rat brain glucose and lactate: a microdialysis study. J Physiol. 1996;496:49–57. doi: 10.1113/jphysiol.1996.sp021664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbo H, Kjaer M, Secher NH. Cardiovascular, ventilatory and catecholamine responses to maximal dynamic exercise in partially curarized man. J Physiol. 1987;389:557–568. doi: 10.1113/jphysiol.1987.sp016672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–741. [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol. 2004;557:331–342. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. [DOI] [PubMed] [Google Scholar]
- Ide K, Boushel R, Sorensen HM, Fernandes A, Cai Y, Pott F, Secher NH. Middle cerebral artery blood velocity during exercise with b-1 adrenergic and unilateral stellate ganglion blockade in humans. Acta Physiol Scand. 2000;170:33–38. doi: 10.1046/j.1365-201x.2000.00757.x. [DOI] [PubMed] [Google Scholar]
- Ide K, Horn A, Secher N. Cerebral metabolic response to submaximal exercise. J Appl Physiol. 1999;87:1604–1608. doi: 10.1152/jappl.1999.87.5.1604. [DOI] [PubMed] [Google Scholar]
- Jørgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol. 1992a;72:1123–1132. doi: 10.1152/jappl.1992.72.3.1123. [DOI] [PubMed] [Google Scholar]
- Jørgensen LG, Perko M, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol. 1992b;73:1825–1830. doi: 10.1152/jappl.1992.73.5.1825. [DOI] [PubMed] [Google Scholar]
- Kemppainen J, Aalto S, Fujimoto T, Kalliokoski KK, Langsjo J, Oikonen V, Rinne J, Nuutila P, Knuuti J. High intensity exercise decreases global brain glucose uptake in humans. J Physiol. 2005;568:323–332. doi: 10.1113/jphysiol.2005.091355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer M, Secher NH, Galbo H. Physical stress and catecholamine release. Bailliere's Clin Endocrin Metab. 1987;1:279–298. doi: 10.1016/s0950-351x(87)80064-2. [DOI] [PubMed] [Google Scholar]
- Lamperti M, Cortellazzi P, D'Onofrio G, Subert M, Falcone C, Filippini G, Caldiroli D. An outcome study on complications using routine ultrasound assistance for internal jugular vein cannulation. Acta Anaesthesiol Scand. 2007;51:1327–1330. doi: 10.1111/j.1399-6576.2007.01442.x. [DOI] [PubMed] [Google Scholar]
- MacKenzie ET, McCulloch J, Harper AM. Influence of endogenous norepinephrine on cerebral blood flow and metabolism. Am J Physiol. 1976;231:489–494. doi: 10.1152/ajplegacy.1976.231.2.489. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19:393–400. doi: 10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bülow J, Holm S, Wildschiødtc G, Paulson OB, Lassen NA. Persistent resetting of the cerebral oxygen/glucose uptake by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1995;15:485–491. doi: 10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58:541–560. doi: 10.1016/s0301-0082(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Vorstrup S, Schmidt JF, Paulson OB. Effect of acute and prolonged treatment with propranolol on cerebral blood flow and cerebral oxygen metabolism in healthy volunteers. Eur J Clin PharmacolKF. 1990;39:295–297. doi: 10.1007/BF00315115. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Morrison JH, Shoemaker WJ, Sapin V, Bloom FE. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proc Natl Acad Sci U S A. 1981;78:6535–6539. doi: 10.1073/pnas.78.10.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylecharane EJ, Raper C. Further studies on the adrenergic neuron blocking activity of some -adrenoceptor antagonists and guanethidine. J Pharm Pharmacol. 1973;25:213–220. doi: 10.1111/j.2042-7158.1973.tb10627.x. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B, Blomstrand E, Moller K, Secher N. Neurohumoral responses during prolonged exercise in humans. J Appl Physiol. 2003;95:1125–1131. doi: 10.1152/japplphysiol.00241.2003. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, O-Yurvati A, Raven PB. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol. 2005;569:697–704. doi: 10.1113/jphysiol.2005.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen J, Hougard K, Hertz M. Isoproterenol and propranolol: ability to cross the blood–brain barrier and effects on cerebral circulation in man. Stroke. 1978;9:344–349. doi: 10.1161/01.str.9.4.344. [DOI] [PubMed] [Google Scholar]
- Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. J Appl Physiol. 1992;73:1838–1846. doi: 10.1152/jappl.1992.73.5.1838. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre K, Debernardi R, Magistretti PJ, Pellerin L. Noradrenaline enhances monocarboxylate transporter 2 expression in cultured mouse cortical neurons via a translational regulation. J Neurochem. 2003;86:1468–1476. doi: 10.1046/j.1471-4159.2003.01964.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Plomgaard P, Krogh-Madsen R, Kim YS, van Lieshout JJ, Secher NH, Quistorff B. MCA Vmean and the arterial lactate-to-pyruvate ratio correlate during rhythmic handgrip. J Appl Physiol. 2006;101:1406–1411. doi: 10.1152/japplphysiol.00423.2006. [DOI] [PubMed] [Google Scholar]
- Schmalbruch IK, Linde R, Paulson OB, Madsen PL. Activation-induced resetting of cerebral metabolism and flow is abolished by b-adrenergic blockade with propranolol. Stroke. 2002;33:251–255. doi: 10.1161/hs0102.101233. [DOI] [PubMed] [Google Scholar]
- Schneider DA, Kamimori GH, Wu SY, McEniery MT, Solomon C. Plasma catecholamine and ventilatory responses to cycling after propranolol treatment. Med Sci Sports Exerc. 1995;27:1616–1620. [PubMed] [Google Scholar]
- Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol. 2008;104:306–314. doi: 10.1152/japplphysiol.00853.2007. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci U S A. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutin J, Minneman KP. Adrenergic beta receptors are not uniformly distributed in the cerebellar cortex. J Comp Neurol. 1985;236:547–554. doi: 10.1002/cne.902360410. [DOI] [PubMed] [Google Scholar]
- Unsworth K, Hicks A, McKelvie R. The effect of beta-blockade on plasma potassium concentrations and muscle excitability following static exercise. Pflugers Arch. 1998;436:449–456. doi: 10.1007/s004240050656. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Fabricius-Bjerre A, Overgaard A, Stromstad M, Bjarrum M, Carlson C, Petersen NT, Rasmussen P, Secher NH, Nielsen HB. The cerebral metabolic ratio is not affected by oxygen availability during maximal exercise in humans. J Physiol. 2008;586:107–112. doi: 10.1113/jphysiol.2007.142273. [DOI] [PMC free article] [PubMed] [Google Scholar]