Abstract
The importance of soluble N-ethyl maleimide (NEM)-sensitive fusion protein (NSF) attachment protein (SNAP) receptors (SNAREs) in synaptic vesicle exocytosis is well established because it has been demonstrated that clostridial neurotoxins (NTs) proteolyze the vesicle SNAREs (v-SNAREs) vesicle-associated membrane protein (VAMP)/brevins and their partners, the target SNAREs (t-SNAREs) syntaxin 1 and SNAP25. Yet, several exocytotic events, including apical exocytosis in epithelial cells, are insensitive to numerous clostridial NTs, suggesting the presence of SNARE-independent mechanisms of exocytosis. In this study we found that syntaxin 3, SNAP23, and a newly identified VAMP/brevin, tetanus neurotoxin (TeNT)-insensitive VAMP (TI-VAMP), are insensitive to clostridial NTs. In epithelial cells, TI-VAMP–containing vesicles were concentrated in the apical domain, and the protein was detected at the apical plasma membrane by immunogold labeling on ultrathin cryosections. Syntaxin 3 and SNAP23 were codistributed at the apical plasma membrane where they formed NEM-dependent SNARE complexes with TI-VAMP and cellubrevin. We suggest that TI-VAMP, SNAP23, and syntaxin 3 can participate in exocytotic processes at the apical plasma membrane of epithelial cells and, more generally, domain-specific exocytosis in clostridial NT-resistant pathways.
INTRODUCTION
The involvement of N-ethyl maleimide (NEM)-sensitive fusion protein (NSF), soluble NSF attachment proteins (SNAPs), and their receptors on membranes (so-called SNAREs) (Söllner et al., 1993a,b) in most membrane fusion events has been extensively documented in yeast and mammalian cells (for review, see Johannes and Galli, 1998). In particular, the neuronal vesicle SNAREs (v-SNAREs), vesicle-associated membrane protein (VAMP)/synaptobrevin 1 and 2 and their plasma membrane targets (t-SNAREs) SNAP25 and syntaxin 1 have been shown to be involved in calcium-dependent synaptic vesicle exocytosis (Schiavo et al., 1992; Blasi et al., 1993a,b). Interestingly, nonneuronal cells possess homologues of these neuronal SNAREs. These include the v-SNARE cellubrevin (McMahon et al., 1993) and the t-SNAREs syntaxins 2–7 (Bennett et al., 1993; Bock et al., 1996; Wang et al., 1997) and SNAP23 (Ravichandran et al., 1996; Wong et al., 1997). An understanding of the role of the brevins syntaxin 1 and SNAP25 in exocytosis has been greatly aided by the use of clostridial neurotoxins (NTs), i.e., tetanus NT (TeNT) and botulinum NT (BoNT), which specifically proteolyze these proteins (for review, see Niemann et al., 1994; Schiavo et al., 1994). These toxins also have effects on several exocytotic pathways in nonneuronal cells. For instance, TeNT, which cleaves cellubrevin, inhibits transferrin receptor exocytosis in fibroblasts (Galli et al., 1994). Strikingly, several exocytotic events are at least partially insensitive to some NTs (for review, see Johannes and Galli, 1998). For example, although TeNT and BoNT F can inhibit transport of vesicular stomatis virus (VSV) G protein to the basolateral plasma membrane in Madin–Darby canine kidney (MDCK) cells, apical transport of influenza hemagglutinin (HA) is insensitive to both NTs (Ikonen et al., 1995). This led the authors to propose a mechanism of membrane fusion at the apical plasma membrane of epithelial cells independent of v- and t-SNAREs, although they had found HA transport to be sensitive to NEM (Simons and Ikonen, 1997). On the contrary, several groups have localized syntaxin isoforms at the apical plasma membrane of epithelial cells (Gaisano et al., 1996; Low et al., 1996; Mandon et al., 1996; Delgrossi et al., 1997) and proposed a role for these SNAREs in apical docking and fusion (Weimbs et al., 1997). We have searched for a TeNT-insensitive v-SNARE that could be a partner of apical t-SNAREs in epithelial cells. Recently, D’Esposito et al. (1996) identified SYBL1, a synaptobrevin-like gene in Xq28 pseudoautosomal region, which undergoes X inactivation. We find that SYBL1p is TeNT insensitive and propose to name it TeNT-insensitive VAMP (TI-VAMP). Hence, we have tested the localization and biochemical properties of TI-VAMP in CaCo-2 epithelial cells.
MATERIALS AND METHODS
Antibodies
Antibodies against syntaxin 3 (TG1), SNAP23 (TG7), and TI-VAMP (TG11) were raised in rabbit against the cytoplasmic domain of rat syntaxin 3 fused to glutathione S-transferase (GST) (Calakos et al., 1994), recombinant human SNAP23, and the recombinant cytoplasmic domain of human TI-VAMP fused to GST, respectively, and purified on the corresponding recombinant proteins. Antibodies against human cellubrevin (TG2) were obtained in rabbit against the N-terminal peptide MSTGPTAATGSNC of cellubrevin and affinity purified on the cytoplasmic domains of human cellubrevin fused to GST. All of these antibodies reacted specifically with their corresponding antigen by Western blotting and immunoprecipitation (see Results). Monoclonal antibodies against E-cadherin/uvomorulin (DECMA), sec8 (clone 14), ZO-1, and transferrin receptor (H68.4) were from Sigma (St. Louis, MO), Transduction Laboratory (Lexington, KY), Dr. M. Mooseker (Yale University, New Haven, CT), and Dr. I. Trowbridge (Salk Institute, La Jolla, CA), respectively. Secondary Cy-2 or Texas Red–labeled goat anti-rat, anti-mouse, and anti-rabbit immunoglobulins were from Jackson ImmunoResearch (West Grove, PA).
cDNA Constructs and Recombinant Proteins
Full-length cDNA of the human isoforms of cellubrevin, syntaxin 3, SNAP23, and TI-VAMP were obtained by reverse transcription-PCR on CaCo-2 mRNA using standard procedures and the following sets of oligonucleotides: 5′-ATGTCTACAGGTCCAACTGCTG-3′ (hcb5′) with 5′-GCTGGTTCTTCATGAAGAGACA-3′ (hcb3′), 5′-ATGAAGGACCGTCTGGAGCAG-3′ (hstx3-5′) with 5′-TTAATTCAGCCCAACGGAAAG-3′ (hstx3-3′), 5′-TGGGCTTCAGGATGAAGGA-3′ (hstx3-5′ups) with 5′-GCTAGATTGTTAGCTGAGTCA-3′ (hstx3-3′dos), 5′-ATGGATAATCTGTCATCAGAAGAA-3′ (SNAP23-5′) with 5′-TTAGCTGTCAATGAGTTTCTT-3′ (SNAP23-3′), and 5′-AGACTGAAGCCATGGCGATT-3′ (TI-VAMP 5′) with 5′-CTATTTCTTCACACAGCTTGGC-3′ (TI-VAMP3′), respectively. In the case of human syntaxin 3, all the clones that we obtained either with the oligonucleotides hstx3-5′ and hstx3-3′ or with hstx3-5′ups and hstx33-3′dos, which correspond to regions upstream of the starting ATG and downstream of the stop codon, had the following modifications compared with the human syntaxin 3 cDNA sequence deposited in GenBank (accession number U32315): position 519, CACGTC → CAGCTC, which translates into HV → QL; and position 816, AGC → ACG, which translates into S → T. The rat sequence is QL and T at the same positions. In the case of human SNAP23, all our clones had the following modification compared with the sequence deposited in GenBank (accession number U55936): 207GAA → GAG, which is conservative; and 462 GTC → GCC, which translates into V → A, as seen in SNAP23A (GenBank accession number Y09567). No modification was observed in our human clones of cellubrevin, identical to synaptobrevin 3 (GenBank accession number U64520), and in TI-VAMP (GenBank accession number M90418). Fragments of human cellubrevin predicted to contain only the first coiled coil domain (amino acids 1–52, hCB1) and both coiled coil domains (amino acids 1–71, hCB2) and of human TI-VAMP predicted to contain both coiled coils (amino acids 1–179) were obtained by cloning the corresponding cDNA into pGEX vectors (Pharmacia, Saclay, France). SNAP25 and syntaxin 1a clones were described previously (Hayashi et al., 1994).
Immunofluorescence on Cells Grown on Filters
CaCo-2 cells were seeded at confluency (3.105 cells/cm2) on Transwell-Clear filters (Costar, Cambridge, MA) and grown 7–10 d to full polarization (transepithelial resistance, 800 Ω · cm2). The cells were washed in PBS supplemented with 0.1 mM CaCl2, 0.1 mM MgCl2 (PBS+), fixed with methanol at −20°C for 3 min, and rehydrated in PBS+. The filter was then cut and incubated over a drop of PBS containing the primary antibodies, washed three times with PBS, incubated with Cy2-conjugated goat anti-rabbit antibody and Texas Red–conjugated goat anti-mouse or anti-rat antibody, washed three times, and mounted in 90% glycerol in PBS. Confocal laser scanning microscopy was performed using a Leica (Nussloch, Germany) TCS microscope. The images were assembled without modification using Adobe (Mountain View, CA) Photoshop.
Immunogold Labeling on Ultrathin Cryosections
CaCo-2 cells were fixed with 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 1 h at room temperature and processed for ultracryomicrotomy as described elsewhere (Raposo et al., 1997). Ultrathin cryosections were collected using a mixture of 2.3 M sucrose and methylcellulose (vol/vol) and were immunogold labeled with antibodies against syntaxin 3, SNAP23, or TI-VAMP and protein A-gold conjugates (PAG 10, Department of Cell Biology, Utrecht University, Utrecht, The Netherlands) (Raposo et al., 1997). No labeling was observed with protein A-gold alone or nonimmune serum and protein A-gold.
In Vitro Assays
The effect of light chains of the clostridial NTs was tested according to the procedure of Hayashi et al. (1994). Briefly, the cDNAs of syntaxin 3, cellubrevin, and TI-VAMP cloned in pCR3 (Invitrogen, NV Leek, The Netherlands), of SNAP23 cloned in pET15b (Novagen, R & D Systems, Abingdon, UK), and of SNAP25 and syntaxin 1a (Hayashi et al., 1994) were translated in vitro using T7 polymerase and rabbit reticulocyte lysate in the presence of [35S]methionine. The proteins were incubated with the light chains of TeNT (2 μM) and BoNT A (0.8 μM), B (2 μM), C (0.9 μM), D (2 μM), E (0.4 μM), F (2 μM), and G (2 μM) in 150 mM potassium glutamate, 10 mM HEPES-KOH, pH 7.2, for 1 h at 37°C. The cleavage was analyzed after the products were separated on 15% SDS-PAGE gels followed by visualization of bands by autoradiography.
For binding assays, full-length human syntaxin 3 and SNAP23 were translated in vitro using the Quick T7 kit (Promega, Lyon, France) according to the manufacturer’s procedure. The resulting extract (8 μl) was incubated with glutathione beads coated with GST, GST-hCB1, GST-hCB2, or GST–TI-VAMP (1 μM final concentration) in 100 μl of binding buffer (4 mM HEPES-NaOH, pH 7.4, 100 mM NaCl, 3.5 mM CaCl2, 3.5 mM MgCl2, 1 mM EDTA, 0.1% NP-40) for 12 h at 4°C as described previously (Hayashi et al., 1994). The beads were collected by centrifugation and washed six times with washing buffer (50 mM Tris-HCl, pH 8.0, 100 mM NaCl, 2.5 mM Mg Cl2, 0.1% NP-40), and the final bead fraction was eluted with gel sample buffer consisting of 60 mM Tris, pH 6.8, 2% (wt/vol) SDS, 10% (wt/vol) glycerol, 0.07% bromophenol blue, and 5% β-mercaptoethanol. The resulting samples were either kept at room temperature for 30 min or boiled for 10 min and analyzed using SDS-PAGE (Schagger and von Jagow, 1987) followed by visualization of bands by autoradiography.
Immunoprecipitation from CaCo-2 Cells
CaCo-2 cells grown on 25-mm-diameter wells to confluency were washed twice briefly in PBS+ and were treated in PBS+ with 1 mM NEM or 1 mM NEM plus 2 mM DTT for 15 min on ice. In the first condition, NEM was quenched by 2 mM DTT for 15 min on ice. The cells were then washed in PBS+, further incubated in culture medium for 30 min at 37°C, lysed in solubilization buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 10 mM EDTA, 1 mM PMSF, 1 mM benzamidine, 1 μg/ml pepstatin, 1 μg/ml antipain, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1% Triton X-100), and centrifuged at 200,000 × g for 15 min. The resulting supernatant was incubated overnight at 4°C with rotation with anti-syntaxin 3, anti-GST, anti-cellubrevin, or anti–TI-VAMP immunobeads, which had previously been prepared as described (Chilcote et al., 1995) by covalent coupling of corresponding affinity-purified antibodies. The beads were then pelleted for 1 min at 1000 × g and washed with solubilization buffer containing 0.5% Triton X-100. Finally, the beads were eluted with 100 mM glycine, pH 2.7, 0.5% Triton X-100, and the corresponding eluate was neutralized with Tris, pH 8, supplemented with gel sample buffer, boiled for 5 min, and run on SDS-PAGE gels (Schagger and von Jagow, 1987).
RESULTS
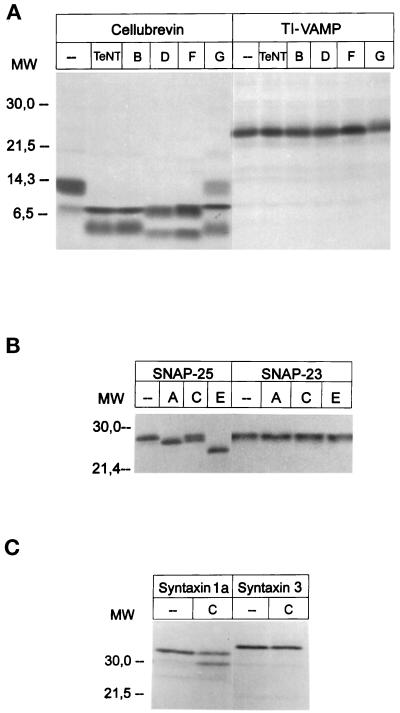
Best-fit comparison (Genetics Computer Group [GCG], Madison, WI) of human TI-VAMP with the human brevins shows that the two putative coiled coils of this protein are 64 and 57%, respectively, similar to corresponding domains in the VAMP/brevins (Figure 1A). Overall, we found that the similarity is 59% with synaptobrevin 2 and 56% with SNC1p, a v-SNARE implicated in Golgi to the plasma membrane transport in yeast (Protopopov et al., 1993). The similarity between TI-VAMP and ERS-24, GOS28, human ykt6, or rsec22 v-SNAREs found in the endoplasmic reticulum and/or in the Golgi is <50% (46, 40, 49, and 43%, respectively). Two sets of four hydrophobic residues present in hepta repeats that overlap with the two coiled coils are found both in this new molecule and in the VAMP/brevins (Figure 1A). We found that TI-VAMP, translated in vitro, is insensitive to TeNT and to BoNTs B, D, F, and G, which all proteolyze cellubrevin (Figure 2A), and has structural properties of v-SNAREs (see below). Moreover, it is noteworthy that the first 90 amino acids of TI-VAMP define a domain that is not found in any other v-SNARE. In this domain, we have identified a region of 40 amino acids that has 47.5% similarity and 27.5% identity with the first annexin repeat of annexin XIII (Figure 1B) which forms part of an important calcium- and phospholipid-binding site (Raynal and Pollard, 1994; Smith and Moss, 1994). We also showed that, similar to the VAMP/brevins, TI-VAMP is a type II membrane protein, because the protein inserted into microsomes is sensitive to proteinase K (our unpublished results). We have generated in rabbit an antibody directed against the putative cytoplasmic domain of TI-VAMP (amino acids 1–179) fused to GST (serum referred to as TG11). This antibody reacts specifically with its corresponding antigen, recognizes a band of the expected size (25 kDa) in CaCo-2 cell extract by Western blotting, and specifically immunoprecipitates TI-VAMP (Figure 1C).
Figure 1.
(A) TI-VAMP belongs to the VAMP/brevin family. Alignment of human TI-VAMP protein with the human brevins was made using Pileup software (GCG). The two potential coiled coils of TI-VAMP predicted by COILS (version 2.1) (Lupas et al., 1991) are underlined. Four hepta repeats of hydrophobic residues (marked by * under the corresponding amino acid in TI-VAMP) conserved within the VAMP/brevin family overlap with these domains. The domains involved in the binding of clostridial NTs are residues 38–47 (domain V1) and 62–71 (domain V2) in synaptobrevin 2 (Rossetto et al., 1994). Key amino acids (underlined below) are changed in the clostridial NT binding domains of TI-VAMP: 43VDIMR47 in domain V1 of synaptobrevin 2 → 137KGIMV141 in TI-VAMP and 62ELDDRADALQ71 in domain V2 of synaptobrevin 2 → 56LLIDKTENLV65 and in the cleavage sites. (B) A domain in the N-terminal portion of TI-VAMP (amino acids 80–119) shares similarity with human annexin XIII (GenBank accession number P27216) within its first annexin repeat. The alignment was performed using Bestfit (GCG). The similarity is 47.5%, and the identity is 27.5%. (C) Identification of TI-VAMP in CaCo-2 cell extract by TG11 rabbit serum. A CaCo-2 cell extract was immunoprecipitated with anti-human cellubrevin (ip hCB) as a control or with anti–TI-VAMP (ip TI-VAMP) (the pellet fractions represent a 10-fold enrichment compared with the amount in the extract). The bound fractions were run on SDS-PAGE (Schagger and von Jagow, 1987) and analyzed by Western blotting with TG11 rabbit serum. TI-VAMP appears as a 25-kDa band specifically immunoprecipitated by anti–TI-VAMP immunobeads.
Figure 2.
Effects of recombinant light chain of clostridial NTs on SNARE proteins. Cellubrevin, TI-VAMP (A), SNAP25, SNAP23 (B), syntaxin 1a, and syntaxin 3 (C) were translated in vitro in the presence of [35S]methionine and then incubated with the various light chains of the NTs in 150 mM potassium glutamate, 10 mM HEPES-KOH, pH 7.2, for 12 h at 37°C. The cleavage was analyzed after the products were separated by SDS-PAGE and visualization of bands by autoradiography. The concentrations of the toxins used were as follows: TeNT, BoNT/B, D, F, and G, 2 μM; BoNT A, 0.8 μM; BoNT C, 0.9 μM; and BoNT E, 0.4 μM.
Potential partners of TI-VAMP, SNAP23, and syntaxin 3 are produced in most cell types, including epithelial cells (see Figure 7; our unpublished observations) and are also insensitive to NTs (Figure 2, B and C). In vitro–translated SNAP23 is not cleaved by BoNTs A, C1, or E, whereas these toxins very efficiently cleaved SNAP25 (Niemann et al., 1994; Schiavo et al., 1994; Osen-Sand et al., 1996) (Figure 2B), a neuronal isoform of SNAP23. We have reinvestigated the effect of BoNT C1 on syntaxin 3. We found that contrary to syntaxin 1a, human syntaxin 3 is insensitive to BoNT C1 at a concentration as high as 0.9 μM (Figure 2C), a 10-fold excess over the concentration required for maximal cleavage of syntaxin 1a. A study with increasing concentrations of BoNT C1 (ranging from 1 nM up to 3.8 μM) showed that rat syntaxin 3 is not cleaved by this toxin even at the highest concentration tested (our unpublished observations).
Figure 7.
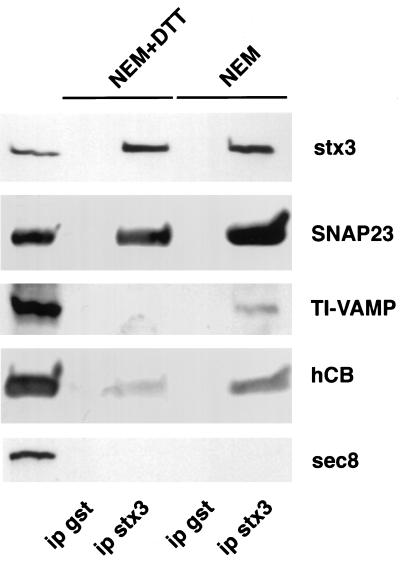
Effect of NEM on the formation of SNARE complexes in CaCo-2 cells. CaCo-2 cells grown to confluency were treated with 1 mM NEM for 15 min and then with 2 mM DTT for 15 min (NEM) or with 1 mM NEM plus 2 mM DTT for 30 min (NEM+DTT) on ice. The cells were then further incubated in culture medium for 30 min at 37°C and lysed in the presence of 1% Triton X-100 in conditions in which proteins are diluted enough so that the SNARE complex does not form spontaneously (Banerjee et al., 1996). The cell extract was immunoprecipitated with anti-syntaxin 3 (ip stx3) or anti-GST (ip gst) (the pellet fractions represent a fivefold enrichment compared with the amount in the extract). The bound fractions were run on SDS-PAGE (Schagger and von Jagow, 1987) and analyzed by Western blotting with specific SNARE (syntaxin 3, stx3; hCB, human cellubrevin) antibodies. Note that syntaxin 3 is entirely immunoprecipitated and that NEM treatment greatly stimulates the recovery of SNAP23, cellubrevin, and TI-VAMP in the syntaxin 3 immunobead pellets.
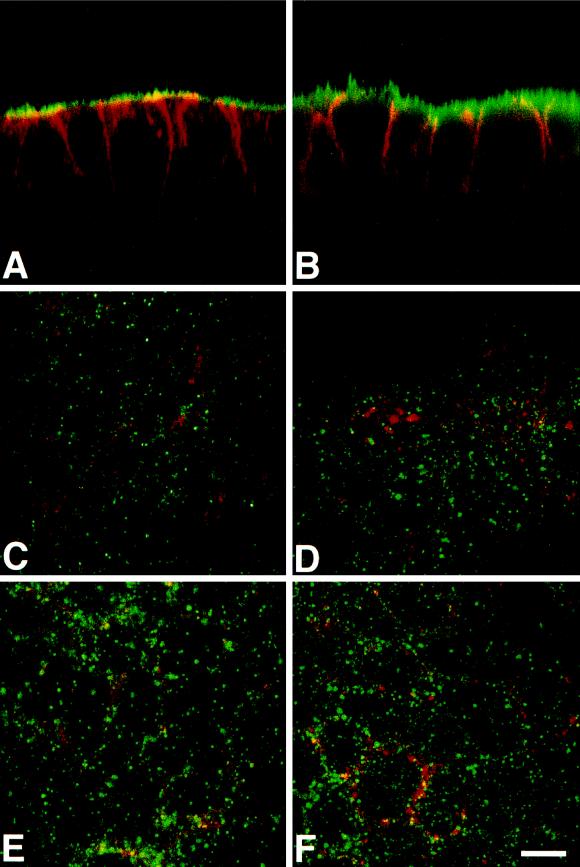
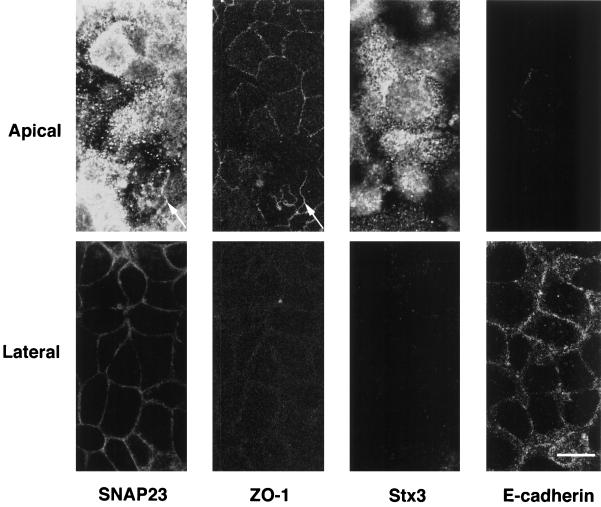
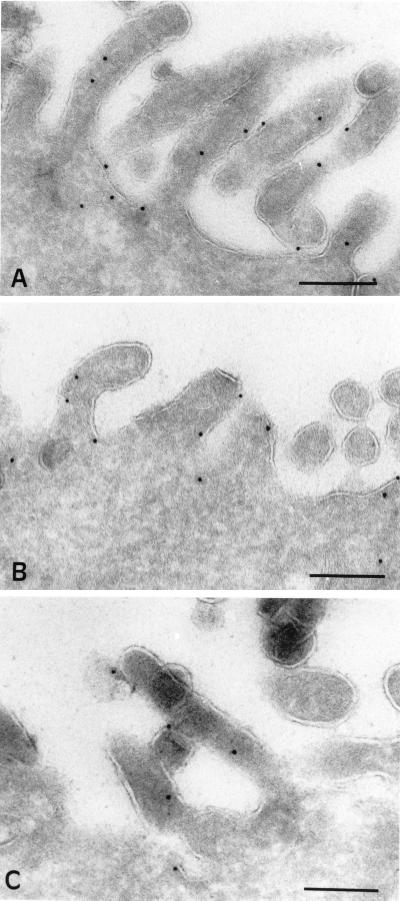
Because it was shown that apical transport of HA is insensitive to clostridial NTs (Ikonen et al., 1995), we investigated the subcellular distribution of clostridial NT-insensitive SNAREs in the human epithelial cell line CaCo-2, a well-documented model of polarized intestinal cells (Weimbs et al., 1997). By confocal microscopy, we found that syntaxin 3 is present at high concentration at the apical plasma membrane and is not detected in the lateral plasma membrane, a region where E-cadherin is specifically found (Figures 3A and 4). In rat kidney and intestine sections, syntaxin 3 is strictly localized in the microvilli structures of the apical domain (our unpublished results). The bulk of SNAP23 is found at the apical plasma membrane by confocal microscopy (Figures 3B and 4). Small amounts of SNAP23 were found in tight junctions, where it colocalizes with ZO-1 (Figure 4), and along the lateral plasma membrane (Figures 3B and 4), by confocal microscopy. Immunoelectron microscopy showed that syntaxin 3 (Figure 5A) and SNAP23 (Figure 5B) are present in the apical plasma membrane rather than in intracellular structures underneath and that syntaxin 3 labeling is strictly restricted to the apical plasma membrane. In CaCo-2 cells, cellubrevin and TI-VAMP localize to small vesicular structures dispersed throughout the peripheral cytoplasm, along the longitudinal axis with a pronounced enrichment of both v-SNAREs in the apical domain (Figure 3, C–F). We did not observe any significant codistribution of these v-SNAREs either with CTR433, a medial Golgi marker (Jasmin et al., 1989), or with lamp-2, a lysosomal marker (Chen et al., 1985). Strikingly, we noticed that neither cellubrevin nor TI-VAMP codistributed with Tf receptor (Figure 3, C–F) or with Tf-containing organelles (our unpublished observations) as seen by confocal microscopy. Because Tf receptor is known to recycle mainly between basolateral endosomes and plasma membrane (Hughson and Hopkins, 1990), we investigated whether TI-VAMP could be detected in the apical plasma membrane as expected if TI-VAMP–containing vesicles dock and fuse at this site. For this purpose, we have analyzed the localization of TI-VAMP by immunoelectron microscopy with gold-labeled antibodies in CaCo-2 cells, and we observed labeling of the apical plasma membrane, suggesting the presence of a pool of this protein (Figure 5C).
Figure 3.
Localization of v- and t-SNARES in vertical (A and B) and horizontal (C–F) confocal sections of CaCo-2 cells. Syntaxin 3 (green, A) and SNAP23 (green, B) are present at the apical plasmalemma. The staining for E-cadherin, a protein of the basolateral membrane, is in red (A and B). Note the occurrence of a low amount of SNAP23 in the top part of the lateral plasma membrane (yellow, B). Cellubrevin (green, C, E) and TI-VAMP (green, D, F) are present on intracellular organelles localized both in the lateral plane (C and D) and in the apical domain (E and F). Note the very low degree of colocalization (yellow) with transferrin receptor (monoclonal antibody H68.4, red, C and D) of both v-SNAREs. Bar, 10 μm.
Figure 4.
Localization of t-SNARES in horizontal confocal sections of CaCo-2 cells. Double immunofluorescence micrographs of CaCo-2 cells stained for SNAP23 and ZO-1, a tight junction marker (left) or syntaxin 3(Stx3) and E-cadherin, a basolateral marker (right). Syntaxin 3 is restricted to the apical plasma membrane (note the lack of staining of the lateral plasma membrane that is positive for E-cadherin in bottom right micrographs). SNAP23 is highly concentrated at the apical plasmalemma and is present in tight junctions (top left micrographs; arrow indicates colocalization with ZO-1) and at low level in the lateral plasma membrane below tight junctions (bottom left). Bar, 10 μm.
Figure 5.
Immunogold localization of syntaxin 3, SNAP23, and TI-VAMP in CaCo-2 cells. Syntaxin 3 (A), SNAP23 (B), and TI-VAMP (C) visualized with their respective affinity-purified antibody and PAG 10 are localized at the cytoplasmic side of the microvillar membrane of polarized CaCo-2 cells. Bars, 200 nm.
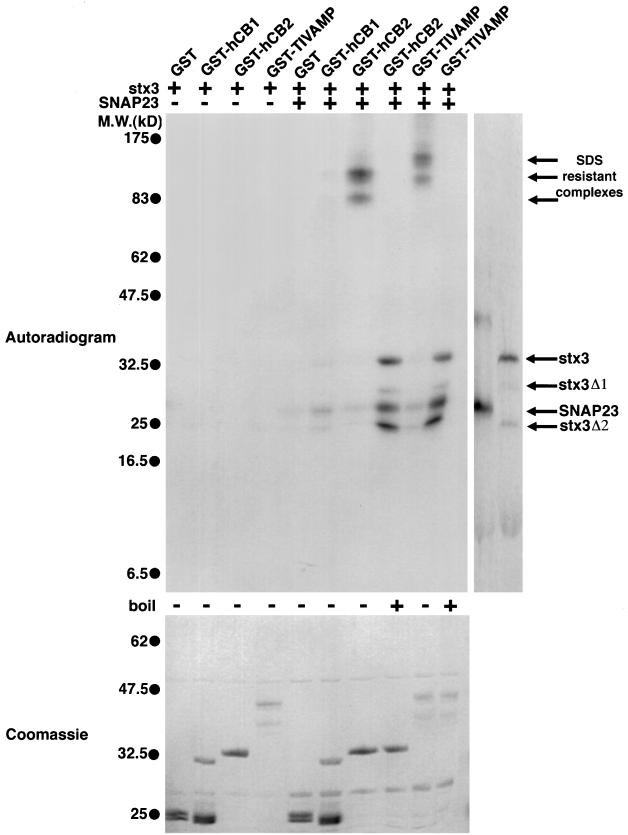
We then studied the capacity of TI-VAMP and cellubrevin to interact with t-SNAREs in vitro. Using recombinant fragments of the cytoplasmic domains of these v-SNAREs fused to GST, we found that cellubrevin and TI-VAMP were both able to form SDS-resistant SNARE complexes (Hayashi et al., 1994) with syntaxin 3 and SNAP23 translated in vitro in the presence of [35S]methionine (Figure 6). SDS resistance of the brain syntaxin 1/SNAP25/synaptobrevin 2 SNARE complex is thought to be a key property of SNARE complexes that is probably attributable to the high stability of the coiled–coiled interactions (Hayashi et al., 1994). We also found that both cellubrevin and TI-VAMP interacted poorly with syntaxin 3 alone (Figure 6) or SNAP23 alone (our unpublished results), in agreement with a previous report showing the lack of interaction between syntaxin 3 and the synaptobrevins (Calakos et al., 1994). Interestingly, a shorter fragment of the cytoplasmic domain of cellubrevin, excluding the second coiled coil fused to GST (GST-hCB1), was only slightly less efficient in this assay (Figure 6), as was expected, because deletion of the second putative α helix in synaptobrevin 2 did not prevent association of SNAP25 (Regazzi et al., 1996). These data clearly indicate that cellubrevin and TI-VAMP have the capacity to form SNARE complexes with the same t-SNAREs, including the NT-insensitive syntaxin 3 and SNAP23.
Figure 6.
In vitro formation of SNARE complexes of cellubrevin and TI-VAMP with SNAP23 and syntaxin 3. Glutathione beads coated with GST, GST-hCB1 (first coiled coil only), GST-hCB2 (whole cytoplasmic domain), or GST–TI-VAMP (whole cytoplasmic domain) (1 μM, 100 μl) were incubated overnight with radiolabeled in vitro–translated human syntaxin 3 (stx3) alone or together with human SNAP23 (8 μl of translated proteins). The fractions bound to glutathione beads were eluted, separated by SDS-PAGE, and visualized by autoradiography. The shorter forms of stx3 (stx3Δ1 and stx3Δ2) are likely to be generated by initiation of translation at internal methionine residues. Binding of syntaxin 3 alone to the v-SNAREs is at the limit of detection. The molecular weights of the SDS stable complexes are 85 and 120 kDa in the case of GST-hCB2 and 115 and 150 kDa in the case of GST–TI-VAMP. Note also that the GST-hCB1 SNARE complex is not SDS stable.
To show that exocytic organelles can dock at the apical plasma membrane, we set up an immunoprecipitation assay based on the observation that syntaxin 3 is found strictly at the apical plasma membrane (see above). NEM treatment was used to block NEM-dependent ATPases, including NEM-sensitive NSF (Beckers et al., 1989), and therefore should elicit accumulation of assembled SNARE complexes as seen in PC12 cells (Banerjee et al., 1996). NEM has also been shown to inhibit the binding of nSec1 to syntaxin 1 in vitro (Meffert et al., 1996), an effect that is also expected to enhance SNARE complex formation, because nSec1 prevents syntaxin 1 from forming SNARE complexes (Pevsner et al., 1994). To compare a control condition with a condition in which SNARE complexes are accumulated, we pretreated CaCo-2 cells grown on filters at 4°C with either 1) 1 mM NEM plus 2 mM DTT for 30 min (control conditions) or 2) 1 mM NEM (15 min) followed by 2 mM DTT (15 min) and allowed vesicles to accumulate at their target membrane by returning the cells to culture medium at 37°C as reported previously in the case of PC12 cells (Banerjee et al., 1996). First, we observed by immunofluorescence that syntaxin 3 and SNAP23 are still localized at the apical plasma membrane after treatment with NEM. Second, using this experimental approach, we could immunoprecipitate virtually all of the syntaxin 3 present in the cell lysate using anti-syntaxin 3 immunobeads (Figure 7). Western-blotting analysis of the fractions bound to the syntaxin 3 immunobeads showed a dramatic simultaneous increase of the amounts of SNAP23 and cellubrevin coimmunoprecipitated with syntaxin 3 when the cells were pretreated with NEM (Figure 7). Third, TI-VAMP was found to coimmunoprecipitate with syntaxin 3 only from NEM-pretreated cell extracts (Figure 7). None of the SNAREs were detected in pellet fractions recovered with anti-GST immunobeads, and Sec8, a protein that can be found in association with syntaxin 1 to a small extent (Hsu et al., 1996), was also absent from all bead fractions (Figure 7), therefore showing the high specificity of the experimental procedure. This result clearly indicates that the t-SNAREs present at the apical plasma membrane, syntaxin 3 and SNAP23, allow NEM-dependent SNARE complex formation, which includes cellubrevin or TI-VAMP (Figure 7).
DISCUSSION
Our results suggest the involvement of the clostridial NT-resistant t-SNAREs syntaxin 3 and SNAP23 in exocytosis at the apical plasma membrane of epithelial cells.
First, we have found that in contrast to their neuronal counterparts, SNAP23 and syntaxin 3 were not proteolyzed by clostridial NTs. Our finding that syntaxin 3 was insensitive to BoNT C1 is in contrast to a report showing the cleavage of a synaptosomal protein recognized by an anti-syntaxin 3 antibody by BoNT C1 (Schiavo et al., 1995). It should be mentioned that the antibody used in the latter study also weakly recognizes syntaxin 1 (Gaisano et al., 1996). In the present article, we report the lack of effect of the toxins on full-length in vitro–translated human SNARE proteins. This method avoids the kind of technical problems described above. The resistance of SNAP23 and syntaxin 3 to clostridial NTs is surprising given the high degree of similarity between SNAP25 and SNAP23 (72%) and between syntaxin 1 and syntaxin 3 (77%). The lack of cleavage by NTs of SNAP23 and syntaxin 3 might be the result of the inability of the toxins either to bind to the putative coiled coils of these SNAREs or to recognize them as substrates because of key amino acid substitutions within the cleavage sites (Pellizzari et al., 1996).
Second, by confocal and immunoelectron microscopy, we have found that endogenous syntaxin 3 and SNAP23 were both localized at the apical plasma membrane of CaCo-2 cells. In the case of syntaxin 3, we were unable to detect any intracellular or basolateral plasma membrane staining either by confocal microscopy or by immunoelectron microscopy on fully polarized and differentiated cells. This is in contrast to two studies on MDCK (Low et al., 1996) and CaCo-2 cells (Delgrossi et al., 1997) overexpressing syntaxin 3 in which significant intracellular labeling was detected, including lysosome labeling (Low et al., 1996). In our case, we observed syntaxin 3-positive vesicular structures only in CaCo-2 cells that were not confluent and not yet fully polarized (our unpublished observations). We believe therefore that intracellular syntaxin 3 labeling might represent the newly synthesized pool or a pool resulting from overexpression of the protein or both. In the case of the partner t-SNARE SNAP23, we found that this protein is localized mainly at the apical plasma membrane on fully polarized CaCo-2 cells, with a minor labeling of the lateral plasma membrane as shown by longitudinal confocal sections. We found a major association of SNAP23 with syntaxin 3 by coimmunoprecipitation. This demonstrates that syntaxin 3 and SNAP23 act in concert as apical plasma membrane t-SNAREs. The lateral pool of SNAP23 is probably associated with another syntaxin isoform, because SNAP23 was already found to associate with syntaxin 4 (Ravichandran et al., 1996), a basolateral t-SNARE in MDCK cells (Low et al., 1996). Because we could not detect syntaxin 4, 2, or 1 by immunofluorescence microscopy in CaCo-2 cells (our unpublished observations), we have not investigated this point in the present study.
Third, we show that syntaxin 3 and SNAP23 form apical SNARE complexes and provide the first evidence for the involvement of cellubrevin and a new v-SNARE, TI-VAMP, in apical SNARE complex formation. We have taken advantage of the specific localization of syntaxin 3 at the apical plasma membrane of polarized CaCo-2 cells to immunoprecipitate apical SNARE complexes containing syntaxin 3. We found that NEM pretreatment of CaCo-2 cells increased the recovery of cellubrevin and SNAP23 associated with syntaxin 3. We have also found that in NEM-pretreated cell extracts, SNAP23 and syntaxin 3 form SNARE complexes with TI-VAMP, a new member of the VAMP/brevin family that is the first to be shown to be insensitive to all clostridial NTs that proteolyze the brevins. Because of the restricted localization of syntaxin 3 to the apical plasma membrane and cellubrevin and TI-VAMP on intracellular vesicles, we believe that the most likely explanation of our immunoprecipitation experiments is that cellubrevin- and TI-VAMP–containing vesicles dock at the apical plasma membrane through the NEM-dependent formation of SNARE complexes, which include SNAP23 and syntaxin 3. Further evidence was obtained in the case of TI-VAMP by our observation of a pool of the protein at the apical plasma membrane revealed by immunogold labeling on ultrathin cryosections. In CaCo-2 cells, the syntaxin 3/SNAP23/cellubrevin and syntaxin 3/SNAP23/TI-VAMP SNARE complexes appear as the analogues of the syntaxin 1/SNAP25/synaptobrevin complex found in the nerve terminal.
The properties of TI-VAMP, a new member of the VAMP/brevin family, have not been examined previously. It is insensitive to NTs, binds to plasma membrane t-SNAREs, and localizes to apical organelles in epithelial cells. This v-SNARE has a unique N-terminal domain that resembles the lipid/Ca2+ binding domain of annexin XIII and therefore may be a v-SNARE with additional lipid/Ca2+ binding properties. In any case, the N-terminal domain of TI-VAMP probably confers to this molecule specific features that would distinguish it from other members of the VAMP/brevin family. It is intriguing that the weak but nonetheless significant similarity we found in the N-terminal part of TI-VAMP establishes a relationship between this new v-SNARE and annexin XIII, an intestine-specific annexin (Wice and Gordon, 1992), which has been proposed to play a role in apical transport of HA in MDCK cells (Fiedler et al., 1995). Future studies should explore the functional relevance of the similarity between TI-VAMP and annexin XIII. Another remarkable property of TI-VAMP is that it is resistant to TeNT and to BoNTs B, D, F, and G. As in the case of SNAP23 and syntaxin 3, this might result from lack of binding of the NTs or from the differences in amino acid sequence observed in the NT cleavage sites (see the legend of Figure 1A). In this study in epithelial cells, despite the biochemical specificities of TI-VAMP depicted above, we have not found major localization or biochemical differences regarding the t-SNARE partners between TI-VAMP and cellubrevin. This may be because these proteins have overlapping functions in the same apical exocytotic pathway or because our level of analysis could not distinguish different apical exocytotic pathways. Our observations 1) that cellubrevin and TI-VAMP could both form SNARE complexes with the same apical t-SNAREs but that these v-SNAREs were not complexed together and 2) that TI-VAMP was found associated with syntaxin 3 only in NEM-pretreated cells could be seen to favor the second hypothesis. In CaCo-2 cells, sucrase isomaltase was shown to be transported directly from the trans-Golgi network to the apical plasma membrane, whereas dipeptidyl-peptidase IV is first transported to the lateral membrane and then sorted to the apical plasma membrane (Matter et al., 1990). Given the role of cellubrevin in transferrin receptor recycling in fibroblasts (Galli et al., 1994) and the lack of effect of TeNT on transport of HA from the trans-Golgi network to the apical plasma membrane in MDCK cells (Ikonen et al., 1995), an attractive but speculative hypothesis could be that cellubrevin is involved in recycling of apical proteins and TI-VAMP in the direct trans-Golgi network to the apical plasma membrane pathway. Obviously, it will be important to characterize by electron microscopy the vesicular pools of cellubrevin and TI-VAMP in epithelial cells, to test whether TI-VAMP distributes on HA-containing vesicles and whether it is involved in apical docking/fusion of these vesicles with the apical plasma membrane in MDCK cells.
In conclusion, our data are in favor of the existence of SNARE-dependent exocytotic events at the apical plasma membrane of epithelial cells. They also imply that NTs should be unable to impair multiple exocytotic events in different cell types. The NT-resistant SNAREs described here have a very broad tissue and cell distribution. Our work opens the way for studying NT-resistant secretory pathways in nonneuronal cells and in neurons (Osen-Sand et al., 1996) and in particular the role played by TI-VAMP in these cell types.
ACKNOWLEDGMENTS
We are indebted to Mark Bennett for the rat syntaxin 3 clone; to Michel Bornens for the CTR433 antibody; to Ian Trowbridge for the H68.4 antibody; to Monique Arpin for helpful discussions; to Dominique Morineau, Ahmed El Marjou, and Lucien Cabanié for excellent technical assistance; and to Margaret Butler and Roy Golsteyn for critical reading of this manuscript. This work was supported in part by an Association pour la Recherche contre le Cancer grant to A.Z., by an Alexander-von-Humboldt Foundation fellowship to V.V.V., by a Human Frontier Science Program Postdoctoral fellowship to J.T., by a Fonds der Chemischen Industrie grant to H.N., and by National Institutes of Health grants to M.K.
Footnotes
Abbreviations used: BoNTs, botulinum NTs; HA, influenza hemagglutinin; NEM, N-ethyl maleimide; NSF, NEM-sensitive fusion protein; NTs, neurotoxins; SNARE, SNAP receptor; SNAPs, soluble NSF attachment proteins; t-SNARE, target SNARE; TeNT, tetanus NT; TI-VAMP, tetanus neurotoxin-insensitive VAMP; VAMP, vesicle-associated membrane protein; v-SNARE, vesicle SNARE.
REFERENCES
- Banerjee A, Barry VA, DasGupta BR, Martin TFJ. N-Ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J Biol Chem. 1996;271:20223–20226. doi: 10.1074/jbc.271.34.20223. [DOI] [PubMed] [Google Scholar]
- Beckers CJ, Block MR, Glick BS, Rothman JE, Balch WE. Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature. 1989;339:397–398. doi: 10.1038/339397a0. [DOI] [PubMed] [Google Scholar]
- Bennett MK, García-Arrarás JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Südhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993a;365:160–163. doi: 10.1038/365160a0. [DOI] [PubMed] [Google Scholar]
- Blasi J, Chapman ER, Yamasaki S, Binz T, Niemann H, Jahn R. Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J. 1993b;12:4821–4828. doi: 10.1002/j.1460-2075.1993.tb06171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicles. J Biol Chem. 1996;271:17961–17965. doi: 10.1074/jbc.271.30.17961. [DOI] [PubMed] [Google Scholar]
- Calakos N, Bennett MK, Peterson KE, Scheller RH. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- Chen JW, Murphy TL, Willingham MC, Pastan I, August JT. Identification of two lysosomal membrane glycoproteins. J Cell Biol. 1985;101:85–95. doi: 10.1083/jcb.101.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcote TJ, Galli T, Mundigl O, Edelmann L, McPherson PS, Takei K, De Camilli P. Cellubrevin and synaptobrevins: similar subcellular localization and biochemical properties in PC12 cells. J Cell Biol. 1995;129:219–231. doi: 10.1083/jcb.129.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgrossi MH, Breuza L, Mirre C, Chavrier P, LeBivic A. Human syntaxin 3 is localized apically in human intestinal cells. J Cell Sci. 1997;110:2207–2214. doi: 10.1242/jcs.110.18.2207. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Ciccodicola A, Gianfrancesco F, Esposito T, Flagiello L, Mazzarella R, Schlessinger D, D’Urso M. A synaptobrevin-like gene in the Xq28 pseudoautosomal region undergoes X inactivation. Nat Genet. 1996;13:227–229. doi: 10.1038/ng0696-227. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Lafont F, Parton RG, Simons K. Annexin XIIIb: a novel epithelial specific annexin is implicated in vesicular traffic to the apical plasma membrane. J Cell Biol. 1995;128:1043–1053. doi: 10.1083/jcb.128.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol Biol Cell. 1996;7:2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T, Chilcote T, Mundigl O, Binz T, Niemann H, De Camilli P. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof TC, Niemann H. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SC, Ting AE, Hazuka CD, Davanger S, Kenny JW, Kee Y, Scheller RH. The mammalian brain rsec6/8 complex. Neuron. 1996;17:1209–1219. doi: 10.1016/s0896-6273(00)80251-2. [DOI] [PubMed] [Google Scholar]
- Hughson EJ, Hopkins CR. Endocytic pathways in polarized Caco-2 cells: identification of an endosomal compartment accessible from both apical and basolateral surfaces. J Cell Biol. 1990;110:337–348. doi: 10.1083/jcb.110.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E, Tagaya M, Ullrich O, Montecucco C, Simons K. Different requirements for NSF, SNAP, and rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995;81:571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Jasmin BJ, Cartaud J, Bornens M, Changeux JP. Golgi apparatus in chick skeletal muscle: changes in its distribution during end plate development and after denervation. Proc Natl Acad Sci, USA. 1989;86:7218–7222. doi: 10.1073/pnas.86.18.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes L, Galli T. Exocytosis: SNAREs drum up! Eur J Neurosci. 1998;10:415–422. doi: 10.1046/j.1460-9568.1998.00081.x. [DOI] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science. 1991;252:1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- Mandon B, Chou CL, Nielsen S, Knepper MA. Syntaxin-4 is localized to the apical plasma membrane of rat renal collecting duct cells: possible role in aquaporin-2 trafficking. J Clin Invest. 1996;98:906–913. doi: 10.1172/JCI118873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Brauchbar M, Bucher K, Hauri HP. Sorting of endogenous plasma membrane proteins occurs from two sites in cultured human intestinal epithelial cells (Caco-2) Cell. 1990;60:429–437. doi: 10.1016/0092-8674(90)90594-5. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Sudhof TC. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Calakos NC, Scheller RH, Schulman H. Nitric oxide modulates synaptic vesicle docking/fusion reactions. Neuron. 1996;16:1229–1236. doi: 10.1016/s0896-6273(00)80149-x. [DOI] [PubMed] [Google Scholar]
- Niemann H, Blasi J, Jahn R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 1994;4:179–185. doi: 10.1016/0962-8924(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Osen-Sand A, Staple JK, Naldi E, Schiavo G, Rossetto O, Petitpierre S, Malgaroli A, Montecucco C, Catsicas S. Common and distinct fusion proteins in axonal growth and transmitter release. J Comp Neurol. 1996;367:222–234. doi: 10.1002/(SICI)1096-9861(19960401)367:2<222::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pellizzari R, Rossetto O, Lozzi L, Giovedí S, Johnson E, Shone CC, Montecucco C. Structural determinants of the specificity for synaptic vesicle-associated membrane protein/synaptobrevin of tetanus and botulinum type B and G neurotoxins. J Biol Chem. 1996;271:20353–20358. doi: 10.1074/jbc.271.34.20353. [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu S-C, Braun JEA, Calakos N, Ting AE, Bennett MK, Scheller RH. Specificity and regulation of a synaptic vesicle docking complex. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- Protopopov V, Govindan B, Novick P, Gerst JE. Homologs of the synaptobrevin/VAMP family of synaptic vesicle proteins function on the late secretory pathway in S. cerevisiae. Cell. 1993;74:855–861. doi: 10.1016/0092-8674(93)90465-3. [DOI] [PubMed] [Google Scholar]
- Raposo G, Kleijmer KJ, Posthuma G, Slot JW, Geuze HJ. Immunogold labeling of ultrathin cryosections: application in immunology. In: Herzenberg LA, Weir DM, Blackwell C, editors. Handbook of Experimental Immunology. Malden, MA: Blackwell; 1997. pp. 1–11. [Google Scholar]
- Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996;271:13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- Raynal P, Pollard HB. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Regazzi R, Sadoul K, Meda P, Kelly RB, Halban PA, Wollheim CB. Mutational analysis of VAMP domains implicated in Ca2+- induced insulin exocytosis. EMBO J. 1996;15:6951–6959. [PMC free article] [PubMed] [Google Scholar]
- Rossetto O, Schiavo G, Montecucco C, Poulain B, Deloye F, Lozzi L, Shone CC. SNARE motifs and neurotoxins. Nature. 1994;372:415–416. doi: 10.1038/372415a0. [DOI] [PubMed] [Google Scholar]
- Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta BR, Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Rossetto O, Benfenati F, Poulain B, Montecucco C. Part III. Botulinum and tetanus toxins. Tetanus and botulinum neurotoxins are zinc proteases specific for components of the neuroexocytosis apparatus. Ann NY Acad Sci. 1994;710:65–75. doi: 10.1111/j.1749-6632.1994.tb26614.x. [DOI] [PubMed] [Google Scholar]
- Schiavo G, Shone CC, Bennett MK, Scheller RH, Montecucco C. Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem. 1995;270:10566–10570. doi: 10.1074/jbc.270.18.10566. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Smith PD, Moss SE. Structural evolution of the annexin supergene family. Trends Genet. 1994;10:241–246. doi: 10.1016/0168-9525(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993a;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993b;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Wang H, Frelin L, Pevsner J. Human syntaxin 7: a Pep12p/Vps6p homologue implicated in vesicle trafficking to lysosomes. Gene. 1997;199:39–48. doi: 10.1016/s0378-1119(97)00343-0. [DOI] [PubMed] [Google Scholar]
- Weimbs T, Low SH, Chapin SJ, Mostov KE. Apical targeting in polarized cells: there’s more afloat than rafts. Trends Cell Biol. 1997;7:393–399. doi: 10.1016/S0962-8924(97)01130-6. [DOI] [PubMed] [Google Scholar]
- Wice BM, Gordon JI. A strategy for isolation of cDNAs encoding proteins affecting human intestinal epithelial cell growth and differentiation: characterization of a novel gut-specific N-myristoylated annexin. J Cell Biol. 1992;116:405–422. doi: 10.1083/jcb.116.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PPC, Daneman N, Volchuk A, Lassam N, Wilson MC, Klip A, Trimble WS. Tissue distribution of SNAP-23 and its subcellular localization in 3T3–L1 cells. Biochem Biophys Res Commun. 1997;230:64–68. doi: 10.1006/bbrc.1996.5884. [DOI] [PubMed] [Google Scholar]