Abstract
According to the current paradigm, muscle nuclei serve a certain cytoplasmic domain. To preserve the domain size, it is believed that nuclei are injected from satellite cells fusing to fibres undergoing hypertrophy, and lost by apoptosis during atrophy. Based on single fibre observations in and ex vivo we suggest that nuclear domains are not as constant as is often indicated. Moreover, recent time lapse in vivo imaging of single fibres suggests that at least for the first few weeks, atrophy is not accompanied by any loss of nuclei. Apoptosis is abundant in muscle tissue during atrophy conditions, but in our opinion it has not been unequivocally demonstrated that such nuclei are myonuclei. As we see it, the preponderance of current evidence suggests that disuse atrophy is not accompanied by loss of nuclei, at least not for the first 2 months. Moreover, it has not been proven that myonuclear apoptosis does occur in permanent fibres undergoing atrophy; it seems more likely that it is confined to stromal cells and satellite cells. If muscle atrophy is not related to loss of nuclei, design of intervention therapies should focus on protein metabolism rather than regeneration from stem cells.
Introduction
The understanding of how muscle fibre size is regulated is important, because under most conditions (although not all, see Bruusgaard et al. 2005), size is directly proportional to force, and the ability to develop force is mainly changed by altering fibre size rather than fibre content or the number of fibres. There is an idea which stems from the 19th century that a nucleus serves a certain volume of cytoplasm, and that the so-called ‘karyoplasmatic’ ratio is constant (Strassburger, 1893). Also in modern literature it has been argued that the link between DNA content and cell volume is a fundamental principle (Gregory, 2001). The mechanistic background is unclear, but it has been speculated that each nucleus has only a limited synthetic capacity, or that there is limited transport capacity, for example related to the number of nuclear pores mediating the passage of RNA from the nucleus to the cytoplasm (Cavalier-Smith, 1978, 1980). In the multinucleated muscle fibres each nucleus seems to synthesize protein for a local domain in the vicinity of that nucleus both in vitro (Hall & Ralston, 1989; Pavlath et al. 1989) and in vivo (Gundersen et al. 1993).
Based on this background, the notion that each myonucleus can serve a certain cytoplasmic volume, and consequently that the number of nuclei is linearly correlated to fibre volume, has served as a conceptual framework in the field. Moreover, the current paradigm for regulation of muscle fibre size is that during atrophy, nuclei are lost by apoptosis, and during hypertrophy, new nuclei are injected into the fibres from muscle stem cells (satellite cells) situated under the basal lamina fusing with the fibres (Allen et al. 1999). The number of nuclei could be the causative factor in regulating muscle fibre size, or a consequence of it, but in any instance the nuclear domain size could be maintained.
Although beautifully simple, the paradigm of constant nuclear domains, and in particular the idea that nuclei are lost by apoptosis during atrophy, has been challenged by recent studies.
Is the nuclear domain size constant in muscle?
Muscle fibres are by volume the largest animal cells. In mouse limb muscles we have estimated the volume to be about 5 nl after intracellular dye injection in vivo (Utvik et al. 1999). In large mammals it could easily reach 1000 nl; by comparison a human ovum is about 50 nl, while most other mammalian cells would range between 10−5 and 10−3 nl (Bruusgaard et al. 2003). Muscle cells are one of the few mammalian syncytia, and such large cells could probably not be supported by only one nucleus, but the literature addressing the correlation between fibre size and number of myonuclei inside each fibre is less clear.
We have counted myonuclei per unit of length in live single fibres in the intact animal, while several other groups have made similar studies on chemically treated and isolated fibres. Across fibre types, no correlation was found between fibre cross-sectional area and number of nuclei in rat diaphragm muscles (Aravamudan et al. 2006), while in mouse tibialis anterior a weak correlation between fibre cross-sectional area and number of nuclei was found (Brack et al. 2005). When fibres from the plantaris muscles of young adult rats were sorted into fibre types, a robust correlation between size and nuclear number was observed (Roy et al. 1999); similarly in limb muscles of young mice (≤ 2 months) there was a reasonably good correlation between size and number of nuclei (Bruusgaard et al. 2003, 2006; Wada et al. 2003; Mantilla et al. 2008). More specifically, oxidative IIa fibres from murine soleus muscles fitted a model where the number of nuclei increased linearly with cross-sectional area and with the correlation line transecting the origin (Bruusgaard et al. 2003). Fast glycolytic IIb fibres from the extensor digitorum longus (EDL), on the other hand, displayed nuclear numbers increasing linearly with fibre circumference as if to keep the surface area per nucleus constant. In both cases fibres adhered rather strictly to a model of constant nuclear domain volumes and surfaces, respectively. In contrast, for middle-aged animals (14–18 months) no significant correlation between fibre size and nuclear number was observed in spite of a fourfold variation in cytoplasmic volume (Wada et al. 2003; Bruusgaard et al. 2006; but see Brack et al. 2005). In older animals (23 months) a weak correlation between size and number of nuclei reappeared (Bruusgaard et al. 2006).
We conclude that in normal muscles constant nuclear domains in the strict sense are only displayed under certain circumstances, for example during growth or senescence when the number of nuclei might serve as a bottleneck (discussed in Bruusgaard et al. 2006). During more stable conditions, the correlation can be either rather loose, or not evident at all, in spite of large variability in fibre size.
Are myonuclei lost during atrophy?
Most of the literature dealing with the correlation between size and number of nuclei is not based on the normal variability discussed above, but rather on observations under conditions where muscle fibres are changing. Thus, it has been reported that during growth or hypertrophy the number of nuclei in each muscle fibre increases (Enesco & Puddy, 1964; Moss, 1968; Cheek et al. 1971; Seiden, 1976; Cabric & James, 1983; Cabric et al. 1987; Giddings & Gonyea, 1992; Winchester & Gonyea, 1992; Allen et al. 1995; McCall et al. 1998; Kadi et al. 1999; Roy et al. 1999), while during atrophy the number decreases. In this review we will focus on atrophy.
A large number of studies have suggested that the number of nuclei is reduced under a wide range of conditions leading to atrophy, such as neuromuscular disorders, in particular denervating disorders (reviewed in Tews, 2005), as well as several experimental models including denervation (Tews et al. 1997; Viguie et al. 1997; Yoshimura & Harii, 1999; Borisov & Carlson, 2000; Schmalbruch & Lewis, 2000; Tang et al. 2000; Jin et al. 2001; Alway et al. 2003a; Siu & Alway, 2005; Adhihetty et al. 2007), abolishment of nerve electrical activity (Dupont-Versteegden et al. 1999, 2000), and mechanical muscle unloading (Darr & Schultz, 1989; Allen et al. 1997a,b; Alway et al. 2003b; Siu et al. 2005a; Siu et al. 2005b; Dupont-Versteegden et al. 2006).
Most of the studies suggesting that nuclei are lost are, however, based on conventional histology of muscle cross-sections observed in the light microscope where there may be difficulties in distinguishing between myonuclei and other nuclei. In particular satellite cells with their close proximity to the muscle fibres, create problems. Moreover, changes in nuclear size and shape may influence the number of nuclei per fibre length inferred from cross-sections (Schmalbruch & Lewis, 2000).
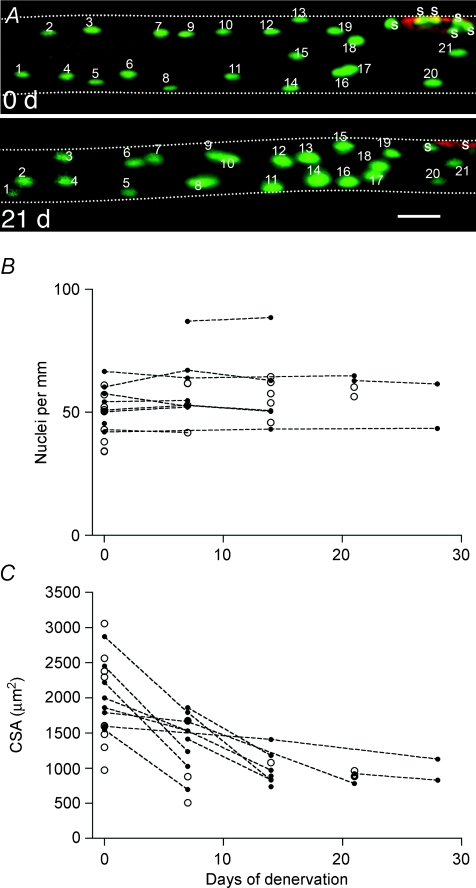
Recently, the idea of nuclear loss was challenged by direct observation by in vivo time lapse imaging of single fibres (Bruusgaard & Gundersen, 2008). Nuclei from single fibres were labelled with GFP using somatic gene transfer by electroporation or intracellular injection and then followed for up to 4 weeks after denervation by re-exposing the muscle and re-imaging the same fibre segments. In these experiments no loss of nuclei was observed in spite of a 50% reduction in fibre volume (Fig. 1). The observations were confirmed by acute intracellular injection of nuclear dyes in both the glycolytic EDL and the oxidative soleus muscle in vivo. Moreover, no loss of nuclei was observed after nerve impulse block by tetrodotoxin or mechanical unloading by tenotomizing antagonist muscles. Similar observations have been made in isolated fibres from normal and denervated mouse plantaris muscles (Wada et al. 2002) and rat diaphragms (Aravamudan et al. 2006). Thus, analysis based on single fibres indicates that there is no loss of nuclei during atrophy in mice, at least not in the short term. For long-term atrophy the situation is less clear. Wada et al. (2002) found no loss of myonuclei for up to 4 months of denervation in mouse plantaris, but in fibre segments isolated from rat EDL muscles denervated for 2–7 months a reduced number of nuclei was observed (Viguie et al. 1997). Among possible explanations for the discrepancies are differences in the methods used to isolate the fibres (mechanical versus chemical), and, related to this, uncertainties with respect to whether the counts include satellite cells or not.
Figure 1. In vivo time lapse observations of myonuclei.
Time-lapse study of nuclei and atrophy in single muscle fibres in the EDL muscle after denervation. A, images of a representative muscle fibre after injection of an expression vector encoding nuclear EGFP (green) showing the same fibre observed at the time of denervation, and again after 21 days. The neuromuscular endplate was stained by α-bungarotoxin (red). At both time points 21 nuclei can be identified within the picture frame; S indicate additional synaptic nuclei. Background staining of EGFP or fluorescent nucleotides in the cytosol made the fibre outline discernable and the cross-sectional area was calculated from the apparent diameter. The fibre boundaries are outlined for clarity. Scale bar: 50 μm. B, individual nuclei were identified from stacks of pictures taken at different focal planes covering the fibre cross-section. Counts are represented as nuclei per millimetre fibre length. Data representing multiple observations from the same fibre are indicated by filled symbols connected with broken lines. Single time-point observations of other fibres are indicated with open symbols. C, cross-sectional areas of the same fibres as shown in B. Figure modified from Bruusgaard & Gundersen (2008).
Has apoptosis of myonuclei been demonstrated?
Apoptosis is normally a process where cells are marked for phagocytosis after having unleashed an intracellular cascade of proteolysis of specific proteins in the cytoplasm and the nucleus, the latter leading to DNA fragmentation. It seems clear that whole muscle fibres or segments thereof can be eliminated by apoptosis during development, and that such elimination is augmented by neonatal denervation (Trachtenberg, 1998). Although the developmental fibre apoptosis wanes during the first two neonatal weeks, it has been suggested that the mechanism may operate in relation to muscle damage in adults, for example after exercise (Podhorska-Okolow et al. 1998), or as a result of disease (Tews, 2005). Elimination of whole fibres or fibre segments is, however, different from atrophy. Atrophy occurs in intact functional fibre syncytia, and apoptosis in such fibres would have to represent a strict elimination of nuclei, or at least would have to occur without phagocytosis or widespread proteolysis. Below we discuss pro et contra evidence for the existence of such a phenomenon during atrophy.
A large number of studies have suggested that myonuclei undergo apoptosis during atrophy. Several of the papers are, however, based only on finding molecular markers of apoptosis in muscle homogenates (Tang et al. 2000; Alway et al. 2003a; Siu & Alway, 2005; Siu et al. 2005a), and the majority of the literature is based on light-microscopy studies without using any staining methods critically differentiating between myonuclei and other nuclei (Allen et al. 1997a; Tews et al. 1997; Yoshimura & Harii, 1999; Alway et al. 2003b; Adhihetty et al. 2007).
Rodrigues & Schmalbruch (1995) reported that myonuclei displayed ultrastructural signs remotely resembling apoptotic nuclei after denervation, but found no evidence of DNA breakage. Two other ultrastructural studies report that myonuclei might display morphological characteristics similar if not identical to nuclei undergoing classical apoptosis, but it is not clear if these nuclei were part of intact muscle fibres or represented whole disintegrating fibres (Borisov & Carlson, 2000; Jin et al. 2001).
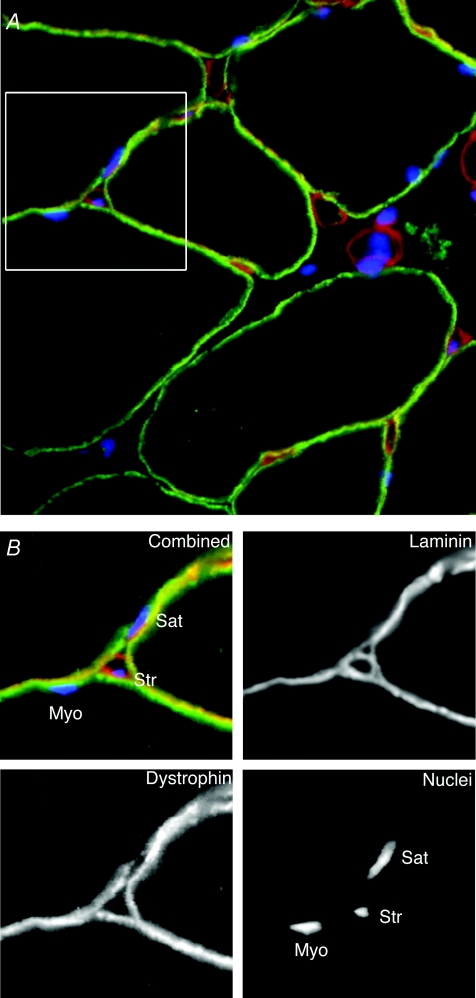
As illustrated in Fig. 2 it is to some degree possible to distinguish between myonuclei, nuclei belonging to satellite cells and nuclei of stromal cells on light microscopy sections by combining nuclear labelling with labelling of laminin and dystrophin. Using such techniques, we observed that denervation and nerve impulse block both led to high levels of apoptotic nuclei in the muscle tissue. These were, however, unlikely to be myonuclei, since virtually none of them had their mass centre inside the dystrophin ring of any muscle fibre (Bruusgaard & Gundersen, 2008). Basal lamina staining suggested that apoptosis occurred both in stromal cells and satellite cells. This apoptotic activity probably reflects an increased turnover rather than net loss of nuclei. It was shown recently that after denervation mitotic activity increased to a similar extent as the rate of apoptosis (Hyatt et al. 2006), and a large body of literature collected over the last 30 years suggest that denervation increases the number of satellite cells (Viguie et al. 1997). What the role might be of the increased turnover and the satellite cell expansion in a situation where myonuclei are not affected remains to be clarified.
Figure 2. Light-microscopical methods to distinguish between different populations of nuclei in muscle Cross-section of rat soleus muscle.
(A) with nuclei stained with Hoechst dye 33342 (blue), and with antibodies against laminin (red) and dystrophin (green), illustrating how different types of nuclei can be identified. The region shown within the 40 μm × 40 μm frame is shown at larger magnification in B, either with all channels combined (upper left) or with the channels separated. When examining sections, nuclei with their mass centre inside the dystrophin ring were defined as myonuclei (Myo), while those outside the laminin ring belonged to stromal cells (Str). Nuclei between the rings are probably in satellite cells (Sat).
To our knowledge, in addition to our own studies on denervation and inactivity (Bruusgaard & Gundersen, 2008), three other studies have utilized antibodies against dystrophin to identify myonuclei. Alway and collaborators (Siu et al. 2004) studied the effect of unloading quail muscles, which had recently been made hypertrophic, and they suggest that a new population of nuclei in hypertrophic muscle is prone to apoptosis. The possibility of a new, less stable, population of nuclei is interesting, but only one example of an apoptotic nucleus inside the dystrophin ring was shown, and the frequency of apoptosis in myonuclei was not reported.
Dupont-Versteegden and colleagues have published two reports on atrophy in the soleus muscle of rats after hindlimb suspension suggesting a high level of apoptosis in myonuclei (Leeuwenburgh et al. 2005; Dupont-Versteegden et al. 2006). If these observations are correct, unloading by hindlimb suspension seems to be different from some other atrophy models. This is not unlikely since hindlimb suspension, in addition to unweighing the muscles, has clear systemic effects on several organ and hormone systems, and is accompanied by a marked weight reduction (Morey-Holton & Globus, 2002; Morey-Holton et al. 2005). Moreover, the procedure leads to stress, with elevated levels of cortisol and muscle cortisol receptors (Steffen & Musacchia, 1987), which influences the atrophy since adrenalectomy leads to decreased atrophy (Aboudrar et al. 1993).
Alternatively, the discrepancies could be related to the fact that TUNEL staining is prone to false positives (Pulkkanen et al. 2000; Garrity et al. 2003). The number of TUNEL-positive myonuclei were quantified in one of the studies (Dupont-Versteegden et al. 2006); and the normal control muscles displayed on average nine TUNEL-positive nuclei per section, of which two-thirds were interpreted to be myonuclei. This contrasts with our own findings in normal mouse muscles where only 0.5 nuclei per section, were TUNEL-positive, none of which were myonuclei (Bruusgaard & Gundersen, 2008). Even if one takes into account that rat muscles contain 3 times as many fibres per section, the apparent apoptotic activity in normal rat muscles seemed to be 6 times as high as in mice.
In order to investigate if this dissimilarity could be due to species differences, we repeated experiments with TUNEL staining of soleus muscles from 6-month-old male Sprague–Dawley rats with the same protocol as we previously used in mice (Bruusgaard & Gundersen, 2008). Our normal muscles displayed only 1.4 ± 1.1 (mean ± s.d. of 9 sections form 3 muscles) TUNEL-positive nuclei per section, which is only 15% of the level reported by Dupont-Versteegden et al. (2006). Given the inherent problems with TUNEL staining (Pulkkanen et al. 2000; Garrity et al. 2003), the discrepancies between the control materials indicate that the experiments with hindlimb suspension should be repeated with alternative methods, or with a control material displaying a lower number of TUNEL-positive nuclei. We also performed a 7-day denervation experiment in the rats, and in the atrophying muscles the number of TUNEL-positive nuclei increased to 16 ± 3 per section (6 sections from 2 muscles), but of the 191 TUNEL-positive nuclei we scrutinized, only one displayed a mass centre that appeared to be inside the dystrophin ring, and thus denervation does not seem to induce apoptosis of myonuclei in the rat.
Conclusions
In our opinion, it has not been unequivocally demonstrated that myonuclei are lost under any atrophy conditions, nor has it been proven that apoptosis of individual nuclei of intact muscle fibres does occur. If nuclei are not lost, atrophy and recovery from it simply reflect changes in the balance between protein synthesis and proteolysis (for reviews see Glass, 2003, 2005; Jackman & Kandarian, 2004), and intervention therapies should concentrate on such mechanisms rather than on regeneration from stem cells. Absence of degeneration may explain why muscle has such a remarkable capability for recovery after prolonged inactivity when electrical activity is restored both in rodents (Hennig & Lømo, 1987) and in man (Kern et al. 2004; Boncompagni et al. 2007).
Acknowledgments
We are grateful to the members of our group, and to Drs Simon M. Hughes and Grace K. Pavlath for comments on previous versions of this manuscript.
References
- Aboudrar S, Sempore B, Koubi H, Dechaud H, Desplanches D. Effects of adrenalectomy or RU-486 on rat muscle fibers during hindlimb suspension. J Appl Physiol. 1993;75:2767–2773. doi: 10.1152/jappl.1993.75.6.2767. [DOI] [PubMed] [Google Scholar]
- Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol. 2007;102:1143–1151. doi: 10.1152/japplphysiol.00768.2006. [DOI] [PubMed] [Google Scholar]
- Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol. 1997a;273:C579–C587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- Allen DL, Linderman JK, Roy RR, Grindeland RE, Mukku V, Edgerton VR. Growth hormone/IGF-I and/or resistive exercise maintains myonuclear number in hindlimb unweighted muscles. J Appl Physiol. 1997b;83:1857–1861. doi: 10.1152/jappl.1997.83.6.1857. [DOI] [PubMed] [Google Scholar]
- Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR. Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol. 1995;78:1969–1976. doi: 10.1152/jappl.1995.78.5.1969. [DOI] [PubMed] [Google Scholar]
- Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350–1360. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Alway SE, Degens H, Krishnamurthy G, Chaudhrai A. Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2003a;58:687–697. doi: 10.1093/gerona/58.8.b687. [DOI] [PubMed] [Google Scholar]
- Alway SE, Martyn JK, Ouyang J, Chaudhrai A, Murlasits ZS. Id2 expression during apoptosis and satellite cell activation in unloaded and loaded quail skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 2003b;284:R540–R549. doi: 10.1152/ajpregu.00550.2002. [DOI] [PubMed] [Google Scholar]
- Aravamudan B, Mantilla CB, Zhan WZ, Sieck GC. Denervation effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol. 2006;100:1617–1622. doi: 10.1152/japplphysiol.01277.2005. [DOI] [PubMed] [Google Scholar]
- Boncompagni S, Kern H, Rossini K, Hofer C, Mayr W, Carraro U, Protasi F. Structural differentiation of skeletal muscle fibers in the absence of innervation in humans. Proc Natl Acad Sci U S A. 2007;104:19339–19344. doi: 10.1073/pnas.0709061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisov AB, Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec. 2000;258:305–318. doi: 10.1002/(SICI)1097-0185(20000301)258:3<305::AID-AR10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Brack AS, Bildsoe H, Hughes SM. Evidence that satellite cell decrement contributes to preferential decline in nuclear number from large fibres during murine age-related muscle atrophy. J Cell Sci. 2005;118:4813–4821. doi: 10.1242/jcs.02602. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Brack AS, Hughes SM, Gundersen K. Muscle hypertrophy induced by the Ski protein: cytoarchitecture and ultrastructure. Acta Physiol Scand. 2005;185:141–149. doi: 10.1111/j.1365-201X.2005.01462.x. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. J Clin Invest. 2008;118:1450–1447. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Liestol K, Ekmark M, Kollstad K, Gundersen K. Number and spatial distribution of nuclei in the muscle fibres of normal mice studied in vivo. J Physiol. 2003;551:467–478. doi: 10.1113/jphysiol.2003.045328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Liestol K, Gundersen K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J Appl Physiol. 2006;100:2024–2030. doi: 10.1152/japplphysiol.00913.2005. [DOI] [PubMed] [Google Scholar]
- Cabric M, Appell HJ, Resic A. Effects of electrical stimulation of different frequencies on the myonuclei and fiber size in human muscle. Int J Sports Med. 1987;8:323–326. doi: 10.1055/s-2008-1025677. [DOI] [PubMed] [Google Scholar]
- Cabric M, James NT. Morphometric analyses on the muscles of exercise trained and untrained dogs. Am J Anat. 1983;166:359–368. doi: 10.1002/aja.1001660309. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Nuclear volume control by nucleoskeletal DNA, selection for cell volume and cell growth rate, and the solution of the DNA C-value paradox. J Cell Sci. 1978;34:247–278. doi: 10.1242/jcs.34.1.247. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. How selfish is DNA? Nature. 1980;285:617–618. doi: 10.1038/285617a0. [DOI] [PubMed] [Google Scholar]
- Cheek DB, Holt AB, Hill DE, Talbert JL. Skeletal muscle cell mass and growth: the concept of the deoxiribonucleic acid unit. Pediatres. 1971;5:312–328. [Google Scholar]
- Darr KC, Schultz E. Hindlimb suspension suppresses muscle growth and satellite cell proliferation. J Appl Physiol. 1989;67:1827–1834. doi: 10.1152/jappl.1989.67.5.1827. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Murphy RJL, Houlè JD, Gurley CM, Peterson CA. Activated satellite cells fail to restore myonuclear number in spinal cord transected and exercised rat. Am J Physiol Cell Physiol. 1999;277:C589–C597. doi: 10.1152/ajpcell.1999.277.3.C589. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Murphy RJ, Houle JD, Gurley CM, Peterson CA. Mechanisms leading to restoration of muscle size with exercise and transplantation after spinal cord injury. Am J Physiol Cell Physiol. 2000;279:C1677–C1684. doi: 10.1152/ajpcell.2000.279.6.C1677. [DOI] [PubMed] [Google Scholar]
- Dupont-Versteegden EE, Strotman BA, Gurley CM, Gaddy D, Knox M, Fluckey JD, Peterson CA. Nuclear translocation of EndoG at the initiation of disuse muscle atrophy and apoptosis is specific to myonuclei. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1730–R1740. doi: 10.1152/ajpregu.00176.2006. [DOI] [PubMed] [Google Scholar]
- Enesco M, Puddy D. Increase in the number of nuclei and weight in skeletal muscle of rats of various ages. Am J Anat. 1964;114:235–244. doi: 10.1002/aja.1001140204. [DOI] [PubMed] [Google Scholar]
- Garrity MM, Burgart LJ, Riehle DL, Hill EM, Sebo TJ, Witzig T. Identifying and quantifying apoptosis: navigating technical pitfalls. Mod Pathol. 2003;16:389–394. doi: 10.1097/01.MP.0000062657.30170.92. [DOI] [PubMed] [Google Scholar]
- Giddings CJ, Gonyea WJ. Morphological observations supporting muscle fiber hyperplasia following weight-lifting exercise in cats. Anat Rec. 1992;233:178–195. doi: 10.1002/ar.1092330203. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Gregory TR. Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biol Rev Camb Philos Soc. 2001;76:65–101. doi: 10.1017/s1464793100005595. [DOI] [PubMed] [Google Scholar]
- Gundersen K, Sanes JR, Merlie JP. Neural regulation of muscle acetylcholine receptor epsilon- and alpha-subunit gene promoters in transgenic mice. J Cell Biol. 1993;123:1535–1544. doi: 10.1083/jcb.123.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ZW, Ralston E. Nuclear domains in muscle cells. Cell. 1989;59:771–772. doi: 10.1016/0092-8674(89)90597-7. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Effects of chronic stimulation on the size and speed of long-term denervated and innervated rat fast and slow skeletal muscles. Acta Physiol Scand. 1987;130:115–131. doi: 10.1111/j.1748-1716.1987.tb08118.x. [DOI] [PubMed] [Google Scholar]
- Hyatt JP, Roy RR, Baldwin KM, Wernig A, Edgerton VR. Activity-unrelated neural control of myogenic factors in a slow muscle. Muscle Nerve. 2006;33:49–60. doi: 10.1002/mus.20433. [DOI] [PubMed] [Google Scholar]
- Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol. 2004;287:C834–C843. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu Z, Tian T, Gu Y. Apoptosis in atrophic skeletal muscle induced by brachial plexus injury in rats. J Trauma. 2001;50:31–35. doi: 10.1097/00005373-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE. Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol. 1999;111:189–195. doi: 10.1007/s004180050348. [DOI] [PubMed] [Google Scholar]
- Kern H, Salmons S, Mayr W, Rossini K, Carraro U. Recovery of long-term denervated human muscles induced by electrical stimulation. Muscle Nerve. 2004;31:98–101. doi: 10.1002/mus.20149. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1288–R1296. doi: 10.1152/ajpregu.00576.2004. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Sill RV, Aravamudan B, Zhan WZ, Sieck GC. Developmental effects on myonuclear domain size of rat diaphragm fibers. J Appl Physiol. 2008;104:787–794. doi: 10.1152/japplphysiol.00347.2007. [DOI] [PubMed] [Google Scholar]
- McCall GE, Allen DL, Linderman JK, Grindeland RE, Roy RR, Mukku VR, Edgerton VR. Maintenance of myonuclear domain size in rat soleus after overload and growth hormone/IGF-I treatment. J Appl Physiol. 1998;84:1407–1412. doi: 10.1152/jappl.1998.84.4.1407. [DOI] [PubMed] [Google Scholar]
- Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- Morey-Holton E, Globus RK, Kaplansky A, Durnova G. The hindlimb unloading rat model: literature overview, technique update and comparison with space flight data. Adv Space Biol Med. 2005;10:7–40. doi: 10.1016/s1569-2574(05)10002-1. [DOI] [PubMed] [Google Scholar]
- Moss FP. The relationship between the dimensions of the fibers and the number of nuclei during normal growth of skeletal muscle in the domestic fowl. Am J Anat. 1968;122:555–564. doi: 10.1002/aja.1001220308. [DOI] [PubMed] [Google Scholar]
- Pavlath GK, Rich K, Webster SG, Blau HM. Localization of muscle gene products in nuclear domains. Nature. 1989;337:570–573. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- Podhorska-Okolow M, Sandri M, Zampieri S, Brun B, Rossini K, Carraro U. Apoptosis of myofibres and satellite cells: exercise-induced damage in skeletal muscle of the mouse. Neuropathol Appl Neurobiol. 1998;24:518–531. doi: 10.1046/j.1365-2990.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- Pulkkanen KJ, Laukkanen MO, Naarala J, Yla-Herttuala S. False-positive apoptosis signal in mouse kidney and liver detected with TUNEL assay. Apoptosis. 2000;5:329–333. doi: 10.1023/a:1009631424351. [DOI] [PubMed] [Google Scholar]
- Rodrigues Ade C, Schmalbruch H. Satellite cells and myonuclei in long-term denervated rat muscles. Anat Rec. 1995;243:430–437. doi: 10.1002/ar.1092430405. [DOI] [PubMed] [Google Scholar]
- Roy RR, Monke SR, Allen DL, Edgerton VR. Modulation of myonuclear number in functionally overloaded and exercised rat plantaris fibers. J Appl Physiol. 1999;87:634–642. doi: 10.1152/jappl.1999.87.2.634. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H, Lewis DM. Dynamics of nuclei of muscle fibers and connective tissue cells in normal and denervated rat muscles. Muscle Nerve. 2000;23:617–626. doi: 10.1002/(sici)1097-4598(200004)23:4<617::aid-mus22>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Seiden D. Quantitative analysis of muscle cell changes in compensatory hypertrophy and work-induced hypertrophy. Am J Anat. 1976;145:459–465. doi: 10.1002/aja.1001450405. [DOI] [PubMed] [Google Scholar]
- Siu PM, Alway SE. Mitochondria-associated apoptotic signalling in denervated rat skeletal muscle. J Physiol. 2005;565:309–323. doi: 10.1113/jphysiol.2004.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Alway SE. Apoptotic responses to hindlimb suspension in gastrocnemius muscles from young adult and aged rats. Am J Physiol Regul Integr Comp Physiol. 2005a;289:R1015–R1026. doi: 10.1152/ajpregu.00198.2005. [DOI] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences the cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2004;288:C338–349. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2005b;288:C338–C349. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- Steffen JM, Musacchia XJ. Disuse atrophy, plasma corticosterone, and muscle glucocorticoid receptor levels. Aviat Space Environ Med. 1987;58:996–1000. [PubMed] [Google Scholar]
- Strassburger E. Ûber die Wirkungssphäre der kerne und die zellgrösse. Histol Beitr. 1893;5:97–124. [Google Scholar]
- Tang H, Cheung WM, Ip FC, Ip NY. Identification and characterization of differentially expressed genes in denervated muscle. Mol Cell Neurosci. 2000;16:127–140. doi: 10.1006/mcne.2000.0864. [DOI] [PubMed] [Google Scholar]
- Tews DS. Muscle-fiber apoptosis in neuromuscular diseases. Muscle Nerve. 2005;32:443–458. doi: 10.1002/mus.20348. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH, Schneider I, Gunkel A, Stennert E, Neiss WF. DNA-fragmentation and expression of apoptosis-related proteins in experimentally denervated and reinnervated rat facial muscle. Neuropathol Appl Neurobiol. 1997;23:141–149. [PubMed] [Google Scholar]
- Trachtenberg JT. Fiber apoptosis in developing rat muscles is regulated by activity, neuregulin. Dev Biol. 1998;196:193–203. doi: 10.1006/dbio.1998.8871. [DOI] [PubMed] [Google Scholar]
- Utvik JK, Nja A, Gundersen K. DNA injection into single cells of intact mice. Hum Gene Ther. 1999;10:291–300. doi: 10.1089/10430349950019075. [DOI] [PubMed] [Google Scholar]
- Viguie CA, Lu DX, Huang SK, Rengen H, Carlson BM. Quantitative study of the effects of long-term denervation on the extensor digitorum longus muscle of the rat. Anat Rec. 1997;248:346–354. doi: 10.1002/(SICI)1097-0185(199707)248:3<346::AID-AR7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wada KI, Katsuta S, Soya H. Natural occurrence of myofiber cytoplasmic enlargement accompanied by decrease in myonuclear number. Jpn J Physiol. 2003;53:145–150. doi: 10.2170/jjphysiol.53.145. [DOI] [PubMed] [Google Scholar]
- Wada KI, Takahashi H, Katsuta S, Soya H. No decrease in myonuclear number after long-term denervation in mature mice. Am J Physiol Cell Physiol. 2002;283:C484–C488. doi: 10.1152/ajpcell.00025.2002. [DOI] [PubMed] [Google Scholar]
- Winchester PK, Gonyea WJ. A quantitative study of satellite cells and myonuclei in stretched avian slow tonic muscle. Anat Rec. 1992;232:369–377. doi: 10.1002/ar.1092320306. [DOI] [PubMed] [Google Scholar]
- Yoshimura K, Harii K. A regenerative change during muscle adaptation to denervation in rats. J Surg Res. 1999;81:139–146. doi: 10.1006/jsre.1998.5504. [DOI] [PubMed] [Google Scholar]