Abstract
Stress urinary incontinence (SUI) development is strongly correlated with vaginal childbirth, particularly increased duration of the second stage of labor. However, the mechanisms of pelvic floor injury leading to SUI are largely unknown. The aim of this study was to determine the effects of increased duration of vaginal distension (VD) on voiding cystometry, leak point pressure testing, and histology. Sixty-nine virgin female rats underwent VD with an inflated balloon for either 1 or 4 h, while 33 age-matched rats were sham-VD controls. Conscious cystometry, leak point pressure testing, and histopathology were determined 4 days, 10 days, and 6 wk after VD. The increase in abdominal pressure to leakage (LPP) during leak point pressure testing was significantly decreased in both distension groups 4 days after distension, indicative of short-term decreased urethral resistance. Ten days after VD, LPP was significantly decreased in the 4-h but not the 1-h distension group, indicating that a longer recovery time is needed after longer distension duration. Six weeks after VD, LPP was not significantly different from sham-VD values, indicating a return toward normal urethral resistance. In contrast, 6 wk after VD of either duration, the distended rats had not undergone the same increase in voided volume as the sham-VD group, suggesting that some effects of VD do not resolve within 6 wk. Both VD groups demonstrated histopathological evidence of acute injuries and tissue remodeling. In conclusion, this experiment suggests pressure-induced hypoxia as a possible mechanism of injury in vaginal delivery.
Keywords: rat, urodynamics, leak point pressure, histology
Vaginal Delivery of Children is often associated with traumatic injury to pelvic floor tissues including muscles, nerves, and connective tissue. This is in turn related to a 40% prevalence of stress urinary incontinence (SUI), the involuntary leakage of urine during moments of increased abdominal pressure, such as when laughing, coughing, sneezing, or lifting (1, 24, 42, 44, 45). Vaginal delivery, especially with a prolonged duration of the second stage of labor, can damage the external urethral sphincter (EUS), the skeletal muscle of the urethra, as well as the smooth muscle of the urethra, vaginal smooth muscle, and other pelvic floor structures via direct compression or indirect hypoxic mechanisms (17, 20, 39, 43). The development of SUI after vaginal delivery might be multifactorial in origin (9, 17), but the precise mechanisms of organ and tissue injury that can eventually lead to incontinence are not yet fully understood.
Animal models could be useful for studying the mechanisms of injury and pathophysiology of SUI. The arrangements of smooth and striated muscles of the urethra are similar in female rats and humans (25, 33, 37), and the relevant innervation has been well studied (23). We have developed a method of measuring the increase in pressure to leakage (LPP) during leak point pressure testing in rats to indicate the development of decreased urethral resistance (5). It has been determined to be useful, reliable, sensitive, and repeatable for this purpose and is presumed to indicate evidence of SUI in rats (5, 7, 11, 12, 31).
It is likely that the function and viability of muscle and nervous tissues are highly dependent on the integrity of blood supply. On the basis of our previous work demonstrating hypoxia of the bladder, urethra, and vagina with vaginal distension (VD) (13), we hypothesized that increased duration of VD in female rats will result in decreased urethral resistance and increased time to recovery in a rat model of simulated childbirth injuries. The present study was undertaken to determine the effects of increased duration of VD on 1) voiding cystometry, 2) leak point pressure testing, and 3) histopathology of the urethra.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee of Loyola University of Chicago Stritch School of Medicine and Hines Department of Veterans Affairs Medical Center. To decrease possible variations in anatomy and function due to prior vaginal deliveries (23), 102 virgin female Sprague-Dawley rats (weight range 180-200 g) were used and randomized into three groups: 1-h VD, 4-h VD, and sham VD. Each group was further divided into three subgroups, with urodynamic testing at 4 days, 10 days, and 6 wk after VD, respectively.
Vaginal distension
Rats underwent VD under intraperitoneal ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia as previously described (7, 14), with supplementation as needed. To avoid rupturing the vagina, it was first accommodated to larger capacities by subsequently inserting and removing increasing sizes (24-32 Fr) of Otis bougie á boule urethral dilators (Allegiance Health Care, McGaw Park, IL) lubricated with Surgilube (E. Fougera, Melville, NY). A modified 10-Fr Foley catheter (Lifetech, Stafford, TX) was then inserted into the vagina, and the balloon was slowly distended with water to 3 ml. After either 1 or 4 h, the catheter was deflated. Those animals undergoing VD for 1 h remained anesthetized for an additional 3 h with the catheter in place but deflated to match the anesthesia conditions of those undergoing VD for 4 h. The vagina of sham-distended rats was accommodated with urethral dilators, and a Foley catheter was inserted into the vagina for 4 h but the balloon was not inflated.
Catheter implantation
Two days before urodynamic testing, all rats underwent suprapubic bladder catheter implantation as previously described (11, 12). The rats were anesthetized as described above, and a circular purse-string suture was placed on the bladder wall. A small incision was made in the bladder wall, and the catheter (PE-50 tubing with a flared tip) was implanted in the bladder dome. The catheter was then tunneled subcutaneously to the back of the neck, where it exited the skin. The catheter was capped, and the incision was closed in two layers.
Conscious cystometry
Conscious cystometry was performed via the suprapubic bladder catheter 4 days, 10 days, or 6 wk after VD as described previously (11, 12). The bladder catheter was connected to both a syringe pump (model 200; KD Scientific, New Hope, PA) and a pressure transducer (model P300; Grass Instruments, West Warwick, RI). All bladder pressures were referenced to air pressure at the level of the bladder. Each bladder was filled with saline via the catheter at 5 ml/h while bladder pressure and voided volume were continuously recorded. A voiding contraction was defined as a bladder pressure increase that resulted in urine loss as detected by a force transducer (model FT10; Grass Instruments) under the cage that was calibrated to measure volume. Three fills and voids were recorded on each rat. Pressure and force signals were amplified (model P122; Grass Instruments), recorded on a chart recorder, and digitized (10 samples/s) for computer data collection. Mean bladder baseline pressure, mean voided volume, mean peak voiding pressure, and mean increase in bladder pressure for voiding (peak voiding pressure minus bladder baseline pressure) were calculated for each animal.
Leak point pressure testing
After cystometry, the animals were anesthetized with urethane (1.2 g/kg body wt ip) and placed supine at the level of zero pressure for leak point pressure testing as previously described (11, 12). Urethane was used because it best maintains voiding reflexes under anesthesia (5). The bladder catheter was connected via a stopcock to the pressure transducer and flow pump. Pressure signals were amplified, recorded on a chart recorder, and digitized for computer data collection (10 samples/s) as above. The bladder was palpated to empty and filled with saline at 5 ml/h via the flow pump. When 0.3 ml was attained (approximately half the capacity of a 200-g rat), gentle pressure was applied with one finger to the rat’s abdomen to increase bladder pressure while bladder pressure was recorded and digitized. Pressure was slowly increased until the rat leaked saline through the urethra. At the first indication of leakage at the urethral meatus, the externally applied abdominal pressure was rapidly removed. Peak pressure at leakage in the absence of a detrusor contraction was recorded. LPP was calculated by subtracting bladder baseline pressure from peak bladder pressure. LPP provides a measure of urethral resistance with consistent results and is not sensitive to volume in the bladder (5, 10, 31). In general, this external application of gentle pressure does not generate a voiding reflex (5, 12). If a voiding reflex occurred, the bladder was emptied and refilled and that test was not included in the results. The bladder was drained and refilled, and the study was repeated three times in each rat. Mean bladder baseline pressure and mean LPP were calculated for each rat.
Histology
Immediately after leak point pressure testing, the anesthetized animals were euthanized and the urethra and vagina were dissected en bloc at the level of the EUS for histological analysis. Tissues from animals euthanized 4 or 10 days after VD were immersion fixed in 10% formalin, processed, embedded in paraffin, sectioned transversely (5 μm), and stained with Masson’s trichrome for qualitative histological analysis to study the effects of VD on the urethra and vagina. Animals euthanized 6 wk after VD underwent intracardiac perfusion fixation as follows. An injection of heparin (0.2 ml) was made in the left ventricle, and perfusion was initiated with a washout of warm phosphate-buffered saline (100 ml), followed by cold fixative (400 ml of 2.5% glutaraldehyde and 0.5% paraformaldehyde in 0.1 M cacodylate buffer). The urethra and vagina were dissected en bloc at the level of the EUS and were postfixed, osmicated (2% osmium tetroxide), embedded in epon-araldite, sectioned transversely (1 μm), and stained with methylene blue azure II for qualitative histological analysis to study the effects of VD on distal nerve fascicle regeneration.
Data analysis
The data are a series of independent measurements made on all animals in this study. All analysis was done with SAS (version 9.1). Quantitative results are displayed as box plots (in which the midline represents the median, the top and bottom of the box are 75th and 25th percentiles, respectively, and the top and bottom bars are 90th and 10th percentiles, respectively). The response measures were log normally distributed; consequently, the log of all responses was taken before analysis. The data were examined with generalized linear models. The independent measures were VD duration and elapsed time after injury. Both of these measurements are continuous; however, to facilitate the computation and comparison of response means it was necessary to treat both VD duration and elapsed time from injury as class variables. Two-way ANOVA was used to compare response means across VD times, elapsed time from injury, and the interaction between the two. The pairwise means comparisons were made with the Tukey-Kramer correction for multiple comparisons. There were fewer significant differences when the test was reduced to pairwise comparisons within the elapsed times and VD durations. In all cases a difference between mean measurements with P > 0.05 was considered significant. Histological data were analyzed qualitatively.
RESULTS
Conscious cystometry
Mean voided volume significantly increased in sham-VD animals 6 wk after distension compared with 4 days and 10 days after sham distension (Fig. 1A). Although bladder baseline pressure increased 4 days after either 1- or 4-h VD, this difference was only significant when the comparison was made between 4 and 10 days after a 1-h distension (Fig. 1B). There were no significant changes in the increase in bladder pressure for voiding between the shamoperated and VD groups at all time points (Fig. 1C).
Fig. 1.
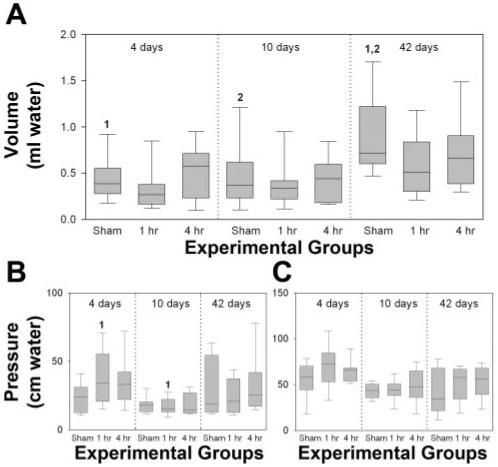
Cystometry results 4 days, 10 days, and 6 wk after a sham, 1 h, or 4 h vaginal distension. A: voided volume. B: bladder baseline pressure. C: increase in bladder pressure for voiding. Each box plot represents the median and 10th, 25th, 75th, and 90th percentiles of data from 11 or 12 individual animals. Paired numbers indicate a significant difference between 2 groups (P < 0.05).
Leak point pressure testing
There were no significant differences in baseline pressure between the sham-operated group and VD groups of either 1-h or 4-h duration at different time points (Fig. 2A). LPP, however, was significantly reduced 4 days after either 1-h or 4-h VD, compared with the sham distension group, indicative of decreased urethral resistance (Fig. 2B). Both 10 days and 6 wk after 1-h VD, LPP was not significantly different compared with the sham-distended group and was significantly increased compared with 4 days after 1 h VD (Fig. 2B). Ten days after a 4-h VD, but not after a 1-h VD, LPP was significantly decreased compared with sham VD, indicating slower recovery time compared with 1-h VD (Fig. 2B). Six weeks after VD, LPP was significantly increased compared with 4 days after either 1-h or 4-h VD and 10 days after 4-h VD and was not significantly different from the sham-VD group (Fig. 2B).
Fig. 2.
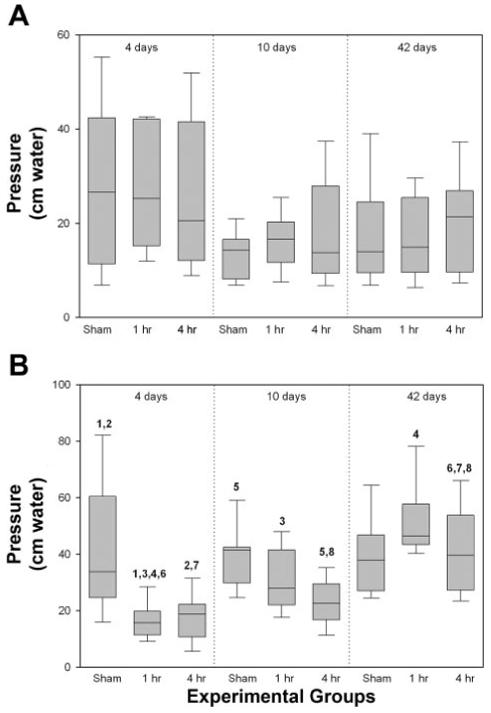
Leak point pressure testing results 4 days, 10 days, and 6 wk after a sham, 1-h, or 4-h vaginal distension. A: bladder baseline pressure. B: increase in abdominal pressure to leak (LPP). Each box plot represents the median and 10th, 25th, 75th, and 90th percentile of data from 11 or 12 individual animals. Paired numbers indicate a significant difference between 2 groups (P < 0.05).
Histology
Four days after either 1-h or 4-h VD, the urethra displayed extensive disruption of the skeletal muscle layer with marked thinning and paucity of EUS muscle fibers compared with the normal-appearing sham-VD group (Fig. 3). Focal inflammatory infiltrates were observed in the submucosa of the vagina, and extravasated red blood cells and vascular proliferations were found in the border between the vagina and urethra of some animals (Fig. 4). Both distension groups also demonstrated a decline in the connective tissue component of the submucosa of the bladder 10 days after VD (Fig. 4). In the stained semithin plastic sections, the previous histological changes as seen in paraffin sections were confirmed as shown at 6 wk (Fig. 5). There was some evidence for increased smooth muscle densities and increased thickness of arterial walls in the urethra 6 wk after distension compared with the sham-distension group (Fig. 5).
Fig. 3.
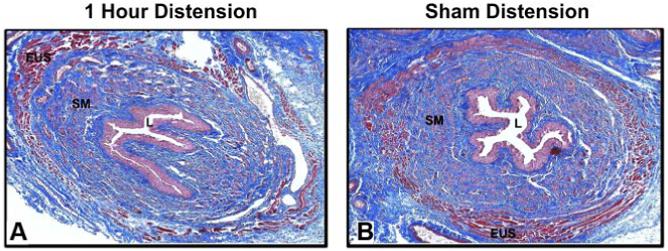
Light micrographs of urethra 4 days after 1-h vaginal distension (A) and sham vaginal distension (B). Stain = Masson’s trichrome. Magnification = ×4. EUS, external urethral sphincter; L, urethral lumen; SM, smooth muscle.
Fig. 4.
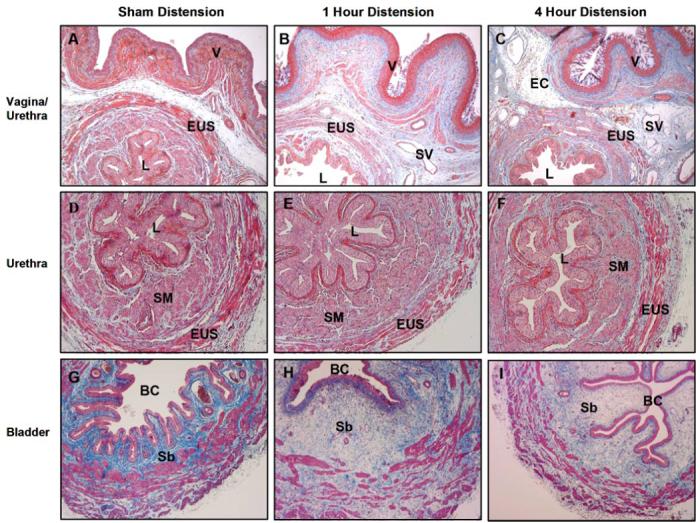
Light micrographs of urethra, bladder, and vagina 10 days after a sham (A, D, G), short-duration (1 h; B, E, H), or long-duration (4 h; C, F, I) vaginal distension. Stain = Masson’s trichrome. Magnification = ×4(A, B, C, G, H, I) or ×10 (D, E, F). BC, bladder cavity; EC, extravasated cells; Sb, submucosa; SV, small vessel; V, vaginal epithelium.
Fig. 5.
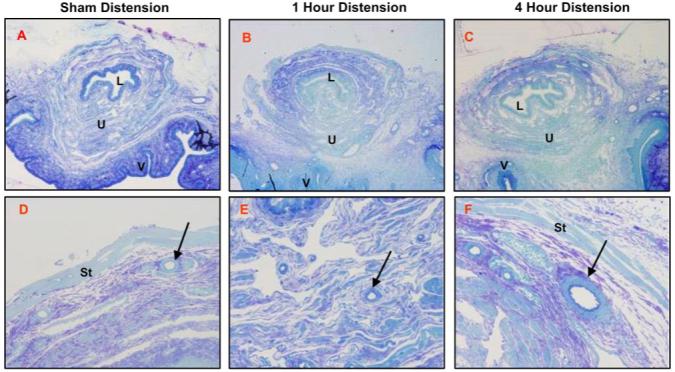
Light micrographs of urethra 6 wk after a sham (A, D), short-duration (1 h; B, E), or long-duration (4 h; C, F) vaginal distension. Stain = methylene blue azure II. Magnification = ×4 (A-C), ×20 (D-F). St, striated muscle; U, urethral submucosa; arrow, blood vessel.
DISCUSSION
SUI is a distressing disorder that results in a restricted lifestyle, impacts confidence and self-perception, and interferes with social relationships. Although symptoms may wax and wane over time, it is very common in women during puerperium (21, 26) and is believed to be considerably underreported (18). Multiple vaginal deliveries can result in multiple injuries because damaged tissue caused by each delivery may not fully recover each time; however, the first vaginal delivery produces the greatest risk of SUI (2). Cesarean sections are believed to have a protective effect on the pelvic floor but probably only for the first delivery and less for multiple births (19, 21, 36). Vaginal delivery, therefore, represents a potent determinant of SUI, carrying from 1.5- to 3-fold the risk of cesarean section (15, 17, 32, 37).
Cystometry involves assessing the bladder and urethra during filling and voiding and is used to determine abnormalities in bladder sensation, capacity, and compliance and inappropriate detrusor activity. These studies can give further insight into the cause of the condition, although multiple technical factors may influence its results, including the choice of distending medium, type of catheter, and bladder fill rate (29, 38). Clinically, leak point pressure is a urodynamic measurement of the minimum intra-abdominal pressure required to cause leakage during sustained strain and is believed to reflect urethral function and predict treatment outcome. Although no cutoff values of LPP measurement have been shown to reliably predict urethral dysfunction, LPP seems to be highly reproducible when performed in a standardized fashion (4, 30).
The rat model of simulated birth trauma allows an assessment of the effects of prolonged dilation and compression of pelvic floor tissues on a variety of outcomes. Behavioral and functional outcomes have been used to demonstrate urethral dysfunction and induced symptoms of SUI after VD, including voiding behavioral studies (22, 23), a sneeze test (6, 28, 41), LPP testing (5, 7), modified LPP testing (3, 41), maximum urethral closure pressure (34), and a vertical tilt table test (10, 16). We have developed and tested a method of mimicking clinical LPP by manually increasing abdominal pressure, which measures urethra resistance to leakage, and have shown that it is a reliable and repeatable measure (7, 12, 31). While LPP may not fully represent the mechanism of SUI in humans, it provides an indicator of urethral resistance and bladder outlet function.
In our study, mean voided volume in sham-distended animals was significantly increased 6 wk after distension. However, both 1-h and 4-h VD groups failed to demonstrate this time-dependent change. This suggests that 1-h and 4-h VD produced damage to the bladder and/or continence mechanism that resulted in reduced bladder capacity and long-term dysfunction. Bakircioglu et al. (3) reported that the bladder capacity 6 wk postpartum in rats was similar to that of virgin rats, although bladder capacity was greater in pregnant and 2-day postpartum rats than in virgin and 6-wk postpartum rats. Higher bladder capacity was also found in day 0 postpartum rats than in virgin rats (40). The increased bladder capacity during pregnancy and 0-2 days postpartum was not observed 4 days after VD in our study. It is therefore likely caused by bladder dysfunction due to hormones or urine retention during pregnancy, or after vaginal delivery. Our data also showed that bladder peak voiding pressure only increased significantly 4 days after both 1-h and 4-h VD compared with sham distension and suggested that the increase in cystometric bladder baseline pressure is a short-term response to VD, possibly due to ischemic injury to the bladder (13), and resolves within 10 days.
LPP was significantly decreased in both 1-h and 4-h VD groups 4 days after distension compared with that in sham-distended rats, indicative of a short-term decrease in urethral resistance. This outcome was comparable to the decrease in modified LPP observed immediately and 2 days postpartum in rats (3, 40), suggesting that this model could be used to study the effects of childbirth. Ten days after distension, LPP was significantly decreased only in the 4-h distension group, indicating that a longer recovery time is needed after a longer distension duration. However, 6 wk after VD, LPP was significantly increased compared with 4 days after either 1- or 4-h VD and 10 days after 4-h VD and was not significantly different from sham-VD values, indicating a return toward normal urethral resistance. The only functional outcome that remained significantly different between the groups 6 wk after VD was cystometric capacity, suggesting that not all the effects of VD fully resolve within 6 wk. In contrast, one study reported that maximum urethral closure pressure was still significantly lower in rats even 9 mo after VD (34).
Clinically, high birth weight, large head circumference, and long duration of the second stage of labor have been correlated with both pelvic floor injuries and incontinence development (14, 21, 35). Our previous work (7) with the same animal model demonstrated that increased duration of VD from 0.5 h to 1 h leads to both increased SUI symptoms and greater tissue damage, suggesting an ischemic mechanism. Similar rat models of simulated vaginal delivery produce symptoms of SUI as well as injury to the EUS (11, 27). In addition, we previously demonstrated (13) that blood flow to the urethra and vagina was significantly decreased just before release of a 1-h VD, then tripled immediately after release of VD; and both 15 min and 1 h after release of VD blood flow to the urethra and vagina was not significantly different from sham distension at the same time points, suggesting a rapid reperfusion as blood flow returned to the urethra and vagina after 1-h distension. The results of the present study are consistent with this previous work in that they demonstrate increased recovery time after longer distension duration, which could be a result of increased ischemic or reperfusion injury. This similarity in correlative clinical and experimental events suggests a specific mechanism of pelvic organ and tissue injury: ischemic and/or reperfusion injury of pelvic floor organs and tissues incurred during vaginal delivery contributes to later development of SUI.
We chose to use a sham-distended group as a control rather than a nondistended control group to control for factors in this study such as distension and accommodation of the vagina. We previously demonstrated (7) no difference in LPP between a 1-h sham-distended group and a nondistended control group; however, we have also shown (13) that sham distension produces hypoxia of the bladder and vagina. In the present study, the only variable to change with time after sham distension was voided volume, which increased 6 wk after distension. This change could be due to a long-term effect of the sham distension. However, if that were true, one would expect to see a greater effect in the VD groups, rather than the lesser effect we observed. Since all animals were age matched at the time of distension, we suspect the increase in voided volume with time is a function of the increased capacity of larger, aged animals 6 wk after distension, compared with 4 or 10 days after distension. The decreased effect in the distension groups 6 wk after distension may be due to urinary leakage, which may have the effect of reducing mean voided volume compared with the sham-distended group.
Hemorrhage and inflammation in the distension groups are both indicative of marked acute injuries that may not completely recover within 6 wk. Focal areas of increased smooth muscle density and increased thickness of arterial walls in the urethra are likely from tissue remodeling produced by exposure to hypoxia, as has been previously demonstrated to occur in VD (13). Resplande et al. (34) reported that increased apoptosis occurred in the urethra and connective tissue increased in the vagina 9 mo after a simulated childbirth injury in rats with VD. Sievert and colleagues (40) demonstrated significantly decreased caveolin-3 at the EUS with delivery of rat pups plus VD, although not with delivery of rat pups alone. Since caveolin-3 is known to concentrate at the neuromuscular junctions of striated muscle (8), this could indicate damage to the neuromuscular junctions from VD, which likely occurred in our experiment as well.
In conclusion, we have demonstrated in a rat model of simulated childbirth that as the duration of vaginal distension is increased the recovery of the resulting dysfunction is also prolonged. Furthermore, the functional and structural recovery from vaginal distension injury is not fully complete by 6 wk.
ACKNOWLEDGMENTS
We thank R. Sam Butler for statistical advice and guidance.
GRANTS
This material is based on work supported by the Office of Research and Development, Rehabilitation Research and Development Service of the Department of Veterans Affairs and National Institute of Child Health and Human Development Grant RO1-HD-038679-05.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked ‘advertisement’ in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen RE, Hosker GL, Smith ARB, Warrell DW. Pelvic floor damage and childbirth: a neurophysiological study. Br J Obstet Gynaecol. 1990;97:770–779. doi: 10.1111/j.1471-0528.1990.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 2.Arya LA, Jackson ND, Myers DL, Verma A. Risk of new onset urinary incontinence after forceps and vacuum delivery in primiparous women. Am J Obstet Gynecol. 2001;185:1318–1323. doi: 10.1067/mob.2001.120365. [DOI] [PubMed] [Google Scholar]
- 3.Bakircioglu ME, Sievert KD, Lau A, Lin CS, Lue TF. The effect of pregnancy and delivery on the function and ultrastructure of the rat bladder and urethra. BJU Int. 2000;85:350–361. doi: 10.1046/j.1464-410x.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- 4.Bump RC, Elser DM, Theofrastous JP, McClish DK. Valsalva leak point pressures in women with genuine stress incontinence: reproducibility, effect of catheter caliber, and correlations with other measures of urethral resistance. Am J Obstet Gynecol. 1995;173:551–557. doi: 10.1016/0002-9378(95)90281-3. [DOI] [PubMed] [Google Scholar]
- 5.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001;69:1193–1202. doi: 10.1016/s0024-3205(01)01182-1. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TW, Sweeney DD, Conway DA, Kamo I, Yoshmura N, Sacks M, Chancellor MB. A tissue-engineered suburethral sling in an animal model of stress urinary incontinence. BJU Int. 2005;96:664–669. doi: 10.1111/j.1464-410X.2005.05702.x. [DOI] [PubMed] [Google Scholar]
- 7.Cannon TW, Wojcik EM, Ferguson CL, Saraga S, Thomas C, Damaser MS. Effects of vaginal distension on urethral anatomy and function. BJU Int. 2002;90:403–407. doi: 10.1046/j.1464-410x.2002.02918.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlson BM, Carlson JA, Dedkov EI, McLennan IS. Concentration of caveolin-3 at the neuromuscular junction in young and old rat skeletal muscle fibers. J Histochem Cytochem. 2003;51:1113–1118. doi: 10.1177/002215540305100901. [DOI] [PubMed] [Google Scholar]
- 9.Chancellor MB, Perkin H, Yoshimura N. Recent advances in the neurophysiology of stress urinary incontinence. Scand J Urol Nephrol. 2005;39:21–24. doi: 10.1080/00365590410002474. [DOI] [PubMed] [Google Scholar]
- 10.Conway DA, Kamo I, Yoshimura N, Chancellor MB, Cannon TW. Comparison of leak point pressure methods in an animal model of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:359–363. doi: 10.1007/s00192-004-1263-4. [DOI] [PubMed] [Google Scholar]
- 11.Damaser MS, Broxton-King C, Ferguson C, Kim FJ, Kerns JM. Functional and neuroanatomical effects of vaginal distention and pudendal nerve crush in the female rat. J Urol. 2003;170:1027–1031. doi: 10.1097/01.ju.0000079492.09716.43. [DOI] [PubMed] [Google Scholar]
- 12.Damaser MS, Kim FJ, Minetti GM. Methods of testing urethral resistance in the female rat. Bladder disease: research concepts and clinical applications. Adv Exp Med Biol. 2003;539:831–839. doi: 10.1007/978-1-4419-8889-8_51. [DOI] [PubMed] [Google Scholar]
- 13.Damaser MS, Whitbeck C, Chichester P, Levin RM. Effect of vaginal distension on blood flow and hypoxia of urogenital organs of the female rat. J Appl Physiol. 2005;98:1884–1890. doi: 10.1152/japplphysiol.01071.2004. [DOI] [PubMed] [Google Scholar]
- 14.Dietz HP. Levator function before and after childbirth. Aust NZ J Obstet Gynaecol. 2004;44:19–23. doi: 10.1111/j.1479-828X.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 15.Foldspang A, Hvidman L, Mommsen S, Nielsen JB. Risk of postpartum urinary incontinence associated with pregnancy and model of delivery. Acta Obstet Gynecol Scand. 2004;83:923–927. doi: 10.1111/j.0001-6349.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 16.Fraser MO, de Groat WC, Chancellor MB. Creation of a new stress incontinence model in the rat by using vertical tilt table and surgical or pharmacological manipulation of internal and external sphincter activity with measurement of leak point pressure (LPP) (Abstract) J Urol Suppl. 2000;163:76. [Google Scholar]
- 17.Fritel X, Fauconnier A, Levet C, Benifla JL. Stress urinary incontinence 4 years after the first delivery: a retrospective cohort survey. Acta Obstet Gynecol Scand. 2004;83:941–945. doi: 10.1111/j.0001-6349.2004.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fultz NH, Burgio K, Diokno AC, Kinchen KS, Obenchian R, Bump RC. Burden of stress urinary incontinence for community-dwelling women. Am J Obstet Gynecol. 2003;189:1275–1282. doi: 10.1067/s0002-9378(03)00598-2. [DOI] [PubMed] [Google Scholar]
- 19.Gilleran JP, Zimmern P. An evidence-based approach to the evaluation and management of stress incontinence in women. Curr Opin Urol. 2005;15:236–243. doi: 10.1097/01.mou.0000172396.54643.96. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg RP, Abramov Y, Botros S, Miller JJ, Gandhi S, Nickolov A, Sherman W, Sand PK. Delivery mode is a major environmental determinant of stress urinary incontinence: results of the Evanston-Northwestern Twin Sisters Study. Am J Obstet Gynecol. 2005;193:2149–2153. doi: 10.1016/j.ajog.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 21.Groutz A, Rimon E, Peled S, Gold R, Pauzner D, Lessing JB, Gordon D. Cesarean section: does it really prevent the development of postpartum stress urinary incontinence? A prospective study of 363 women one year after their first delivery. Neurourol Urodyn. 2004;23:2–6. doi: 10.1002/nau.10166. [DOI] [PubMed] [Google Scholar]
- 22.Heidkamp MC, Leong FC, Brubaker L, Russell B. Pudendal denervation affects the structure and function of the striated, urethral sphincter in female rats. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:88–93. doi: 10.1007/BF01982215. [DOI] [PubMed] [Google Scholar]
- 23.Kerns JM, Damaser MS, Kane JM, Sakamoto K, Benson JT, Shott S, Brubaker L. Effects of pudendal nerve injury in the female rat. Neurourol Urodyn. 2000;19:53–69. doi: 10.1002/(sici)1520-6777(2000)19:1<53::aid-nau7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim HL, Gerber GS, Patel RV, Hollowell CM, Bales GT. Practice patterns in the treatment of female urinary incontinence: a postal and internet survey. Urology. 2001;57:45–48. doi: 10.1016/s0090-4295(00)00885-2. [DOI] [PubMed] [Google Scholar]
- 25.Kim RJ, Kerns JK, Liu S, Nagel T, Zaszczurynski P, Lin DL, Damaser MS. Striated muscle and nerve fascicle distribution in the female rat urethral sphincter. Anat Rec. 2007;290:145–154. doi: 10.1002/ar.20420. [DOI] [PubMed] [Google Scholar]
- 26.Klein MC, Kaczorowski J, Firoz T, Hubinette M, Jorgensen S, Gauthier R. A comparison of urinary and sexual outcomes in women experiencing vaginal and Caesarean births. J Obstet Gynaecol Can. 2005;27:332–339. doi: 10.1016/s1701-2163(16)30459-5. [DOI] [PubMed] [Google Scholar]
- 27.Kuo HC. Effects of vaginal trauma and oophorectomy on the continence mechanism in rats. Urol Int. 2002;69:36–41. doi: 10.1159/000064358. [DOI] [PubMed] [Google Scholar]
- 28.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143–151. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 29.Low JA, Mauger GM, Dragovic J. Diagnosis of the unstable detrusor: comparison of an incremental and continuous infusion technique. Obstet Gynecol. 1985;65:99–103. [PubMed] [Google Scholar]
- 30.McGuire EJ, Fitzpatrick CC, Wan J, Bloom D, Sanvordenker J, Ritchey M, Gormley EA. Clinical assessment of urethral sphincter function. J Urol. 1993;150:1452–1454. doi: 10.1016/s0022-5347(17)35806-8. [DOI] [PubMed] [Google Scholar]
- 31.Peng CW, Chen JJ, Chang HY, de Groat WC, Cheng CL. External urethral sphincter activity in a rat model of pudendal nerve injury. Neurourol Urodyn. 2006;25:388–396. doi: 10.1002/nau.20229. [DOI] [PubMed] [Google Scholar]
- 32.Peyrat L, Haillot O, Bruyere F, Boutin JM, Bertrand P, Lanson Y. Prevalence and risk factors of urinary incontinence in young and middleaged women. BJU Int. 2002;89:61–66. doi: 10.1046/j.1464-4096.2001.01813.x. [DOI] [PubMed] [Google Scholar]
- 33.Praud C, Sebe P, Mondet F, Sebille A. The striated urethra sphincter in female rats. Anat Embryol (Berl) 2003;207:169–175. doi: 10.1007/s00429-003-0340-7. [DOI] [PubMed] [Google Scholar]
- 34.Resplande J, Gholami SS, Graziottin TM, Rogers R, Lin CS, Leng W, Lue TF. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J Urol. 2002;168:323–30. [PubMed] [Google Scholar]
- 35.Rortveit G, Daltveit AK, Hannestad YS, Hunskaar S. Vaginal delivery parameters and urinary incontinence: the Norwegian EPINCONT study. Am J Obstet Gynecol. 2003;189:1268–1274. doi: 10.1067/s0002-9378(03)00588-x. [DOI] [PubMed] [Google Scholar]
- 36.Rortveit G, Kjersti A, Hannestad Y, Hunskaar S. Urinary incontinence after vaginal delivery or Cesarean section. N Engl J Med. 2003;348:900–907. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 37.Russell B, Baumann M, Heidkamp MC, Svanborg A. Morphometry of the aging female rat urethra. Int Urogynecol J Pelvic Floor Dysfunct. 1996;7:30–36. doi: 10.1007/BF01895103. [DOI] [PubMed] [Google Scholar]
- 38.Sand PK, Hill RC, Ostergard DR. Supine urethroscopic and standing cystometry as screening methods for the detection of detrusor instability. Obstet Gynecol. 1987;70:57–60. [PubMed] [Google Scholar]
- 39.Shafik A, El-Sibai O. Study of the levator ani muscle in the multipara: role of levator dysfunction in defecation disorders. J Obstet Gynaecol. 2002;22:187–192. doi: 10.1080/01443610120113391. [DOI] [PubMed] [Google Scholar]
- 40.Sievert KD, Bakircioglu ME, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. I. Functional and structural change. J Urol. 2001;166:311–317. [PubMed] [Google Scholar]
- 41.Sievert KD, Bakircioglu ME, Tsai T, Nunes L, Lue TF. The effect of labor and/or ovariectomy on rodent continence mechanism—the neuronal changes. World J Urol. 2004;22:244–250. doi: 10.1007/s00345-004-0444-6. SE. [DOI] [PubMed] [Google Scholar]
- 42.Snooks SJ, Swash M, Mathers SE, Henry MM. Effect of vaginal delivery on the pelvic floor: a 5-year follow-up. Br J Surg. 1990;77:1358–1360. doi: 10.1002/bjs.1800771213. [DOI] [PubMed] [Google Scholar]
- 43.Testzschner T, Sorensen M, Jonsson L, Lose G, Christiansen J. Delivery and pudendal nerve function. Acta Obstet Gynecol Scand. 1997;76:324–331. doi: 10.1111/j.1600-0412.1997.tb07986.x. [DOI] [PubMed] [Google Scholar]
- 44.Thom DH, van den Eeden SK, Brown JS. Evaluation of parturition and other reproductive variables as risk factors for urinary incontinence in later life. Obstet Gynecol. 1997;90:983–989. doi: 10.1016/s0029-7844(97)00537-1. [DOI] [PubMed] [Google Scholar]
- 45.Turan C, Zorlu CG, Ekin M, Hancerliogullari N, Saracoglu F. Urinary incontinence in women of reproductive age. Gynecol Obstet Invest. 1996;41:132–134. doi: 10.1159/000292059. [DOI] [PubMed] [Google Scholar]


