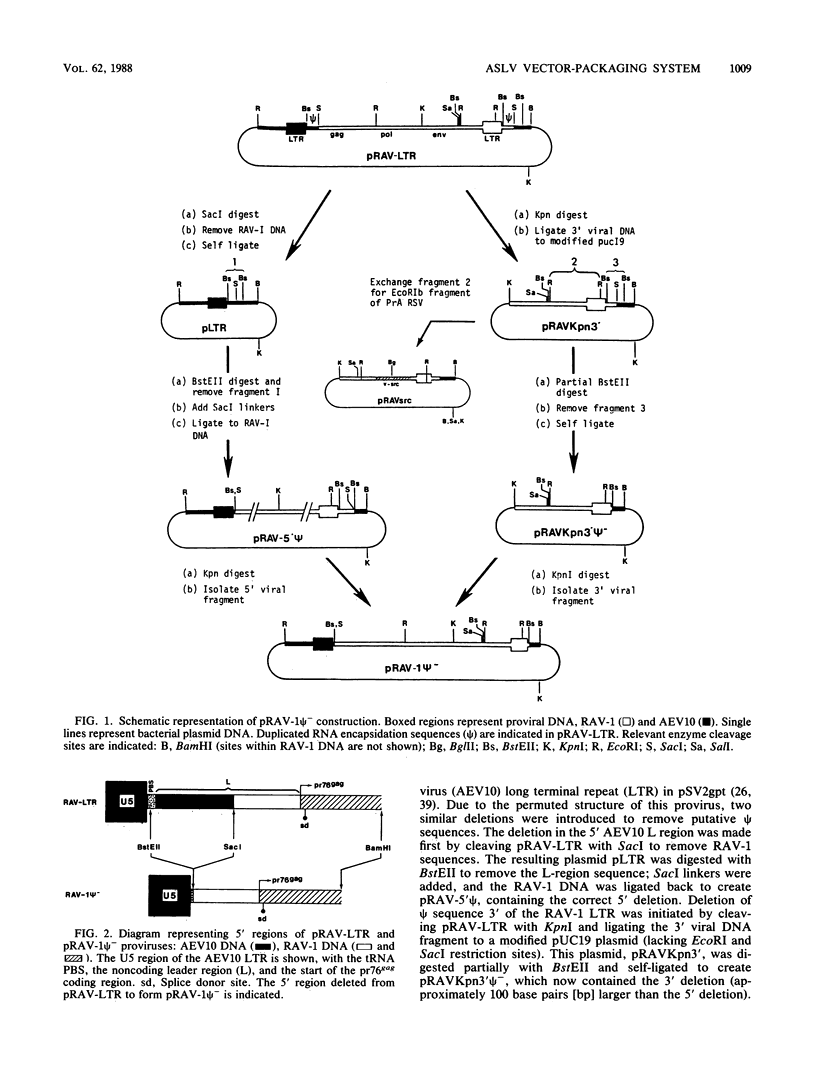
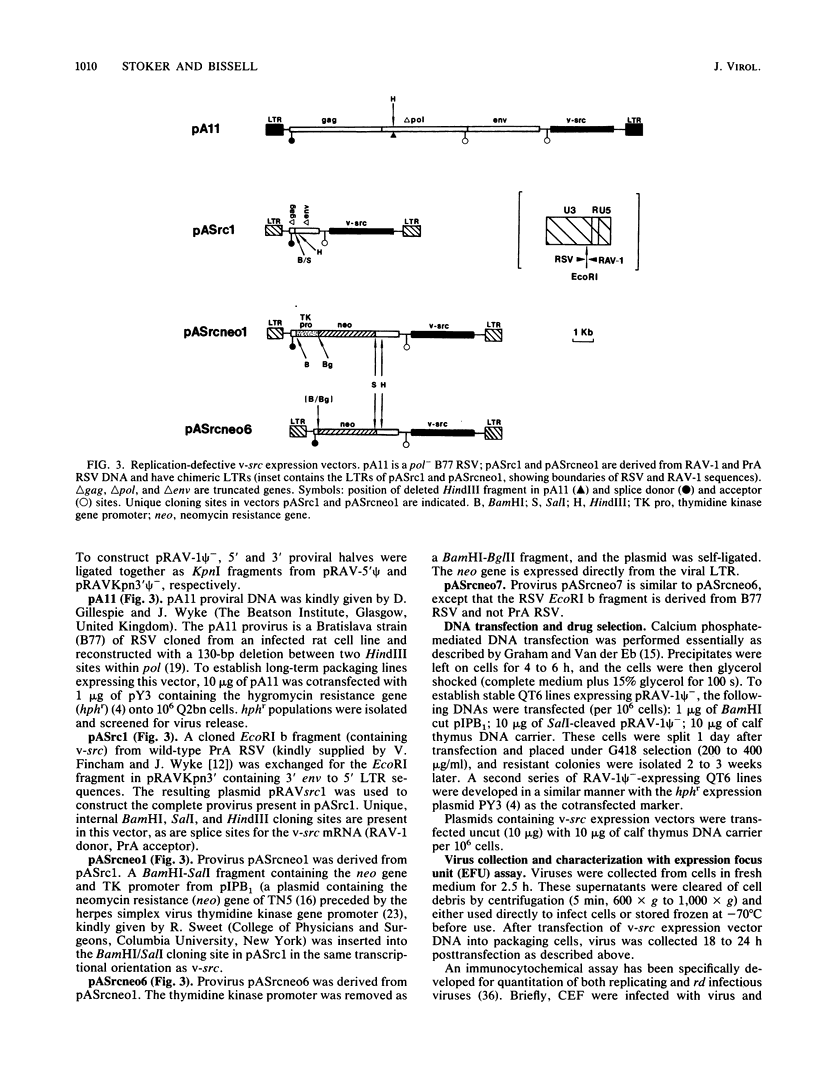
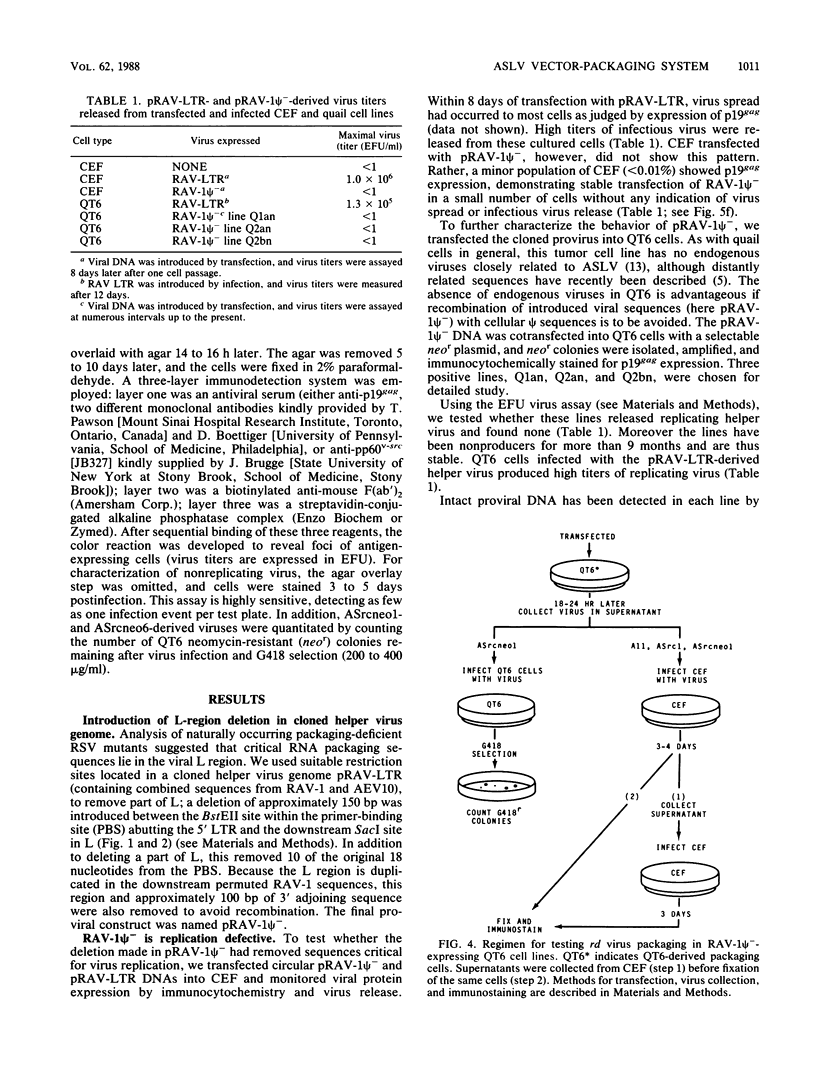
Abstract
We have constructed an avian leukosis virus derivative with a 5' deletion extending from within the tRNA primer binding site to a SacI site in the leader region. Our aim was to remove cis-acting replicative and/or encapsidation sequences and to use this derivative, RAV-1 psi-, to develop vector-packaging cell lines. We show that RAV-1 psi- can be stably expressed in the quail cell line QT6 and chicken embryo fibroblasts and that it is completely replication deficient in both cell types. Moreover, we have demonstrated that QT6-derived lines expressing RAV-1 psi- can efficiently package four structurally different replication-defective v-src expression vectors into infectious virus, with very low or undetectable helper virus release. These RAV-1 psi--expressing cell lines comprise the first prototype avian sarcoma and leukosis virus-based vector-packaging system. The construction of our vectors has also shown us that a sequence present within gag, thought to facilitate virus packaging, is not necessary for efficient vector expression and high virus production. We show that quantitation and characterization of replication-defective viruses can be achieved with a sensitive immunocytochemical procedure, presenting an alternative to internal selectable vector markers.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo S., Yun M., Beemon K. cis-acting regulatory elements within gag genes of avian retroviruses. Mol Cell Biol. 1987 Jan;7(1):388–397. doi: 10.1128/mcb.7.1.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Farson D., Tung A. S. Cell shape and hexose transport in normal and virus-transformed cells in culture. J Supramol Struct. 1977;6(1):1–12. doi: 10.1002/jss.400060102. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Diggelmann H. Hygromycin B phosphotransferase as a selectable marker for DNA transfer experiments with higher eucaryotic cells. Mol Cell Biol. 1984 Dec;4(12):2929–2931. doi: 10.1128/mcb.4.12.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers J. A., Cywinski A., Chen P. J., Taylor J. M. Characterization of Rous sarcoma virus-related sequences in the Japanese quail. J Virol. 1986 Aug;59(2):354–362. doi: 10.1128/jvi.59.2.354-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. M., Okenquist S. Mechanism of transfection of chicken embryo fibroblasts by Rous sarcoma virus DNA. J Virol. 1978 Oct;28(1):45–52. doi: 10.1128/jvi.28.1.45-52.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolberg D. S., Bissell M. J. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984 Jun 7;309(5968):552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- Dolberg D. S., Hollingsworth R., Hertle M., Bissell M. J. Wounding and its role in RSV-mediated tumor formation. Science. 1985 Nov 8;230(4726):676–678. doi: 10.1126/science.2996144. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Fincham V. J., Wyke J. A. Localization of temperature-sensitive transformation mutations and back mutations in the Rous sarcoma virus src gene. J Virol. 1986 May;58(2):694–699. doi: 10.1128/jvi.58.2.694-699.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisby D. P., Weiss R. A., Roussel M., Stehelin D. The distribution of endogenous chicken retrovirus sequences in the DNA of galliform birds does not coincide with avian phylogenetic relationships. Cell. 1979 Jul;17(3):623–634. doi: 10.1016/0092-8674(79)90270-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Terry R. W., Skalka A. M. A conserved cis-acting sequence in the 5' leader of avian sarcoma virus RNA is required for packaging. J Virol. 1986 Jul;59(1):163–167. doi: 10.1128/jvi.59.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Koyama T. Characterization of a Rous sarcoma virus mutant defective in packaging its own genomic RNA: biological properties of mutant TK15 and mutant-induced transformants. J Virol. 1984 Jul;51(1):147–153. doi: 10.1128/jvi.51.1.147-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levantis P., Gillespie D. A., Hart K., Bissell M. J., Wyke J. A. Control of expression of an integrated Rous sarcoma provirus in rat cells: role of 5' genomic duplications reveals unexpected patterns of gene transcription and its regulation. J Virol. 1986 Mar;57(3):907–916. doi: 10.1128/jvi.57.3.907-916.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsick J. S., Ibanez C. E., Baluda M. A. Expression of molecular clones of v-myb in avian and mammalian cells independently of transformation. J Virol. 1986 Aug;59(2):267–275. doi: 10.1128/jvi.59.2.267-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Koyama T., Kawai S. Unusual features of the leader sequence of Rous sarcoma virus packaging mutant TK15. J Virol. 1985 Sep;55(3):881–885. doi: 10.1128/jvi.55.3.881-885.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. A., Coffin J. M. Bacterial beta-galactosidase as a marker of Rous sarcoma virus gene expression and replication. Mol Cell Biol. 1985 Feb;5(2):281–290. doi: 10.1128/mcb.5.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugatsch T., Stacey D. W. Analysis by microinjection of the biological effects of site-directed mutagenesis in cloned avian leukosis viral DNAs. J Virol. 1982 Aug;43(2):503–510. doi: 10.1128/jvi.43.2.503-510.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugatsch T., Stacey D. W. Identification of a sequence likely to be required for avian retroviral packaging. Virology. 1983 Jul 30;128(2):505–511. doi: 10.1016/0042-6822(83)90279-9. [DOI] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Reinsch S. S., Shank P. R. Sequences near the 5' long terminal repeat of avian leukosis viruses determine the ability to induce osteopetrosis. J Virol. 1986 Jul;59(1):45–49. doi: 10.1128/jvi.59.1.45-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., Hughes S. H. Polypurine tract adjacent to the U3 region of the Rous sarcoma virus genome provides a cis-acting function. J Virol. 1982 Aug;43(2):482–488. doi: 10.1128/jvi.43.2.482-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker A. W., Bissell M. J. Quantitative immunocytochemical assay for infectious avian retroviruses. J Gen Virol. 1987 Sep;68(Pt 9):2481–2485. doi: 10.1099/0022-1317-68-9-2481. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Temin H. M. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983 Dec;3(12):2241–2249. doi: 10.1128/mcb.3.12.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]