Abstract
There is comparatively little information about premorbid maturational brain abnormalities in schizophrenia (SCZ). We investigated whether a history of childhood enuresis, a well-established marker of neurodevelopmental delay, is associated with SCZ and with measures of brain abnormalities also associated with SCZ. A Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) based history of enuresis, volumetric brain MRI scans and neuropsychological testing were obtained in patients with SCZ, their non-psychotic siblings (SIB) and non-psychiatric controls (NC). The subjects were 211 patients (79.6% male), 234 of their SIB (43.2% male) and 355 controls (39.2% male). Frequency of enuresis was compared across groups and correlated with cognitive measures. Total and regional brain volumes were determined using voxel-based morphometry on matched subsets of probands (n = 82) with or without enuresis (n = 16, n = 66, respectively) and controls (n = 102) with or without enuresis (n = 11, n = 91, respectively). Patients with SCZ had higher rates of childhood enuresis (21%) compared with SIB (11%; χ2 = 6.42, P = 0.01) or controls (7%; χ2 = 23.65, P < 0.0001) and relative risk for enuresis was increased in SIB (λS = 2.62). Patients with enuresis performed worse on two frontal lobe cognitive tests [Letter Fluency (t = 1.97, P = 0.05, df = 200) and Category Fluency (t = 2.15, P = 0.03, df = 200)] as compared with non-enuretic patients. Voxel-based morphometry analysis revealed grey matter volume reductions in several frontal regions (right BA 9, right BA 10 and bilateral BA 45) and right superior parietal cortex (BA 7) in patients with a history of enuresis as compared with non-enuretic patients (all t > 3.57, all P < 0.001). The high frequency of childhood enuresis associated with SCZ and abnormalities in prefrontal function and structure in patients with a childhood history of enuresis suggest that childhood enuresis may be a premorbid marker for neurodevelopmental abnormalities related to SCZ. These findings add to the evidence implicating prefrontal dysmaturation in this disorder, potentially related to genetic risk factors.
Keywords: schizophrenia, enuresis, development, neuroimaging, frontal lobes
Introduction
Despite a prodromal period in late adolescence and early adulthood and a relatively fixed age of risk for onset, schizophrenia (SCZ) is generally conceptualized as a neurodevelopmental disorder. Pioneering prospective studies by Bender and later Fish described ‘pandysmaturation’ in motor, intellectual and adaptive functioning of infants who subsequently developed SCZ (Bender, 1947; Fish et al., 1965). Developmental delays have been detected in infancy and early childhood in numerous studies both in offspring of patients with SCZ and ‘pre-schizophrenics’, including abnormalities in acquisition and refinement of language, gross motor delays in standing and walking and delayed physical growth (Fish et al., 1992; Jones et al., 1994; Isohanni et al., 2001; Fish and Kendler, 2005).
The acquisition of volitional bladder control, particularly at night, is an important and well-established developmental neurological milestone. It usually occurs around 4 years of age (Johnson, 1998). Rates of enuresis in otherwise healthy children vary from 10% among 6-year-olds to 5% among 10-year-olds (Neveus et al., 2000). In general, enuresis is more common in boys than girls (Johnson, 1998; Neveus et al., 2000). Persistent bedwetting has been associated with delayed achievement of language and motor milestones in otherwise healthy children (Touchette et al., 2005). The Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) characterizes enuresis as a disorder when there is a persistent loss of bladder control after age 5 years. In most cases, delays in the acquisition of volitional bladder control beyond age 5 years, in the absence of medical causes such as diabetes or spina bifida, represent a neurodevelopmental abnormality.
Few studies have explored the relationship between a history of childhood enuresis and SCZ, although Kraepelin, as early as 1919, anecdotally referred to ‘obstinate nocturnal enuresis’ in childhood in cases of dementia praecox (Kraepelin, 1919–71). In a birth cohort study limited by the absence of statistical analysis, 40 ‘pre-schizophrenics’, assessed at regular intervals from 7 through 28 years of age, were said to be more likely enuretic by day at age 3 years and by night at age 5 years than controls (Crow et al., 1995). In addition to a lack of statistical results, this study did not address whether and to what extent bedwetting persisted beyond age 5 years, which DSM-IV criteria require for a diagnosis of enuresis. Another analysis of this cohort showed that at age 7 years, individuals who subsequently went on to develop SCZ were more likely to have incontinence problems than normal controls (P < 0.10) (Jones, 1997). A Finnish study of developmental progress at 1 year of age suggested that females with slower onset of urinary continence had a slightly increased risk of SCZ (Isohanni et al., 2001). A study of child/adolescent-onset SCZ found late onset of urinary incontinence in 36% of subjects with SCZ (n = 61), compared with 18% of patients with affective psychoses (n = 48) and subjects with a preponderance of negative symptoms were more likely to have had incontinence (Hollis, 2003). It is not clear from this study whether the emergence of incontinence in the setting of manifest illness was associated with enuresis prior to illness onset. This is an important point because the biologic meaning of incontinence during acute psychosis and enuresis during development may not be the same. Another study found increased rates of bedwetting at age 4 years in offspring of schizophrenic women compared with controls (Henriksson and McNeil, 2004). Finally, adult patients with SCZ have higher rates of urinary incontinence than patients with mood disorders (Bonney et al., 1997). These various studies provide some evidence for delayed or impaired bladder control in individuals at risk for SCZ or in child/adolescent-onset SCZ. However, there has been no systematic study of the relationship between SCZ diagnosed in adulthood and antecedent childhood enuresis as defined by current criteria for persistent bedwetting beyond the age of 5 years.
A limited number of studies have explored the relationship between childhood enuresis and other psychiatric disorders. Two studies found increased rates of childhood enuresis in children with attention deficit hyperactivity disorder (28–32%) (Biederman et al., 1995; Neveus et al., 2000), one study found increased rates of enuresis in childhood Tourette's syndrome (22%) (Comings and Comings, 1987) and one study found increased rates of enuresis in adult bipolar disorder (18%) (Henin et al., 2007). Persistent enuresis has also been associated with an increased risk of adolescent suicidal behaviour (Liu and Sun, 2005). Persistent enuresis after the age of 10 years has been associated with a small but significantly increased risk of conduct problems, attention deficit behaviours and anxiety/withdrawal in adolescence (Fergusson and Horwood, 1994). Feehan and colleagues (1990) found that secondary enuresis (enuresis recurring after at least 1 year of continence), but not primary enuresis, was associated with an increased rate of behavioural problems (Feehan et al., 1990). In Chinese children, persistent enuresis after 4 years of age is associated with increased behaviour problems, as reported by both parents and teachers (Liu et al., 2000). A similar finding was reported in US children with enuresis persisting after 5 years of age (Byrd et al., 1996). These studies illustrate that enuresis as a putative marker of cerebral developmental delay is not an antecedent for a specific behavioural disorder any more that it is specifically associated with a single childhood neurological disorder.
To define forebrain structures involved in volitional bladder control, traditional clinical-neuropathological studies have relied upon the association between acquired brain lesions and the development of urinary incontinence. Nearly 75% of stroke patients with frontal lobe lesions developed urinary incontinence (Sakakibara et al., 1999). Right superior frontal damage has been linked with transient urinary incontinence while bilateral damage has been associated with permanent incontinence (Mochizuki and Saito, 1990). These studies suggest that the frontal lobes in general, and the superior frontal gyri in particular, are important in volitional bladder control.
Functional neuroimaging studies have more clearly defined the neural network involved in volitional bladder control. While peripheral muscles, nerves, spinal cord and brainstem structures play an important role in bladder control, forebrain structures are also important. In normal controls, the left superior frontal gyrus, supplementary motor area, cingulate gyrus, left orbitofrontal cortex, bilateral frontal opercula and insula were activated during a strong urge to void, using a functional MRI paradigm (Kuhtz-Buschbeck et al., 2005). Depending upon the physiological paradigm employed, PET studies have implicated the mid-cingulate and prefrontal cortices bilaterally; right inferior frontal gyrus, parts of the premotor cortex and cingulate gyri bilaterally; right anterior cingulate gyrus, left superior prefrontal, middle frontal and anterior cingulate gyri, and left insular cortex in the neural regulation of volitional bladder control (Blok et al., 1997, 1998; Nour et al., 2000; Athwal et al., 2001). In summary, functional neuroimaging studies have implicated a complex neural network involving, among other regions, frontal, insular and cingulate cortices in volitional bladder control.
Neuropathology, neuroimaging and neuropsychological studies have consistently implicated the frontal lobes in the pathogenesis of SCZ (Weinberger et al., 2001). We hypothesized that if these abnormalities in SCZ are related to frontal lobe development, then since maturation and functional competence of the frontal lobes also are important in the acquisition of volitional bladder control in children, schizophrenic patients would have a higher rate of childhood enuresis as a secondary manifestation of their frontal lobe developmental abnormalities. In a broad-based family study of SCZ, we assessed the rates of childhood enuresis in probands compared with their non-psychotic siblings (SIB) and normal controls. Assessment of enuresis in siblings allowed us to address the potential heritability of this developmental condition. We also examined neuropsychological test performance and volumetric brain morphometry [voxel-based morphometry (VBM)] of these groups, hypothesizing that subjects with a history of childhood enuresis would have evidence of persistent frontal lobe dysfunction and volumetric abnormalities continuing into adulthood. This is the first comprehensive study to examine the history of childhood enuresis in a large cohort of patients with adult-onset SCZ.
Methods
Subject selection
A total of 800 subjects participated, a subset of participants in the Clinical Brain Disorders Branch ‘Sibling Study’ protocol (NIMH #95-M-0150), an ongoing investigation of neurobiological abnormalities related to genetic risk for SCZ. Subjects were volunteers recruited nationally and through the local community, as well as from the NIMH Inpatient Schizophrenia Research Program. All of the subjects gave written informed consent according to the guidelines of the NIMH/NIH Institutional Review Board and the regulations and ethical guidelines of the NIH Office of Human Subjects Research. Subjects were between 18 and 60 years of age. Participants included 211 patients with SCZ (herein ‘probands’ or SCZ) [82.5% schizophrenia, 17.5% schizoaffective disorder (79.6% male)], 234 of their non-psychotic siblings (SIB) (43.2% male) and 355 non-psychiatric controls (NC) (39.2% male). All subjects were evaluated by a research psychiatrist for lifetime psychiatric illness and substance use according to DSM-IV, using the Structural Clinical Interview for DSM-IV Disorders (First, 1997). Subjects were excluded from any of the three diagnostic groups if they met DSM-IV criteria for substance use/dependence within the past year and also if they had a lifetime history of abuse/dependence that exceeded 5 years. Furthermore, at the time of testing, all subjects received a blood toxicology screening to rule out acute alcohol or drug intoxication. Subjects were also excluded if they had a history of significant medical or neurological disorders, such as epilepsy or traumatic brain injury.
In addition, detailed demographic information and clinical histories, including age of onset of prodromal symptoms and of illness, were compiled on each proband. Specifically, prodromal age and age of onset were determined by merging data from all past medical/psychiatric records, the Structural Clinical Interview for DSM-IV Disorders interview and self/family completed application. In every case, a third party informant (i.e. either psychiatric records or a treating clinician) was used to verify symptoms and onset of illness. A second board-certified psychiatrist confirmed the diagnosis of SCZ for every proband. Additional information on subject recruitment, screening and examination are described elsewhere (Egan et al., 2001). Table 1 shows the demographic data on the subjects.
Table 1.
Demographic data
| Variable | Probands (SCZ) (n = 211) | Siblings (n = 234) | Controls (n = 355) |
|---|---|---|---|
| Diagnostic groups | |||
| Age (years) | 33.7 (9.1) | 35.1 (9.0) | 31.6 (9.5) |
| Sex (M/F) | 168/43 | 68/84 | 139/216 |
| Race # (%) Caucasian | 172 (81.5) | 130 (85.5) | 297 (83.7) |
| Education (years) | 14.0 (2.1) | 15.9 (2.4) | 16.8 (2.4) |
| WAIS-FSIQ | 92.2 (11.4) | 106.0 (11.0) | 106.9 (9.7) |
| GAF | 46.4 (15.6) | 85.9 (6.7) | 86.5 (6.3) |
| Variable | SCZ + Enuresis (n = 45) | SCZ − Enuresis (n = 166) | Group comparison |
|---|---|---|---|
| Probands with and without Enuresis—Neuropsych subset | |||
| Age (years) | 33.2 (9.2) | 33.8 (9.0) | t = 0.43, P = 0.67 |
| Sex (M/F) | 39/6 | 129/37 | χ2 = 1.75, P = 0.19 |
| Race # (%) Caucasian | 38 (84.4) | 134 (80.7) | χ2 = 0.33, P = 0.57 |
| Prodromal age | 17.1 (6.3) | 19.2 (5.6) | t = 2.06, P = 0.04* |
| Age onset illness | 20.3 (4.8) | 21.6 (5.3) | t = 1.39, P = 0.17 |
| Education (years) | 13.7 (2.1) | 14.1 (2.0) | t = 1.21, P = 0.23 |
| GAF | 44.8 (13.9) | 46.8 (16.0) | t = 0.76, P = 0.45 |
| Age offset enuresis | 8.3 (3.3) | – | – |
| Variable | SCZ + Enuresis (n = 16) | SCZ − Enuresis (n = 66) | Group comparison |
|---|---|---|---|
| Probands with and without Enuresis—VBM imaging subset | |||
| Age (years) | 34.7 (11.2) | 33.1 (9.5) | t = −0.60, P = 0.55 |
| Sex (M/F) | 14/2 | 55/11 | Fisher exact, P = 0.51 |
| Race # (%) Caucasian | 14 (87.5) | 53 (80.3) | Fisher exact, P = 0.40 |
| Prodromal age | 17.6 (6.8) | 19.7 (5.4) | t = 1.31, P = 0.20 |
| Age onset illness | 20.9 (6.2) | 21.7 (5.3) | t = 0.53, P = 0.60 |
| Education (years) | 13.6 (2.2) | 14.4 (2.2) | t = 1.44, P = 0.15 |
| GAF | 46.1 (11.8) | 47.7 (15.9) | t = 0.39, P = 0.70 |
| Age offset enuresis | 8.8 (2.6) | – | |
Mean (SD) unless stated otherwise. WAIS-FSIQ = revised Wechsler Adult Intelligence Scale full-scale intelligence quotient; GAF = Global Assessment of Functioning Scale; Prodromal Age = age of onset of prodromal symptoms of SCZ; *P ≤ 0.05.
A history of enuresis was obtained from the mother of each participant by applying DSM-IV criteria (i.e. frequency of wetting >2 times per week for at least three consecutive months at age 5 years or older, not due to a general medical condition or effect of a substance), as part of a birth and developmental history questionnaire. Neurological/medical comorbidity contributing to childhood enuresis was evaluated through subject and maternal interviews. Subject cohorts were subdivided into those with or without a history of childhood enuresis. Rates of enuresis for each subject cohort were then compared. For the group comparisons with SIB, this particular analysis was limited to a subset of siblings (n = 152) consisting of one randomly selected SIB per family.
Neuropsychological tests
All subjects completed a battery of neuropsychological tests in order to assess academic skill (revised Wide Range Achievement Test reading subtest), general intellectual ability (a four-subtest revised Weschler Adult Intelligence Scale short form IQ), psychomotor speed (Trail Making Tests A and B), working memory (1 and 2 back versions of the N-Back), problem-solving (Wisconsin Card Sorting Test), episodic memory (California Verbal Learning Test) and speeded verbal retrieval (Letter and Category Fluency) (Egan et al., 2001).
Magnetic resonance imaging
Subjects
All structural imaging data free of visible artifact, distortion, incomplete acquisition or evidence of neurological abnormality were processed for whole-brain VBM analysis. Subjects had to have both available and usable structural imaging data meeting these quality control criteria and complete enuresis data to be included in the VBM sample. The resulting sample was 82 probands (16 SCZ + enuresis, 66 SCZ−enuresis) and 102 controls (11 NC + enuresis, 91 NC−enuresis). Thus, 20% of probands and 40.3% of controls from the original sample were excluded based on VBM quality control criteria. All probands were on antipsychotic medications at the timing of scan acquisition. Differences in demographic means between each diagnostic group were tested using an ANOVA in SPSS version 13.0 (www.spss.com). There were no significant differences between probands with and without a history of enuresis in age, sex, education, handedness and revised Wechsler Adult Intelligence Scale full-scale intelligence quotient and there were no significant differences between controls with and without a history of enuresis on these measures. Both the proband and control subgroups were matched for age, sex, revised Wechsler Adult Intelligence Scale full-scale intelligence quotient, handedness and education.
Structural image processing and analyses
Image acquisition and data processing software have been described in a previous study from our group (Pezawas et al., 2004) and most specifically in a recent study from this group involving a largely overlapping sample of subjects (Honea et al., 2008). Customized template creation was based on a sample of 100 healthy volunteers, 100 non-psychotic siblings, and 100 probands taken from our study sample. These subjects were grouped to best represent the sample as a whole and were matched for age (NC 35.6 ± 9.97 years; SCZ 36.18 ± 9.54 years; P > 0.05). The iterative procedure used to make the customized template has been described previously (Pezawas et al., 2004).
We used optimized VBM performed in SPM2, including the non-uniformity correction option (Augood et al., 1997; Ashburner and Friston, 2000). Images were modulated by multiplication with the determinant of the Jacobian of the spatial normalization function and an integration of modulated grey matter voxel values resulted in estimates for total grey matter volume. Modulated images were smoothed 10 mm for the whole-brain VBM analysis.
The normalized, modulated and smoothed grey matter images were analyzed using the General Linear Model as implemented in SPM2. For this within-group analysis for NC and SCZ, we used an analysis of covariance model with enuresis history as factor, age and gender as covariates of interest and total grey matter volume and second-order polynomial age expansions as covariates of no interest. Voxel-by-voxel t-tests were computed across the whole brain to determine global differences in grey matter volumes based on enuresis history, contrasting NC + enuresis versus NC−enuresis and SCZ + enuresis versus SCZ−enuresis. There were an insufficient number of siblings to examine volumetric changes between SIB + enuresis and SIB−enuresis in our structural dataset. Significant voxels [thresholded at 0.001 uncorrected, cluster (k) > 5 contiguous voxels] are reported in Talairach space as in prior reports (Callicott et al., 2005). A threshold was set at this level because of the lack of power, small numbers of those with a history of enuresis and the fact that this was a within-group exploratory analysis that has never been performed previously.
Statistics
Statistical analyses of group differences in parameters other than MRI were computed with chi-square analyses or student's t-tests using Statistica 7.1 (Statsoft, Inc., 2005; www.statsoft.com).
Results
Probands had significantly higher rates of enuresis than their non-psychotic siblings (21.3 versus 11.2%, χ2 = 6.42, P = 0.01) and controls (21.3 versus 7.3%, χ2 = 23.7, P < 0.0001). Male probands also had significantly higher rates of enuresis than male siblings (23.2 versus 10.3%, χ2 = 5.15, P = 0.02) or male controls (23.2 versus 11.5%, χ2 = 7.08, P = 0.008). Female probands had significantly higher rates of enuresis than female controls (14 versus 4.6%, χ2 = 5.38, P = 0.02) but not female siblings (14 versus 12%, χ2 = 0.11, P = 0.72). No significant differences in rates of enuresis were found between siblings and controls in the overall sample or male subset but were found in female siblings versus female controls (12 versus 4.6%, χ2 = 5.14, P = 0.02). Siblings of probands with enuresis had significantly higher rates of enuresis than siblings of probands without enuresis (19.1 versus 7.0%, χ2 = 6.56, P = 0.01). Thus, familial relative risk for enuresis in probands with enuresis was calculated to be λS = 2.62 (Risch, 1990).
Probands with enuresis did not differ from probands without enuresis on any demographic variable, with the exception of being significantly younger at age of first prodromal symptoms (17.1 versus 19.2, t = 2.06, P = 0.04). In analyses of cognitive data, we found that probands with enuresis performed significantly worse than probands without enuresis on two measures, both involving speed-dependent verbal retrieval [Letter Fluency (mean score 31.1 versus 35.2, t = 1.97, P = 0.05, df = 200) and Category Fluency (mean score 33.6 versus 37.8, t = 2.15, P = 0.03, df = 200)]. None of the other contrasts approached significance (all P > 0.20, see Table 2). Given the number of tests performed without alpha correction, it is tempting to consider the verbal fluency findings as representing chance results. However, a nearly identical pattern of findings emerged in controls. Controls with enuresis performed significantly worse than controls without enuresis on Letter Fluency (mean score 40.7 versus 45.3, t = 1.96, df = 335, P = 0.05, two-tailed) and showed a trend towards worse performance on Category Fluency (mean score 50.0 versus 53.8, t = 1.71, df = 335, P = 0.09, two-tailed) in comparison to controls without enuresis. No other significant findings were found between the two control groups on neuropsychological measures. Thus, in both subject groups, enuresis was significantly and selectively associated with relatively poorer verbal fluency performance alone. No significant differences were found between siblings with or without enuresis on any neuropsychological measures.
Table 2.
Neuropsychological assessment of probands with and without enuresis
| Variable | SCZ + Enuresis (n = 45) | SCZ − Enuresis (n = 166) | Group comparison |
|---|---|---|---|
| General intelligence | |||
| WAIS-FSIQ | 92.3 (9.3) | 92.2 (12.0) | t = −0.03, P = 0.98 |
| Academic skill | |||
| WRAT reading | 99.6 (12.8) | 101.5 (11.9) | t = 0.95, P = 0.34 |
| Psychomotor speed | |||
| Trails A | 41.8 (18.8) | 38.2 (15.3) | t = −1.29, P = 0.20 |
| Trails B | 95.9 (50.2) | 96.1 (52.2) | t = 0.02, P = 0.98 |
| Working memory | |||
| N-back (1-back) | 0.66 (0.25) | 0.68 (0.25) | t = 0.77, P = 0.77 |
| N-back (2-back) | 0.51 (0.23) | 0.50 (0.23) | t = −0.06, P = 0.95 |
| Problem solving | |||
| WCST %PE | 39.2 (11.7) | 39.4 (12.0) | t = 0.09, P = 0.93 |
| Episodic memory | |||
| CVLT standard | 23.1 (16.8) | 23.4 (15.6) | t = 0.12, P = 0.91 |
| CVLT corrected | 39.3 (12.8) | 39.6 (11.5) | t = 0.16, P = 0.88 |
| Speeded verbal retrieval | |||
| Letter fluency | 31.1 (10.1) | 35.2 (12.6) | t = 1.97, P = 0.05* |
| Category fluency | 33.6 (9.4) | 37.7 (11.7) | t = 2.15, P = 0.03* |
WRAT = Wide Range Achievement Test; WCST = Wisconsin Card Sorting Test; %PE = percentage of perseverative errors; CVLT = California Verbal Learning Test; *P ≤ 0.05.
Magnetic resonance imaging
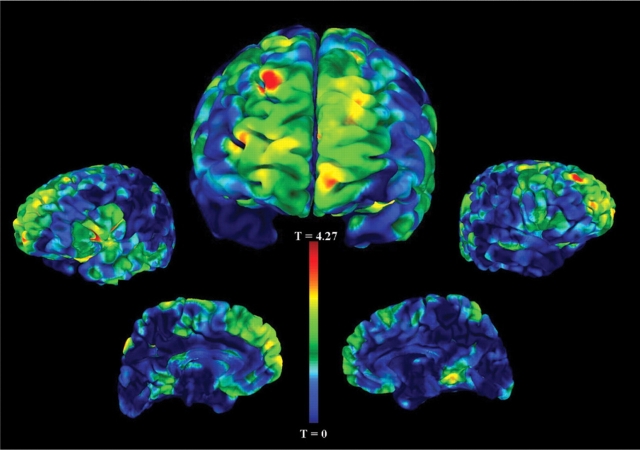
The VBM analysis of probands revealed frontal grey matter decreases in those with a history of enuresis (16 probands with enuresis and 66 probands without enuresis). Specifically, we found reductions of grey matter volume in the right superior frontal gyrus (BA 9, P < 0.001, t = 4.36), right middle frontal gyrus (BA 10, P < 0.001, t = 3.57), bilateral inferior frontal gyrus (BA 45, P < 0.001, t = 3.75) and right superior parietal cortex (BA 7, P < 0.001, t = 3.74) (see Table 3 and Fig. 1).
Table 3.
Voxel-wise statistical analysis of GM maps of decreased grey matter in probands with (n = 16) vs. without enuresis (n = 66), and in healthy controls with (n = 11) and without enuresis (n = 91)
| MNI label | kE | T | Z | P | x | y | z |
|---|---|---|---|---|---|---|---|
| Probands | |||||||
| Right middle frontal gyrus, BA 10 | 84 | 3.57 | 3.33 | 0.000* | 33 | 63 | 13 |
| Right superior frontal gyrus, BA 9 | 404 | 4.36 | 4.10 | 0.000* | 22 | 51 | 39 |
| Left inferior frontal gyrus, BA 45 | 248 | 3.75 | 3.58 | 0.000* | −44 | 27 | 7 |
| Right inferior frontal gyrus, BA 45 | 117 | 3.75 | 3.58 | 0.000* | 47 | 18 | 13 |
| Left superior parietal lobe, BA 7 | 51 | 3.74 | 3.42 | 0.000* | −12 | −79 | 57 |
| Controls | |||||||
| Right medial frontal gyrus, BA 11 | 22 112 | 3.42 | 3.31 | 0.000* | 0 | 20 | −12 |
| Right middle temporal gyrus, BA 21 | 19 033 | 3.42 | 3.31 | 0.000* | 62 | −25 | −9 |
| Left middle temporal gyrus, BA 22 | 25 372 | 4.08 | 3.90 | 0.000* | −53 | −43 | 3 |
| Left cuneus | 1236 | 3.75 | 3.62 | 0.000* | −14 | −76 | 18 |
Peak coordinates listed rostral to caudal. *P < 0.001. kE is the number of voxels in the significant cluster.
Fig. 1.
Grey matter volume decreases in probands with enuresis compared with probands without enuresis. This figure depicts the statistical main effects of decreased regional grey matter volume comparing probands with a history of childhood enuresis to those without a history. The unthresholded VBM results are overlaid on an analysis of functional neuro-images (AFNI) pial surface and colour coded for the statistical t-score. The central figure is the frontal view and the other figures, from left to right, are the left lateral, left medial, right medial and right lateral hemispheric surface views. The most significant cluster of decreased grey matter volume in probands with enuresis is in the right superior frontal gyrus, BA 9 (coordinates x = 22, y = 51, z = 39, P < 0.001). Other clusters that were statistically significant are listed in Table 3.
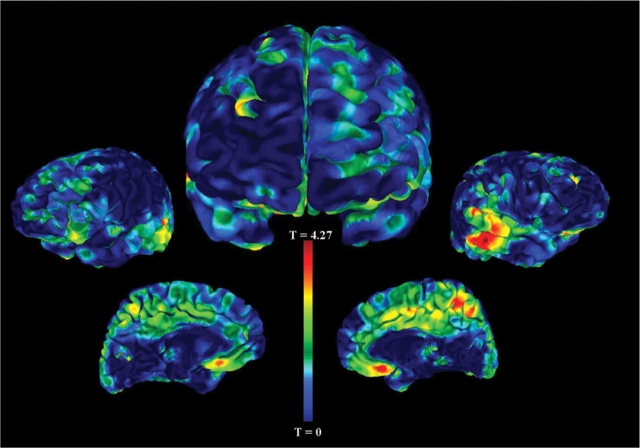
The VBM analysis on the subset of healthy controls also revealed areas of significantly decreased grey matter volume in those with a history of enuresis (11 healthy controls with enuresis and 91 healthy controls without enuresis). More specifically, we found reductions of grey matter volume in the right medial frontal gyrus (BA 11, P < 0.001, t = 3.42), right middle temporal gyrus (BA 21, P < 0.001, t = 3.42), left middle temporal gyrus (BA 22, P < 0.001, t = 4.08) and left cuneus (P < 0.001, t = 3.75) (see Table 3 and Fig. 2).
Fig. 2.
Grey matter volume decreases in controls with enuresis compared with controls without enuresis. This figure depicts the statistical main effects of regional grey matter volume decreases in healthy controls with a history of childhood enuresis versus those without a history. The unthresholded VBM results are overlaid on an analysis of functional neuro-images (AFNI) pial surface and colour coded for the statistical t-score. The central figure is the frontal view and the other figures, from left to right, are the left lateral, left medial, right medial and right lateral hemispheric surface views. The most significant cluster of reduced grey matter volume in healthy controls with enuresis is the left middle temporal gyrus, BA 22 (coordinates x = −53, y = −43, z = 3, P < 0.001). There was also a cluster of decreased grey from the medial frontal gyrus, BA 11 extending into the subgenual cingulate (coordinates x = 0, y = 20, z = −12, P < 0.001). Other clusters that were statistically significant are listed in Table 3.
Discussion
We have found that adult patients with SCZ have an increased rate of childhood enuresis (21%) compared with their healthy siblings (11%) or with normal controls (7%). This is the first and thus largest study to date of childhood enuresis in adult patients with SCZ and the only study of adults with a history of enuresis examined with both neuropsychological testing and structural brain imaging. Indeed, we did find evidence that enuresis is familial (and potentially heritable): the familial risk of enuresis was 2.6 times greater in siblings of enuretic probands than in siblings of non-enuretic probands. Moreover, we found that at least healthy female siblings of patients with SCZ have a significantly increased frequency of enuresis, suggesting that enuresis may be related to genetic risk factors for SCZ. In a preliminary report from our group, we had failed to find an increased rate of enuresis in probands compared with their non-psychotic siblings or controls (Egan et al., 2001). However, that report contained a much smaller sample size, particularly for controls, than the current study and was relatively underpowered.
It is interesting to note that while the age of first diagnosis of SCZ was not significantly earlier for probands with a history of childhood enuresis, prodromal symptoms did appear 2 years earlier in these individuals. These findings suggest that childhood enuresis may be a marker of abnormal brain maturation that predates illness and indicates a predisposition towards the development of SCZ. This is not to say that childhood enuresis is a determinative and specific risk factor for SCZ, as most children with enuresis do not subsequently develop SCZ. The increased familial risk of childhood enuresis in siblings of probands with a history of childhood enuresis suggests that there may be a genetic component underlying this maturational defect. That is, the genetic risk factor may be one for alterations in prefrontal maturation, with childhood enuresis as one consequence and SCZ as another. Since all enuretic subjects in this study eventually gained volitional bladder control, the underlying defect primarily is one of delayed or abnormal maturation, rather than a permanent loss of function.
Our study is not the first to implicate genetic risk in enuresis. In a large Finnish cohort (n = 3206), if fathers were enuretic after age 4 years, the risk of offspring being enuretic was 7.1 times greater (Jarvelin et al., 1988). Comparing monozygotic (n = 1298 pairs) to dizygotic (n = 2419 pairs) twin pairs, the concordance rate for childhood enuresis (at 4 years of age) was significantly higher in monozygotic twins. This was true in male pairs as well as female pairs (Hublin et al., 1998). Twenty-three percent of probands with childhood enuresis had a positive family history of childhood enuresis in a recent study (Wang et al., 2007). In our study, the familial risk of enuresis was 2.6 times greater in siblings of enuretic probands than in siblings of non-enuretic probands. Linkage analysis in families with a high density of enuretic children have implicated a variety of chromosomal locations including 4p16.1, 12q, 13q and 22q11 [see review (von Gontard et al., 2001)]. Additional study is clearly needed to better define the genetic basis of childhood enuresis.
Environmental factors also have been implicated in the aetiology of childhood enuresis. In the past, enuresis was largely attributed to a variety of factors often grouped as ‘psychosocial adversity’ (Stein and Susser, 1965; Oppel et al., 1968). However, Fergusson and colleagues (1986) found no association between childhood enuresis and psychosocial factors including family, social and economic background, family life events or changes in family or residence. While adoption studies might help sort out the relative roles of genes and environment in the aetiology of childhood enuresis, the linkage studies previously cited suggest a strong heritable component.
The VBM analysis on adult probands revealed that a childhood history of enuresis was associated with decreased grey matter in several frontal (right BA 9, right BA 10, bilateral BA 45) and one parietal region (right BA 7). In controls, a somewhat different pattern emerged, with decreased grey matter in frontal (right BA 11) and temporal (right BA 21, left BA 22) cortices and the left cuneus. Lesion studies have associated acquired frontal lobe lesions in adulthood with the development of urinary incontinence (Sakakibara et al., 1999). The findings from subjects with SCZ and controls are in agreement with the proposition that the frontal lobes are intimately involved in the development and maintenance of volitional bladder control. The fact that these structural differences are present in adults with no current incontinence but a childhood history of enuresis suggests that they represent vestigial developmental pathology that is functionally compensated.
The persistence of structural abnormalities in the adult brain related to early postnatal motor development has previously been reported with respect to infant motor development. Ridler and colleagues found that in normal controls, the earlier the age of learning to stand and walk, the greater the grey matter volumes in the premotor cortex, caudate nucleus, thalamus and cerebellum in adulthood (Ridler et al., 2006). Similar to our findings, the study by Ridler and colleagues was notable for demonstrating continuity between behavioural markers of early postnatal development and adult brain structure in humans. Moreover, Ridler and colleagues found that patients with SCZ not only had significant delays in early motor development but also had abnormal associations between early motor development scores and adult premotor cortical grey and fronto-parietal white matter volumes. Similarly, we found that subjects with delayed acquisition of urinary continence had smaller volumes of several cortical regions linked to micturition control. These abnormalities were found in both schizophrenics and normal controls with delayed acquisition of urinary continence, although the precise anatomical structures involved differed somewhat between groups.
Interestingly, right superior frontal damage has been associated with transient urinary incontinence in adulthood (Mochizuki and Saito, 1990). In adult schizophrenic subjects, we found that decreased volume of the right superior frontal gyrus was associated with persistent yet ultimately transient urinary incontinence in childhood. This finding suggests that at least in schizophrenic subjects, delayed or abnormal development of the right superior frontal gyrus (BA9) may mediate childhood enuresis.
Functional MRI and blood-flow PET studies have implicated a wide cortical network in the volitional control of bladder function, with regional activation dependent upon the choice of physiological paradigm. Implicated regions include prefrontal, insular, cingulate and parietal cortices (Mochizuki and Saito, 1990; Blok et al., 1997; Nour et al., 2000; Athwal et al., 2001; Matsuura et al., 2002; Kuhtz-Buschbeck et al., 2005). In both probands and controls with a history of childhood enuresis, there appears to be a developmental precursor within this neural network leading to abnormally small grey matter volumes in multiple prefrontal regions. The differences in the regions implicated by VBM analysis between probands and controls suggest that different genetic and/or environmental factors may be at work, altering the growth and maturation of different aspects of the neural network subserving the development of volitional bladder control in childhood.
Cognitive assessment with neuropsychological tests revealed that probands with a history of enuresis performed significantly worse than probands without enuresis on two measures of timed verbal retrieval (Letter Fluency and Category Fluency). One might have anticipated that a marker of neurodevelopmental compromise (i.e. childhood enuresis) would be associated with cognitive ability and academic skill in a broad sense. That is clearly not the case in this data set; schizophrenic subjects with and without a history of childhood enuresis had very similar scores on the revised Wide Range Achievement Test and revised Wechsler Adult Intelligence Scale estimated Full Scale IQ. Instead, both probands and controls demonstrated specific decreases in both phonemic and semantic fluency. Such selective decreases in verbal fluency would primarily indicate left frontal and temporal lobe impairment based on the lesion and functional imaging literature (Friston, 1995; Mummery et al., 1996; Baldo and Shimamura, 1998; Gourovitch et al., 2000; Baldo et al., 2001, 2006; Costafreda et al., 2006). However, this interpretation is challenged by the fact that we did not observe any hint of an association between a history of childhood enuresis and poorer performance on other putative frontal lobe tests, such as the Wisconsin Card Sorting Test and N-Back (Milner, 1963; Weinberger et al., 1986; Cohen et al., 1997; Callicott et al., 1998; Weiss et al., 2004; Glahn et al., 2005). One possible explanation might be that the Wisconsin Card Sorting Test and N-Back typically activate a broader working memory network involving dorsal prefrontal areas bilaterally as well as superior parietal regions (Glahn et al., 2005), whereas the fluency ‘network’ involves frontal and temporal lobe structures, both of which were implicated in the VBM analysis. Thus, a dissociation of performance on these tasks may be supported by the functional neuroanatomy subserving performance.
Several studies have reported that language functions are less lateralized to the left hemisphere in schizophrenic subjects than controls (Sommer et al., 2004; Weiss et al., 2006). Accordingly, the right frontal VBM abnormalities noted in schizophrenic subjects with a history of enuresis might also mediate their poorer performance on tests of timed verbal retrieval. In contrast, the MRI data showed bilateral temporal lobe volumetric abnormalities in normal subjects with a history of enuresis. Controls with a history of childhood enuresis also performed more poorly on tests of timed verbal retrieval, possibly reflecting abnormal temporal lobe development. Given the relatively broad cortical network subserving volitional bladder control, it is not altogether surprising that different subregions within this network are differentially affected in probands and controls.
We did not observe the same effects on fluency in the sibling cohort. The sibling group is, in many respects, the most problematic of the three groups, presumably having both more risk and protective alleles for SCZ than the controls. This group was also disproportionately female and contains the smallest number of enuretic subjects. Furthermore, it is also clinically heterogeneous. These factors may have obscured any effects on verbal fluency associated with enuresis.
While this report establishes an association between childhood enuresis and late adolescent/adult-onset SCZ, childhood enuresis has also been associated with a variety of childhood, adolescent and adult-onset psychiatric disorders and many neurological disorders of childhood. These include attention deficit hyperactivity disorder (Biederman et al., 1995; Neveus et al., 2000), Tourette's syndrome (Comings and Comings, 1987) and bipolar disorder (Henin et al., 2007), as well as less clearly characterized behavioural disorders of childhood and adolescence (Feehan et al., 1990; Fergusson and Horwood, 1994; Byrd et al., 1996; Liu et al., 2000). These reports are consistent with the view that childhood enuresis is a general reflection of maturational neurodevelopmental abnormalities that impede the acquisition of normal developmental milestones. This is not that surprising, given the complex and widely distributed neural network subserving volitional bladder control. What is novel here is the frequency with which childhood enuresis occurs in individuals who subsequently go on to develop SCZ and the potential heritability of this dysmaturation in their non-schizophrenic siblings. This heritability, in conjunction with the MRI and neuropsychological testing results, suggests that some of the variations in genes that contribute liability towards childhood enuresis also may be involved in frontal lobe growth and development and as such increase the risk of SCZ.
This study has several potential limitations. First, collection of enuresis data by retrospective maternal report may result in a selective recall bias and/or over-reporting of enuresis in subjects with SCZ. However, the fact that siblings as a group and normal control subjects had similar rates of enuresis mitigates against this possibility. The alternative strategy, i.e. prospective recruitment of enuretic subjects who might later develop SCZ would be impractical, and it is unlikely that attempts to gather paediatric records of childhood enuresis for adult subjects would yield much return, if any, given the age of the subjects in the study. Another possible limitation in this study is the discrepancy between male and female ratios for probands and controls. Since enuresis may be more predominant in males, it would be optimal to gather additional male control subjects in order to control for possible gender effects on our findings. However, when analysis was restricted to male subjects, the rates of enuresis were still significantly higher in schizophrenic subjects than their male siblings or male normal controls. Moreover, in the siblings, who were better sex matched with controls, there was a significant difference in frequency of childhood enuresis in the female sibling group.
In summary, childhood enuresis is much more common in schizophrenic subjects than in controls. There may be a heritable component to childhood enuresis in the families of patients with SCZ, as the non-schizophrenic siblings of schizophrenic subjects with enuresis also had a higher rate of enuresis. The genetic trait that underlies a familial predisposition towards childhood enuresis may be involved in prefrontal maturation and development. A history of childhood enuresis may be a marker of frontal cortical maldevelopment, as both adult schizophrenic subjects and controls with a history of childhood enuresis performed poorly on two tests of verbal fluency linked to frontal lobe function. Our anatomic data suggest that there is a persistent abnormality in regional cortical development, particularly involving portions of the frontal lobes. Taken together, the findings from this study advance the concept of SCZ being associated with early developmental difficulties, with particular focus on dysmaturation of the frontal lobes.
Acknowledgements
This study was supported by the NIMH Intramural Research Program, NIH. We acknowledge all the individuals and their families who participated in this study. We acknowledge Joann Berkson, RN, MEd, and Mary Weirich, MSW, for assistance in data collection and John Meyers, BS, for data management.
Glossary
Abbreviations:
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders
- SCZ
schizophrenia
- SIB
non-psychotic sibling
- NC
non-psychiatric control
- VBM
voxel-based morphometry
References
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Athwal BS, Berkley KJ, Hussain I, Brennan A, Craggs M, Sakakibara R, et al. Brain responses to changes in bladder volume and urge to void in healthy men. Brain. 2001;124:369–77. doi: 10.1093/brain/124.2.369. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Westmore K, Emson PC. Phenotypic characterization of neurotensin messenger RNA-expressing cells in the neuroleptic-treated rat striatum: a detailed cellular co-expression study. Neuroscience. 1997;76:763–74. doi: 10.1016/s0306-4522(96)00449-6. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. 2006;12:896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP. Letter and category fluency in patients with frontal lobe lesions. Neuropsychology. 1998;12:259–67. doi: 10.1037//0894-4105.12.2.259. [DOI] [PubMed] [Google Scholar]
- Baldo JV, Shimamura AP, Delis DC, Kramer J, Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc. 2001;7:586–96. doi: 10.1017/s1355617701755063. [DOI] [PubMed] [Google Scholar]
- Bender L. Childhood schizophrenia. Am J Orthopsychiatry. 1947;17:40–56. doi: 10.1111/j.1939-0025.1947.tb04975.x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Santangelo SL, Faraone SV, Kiely K, Guite J, Mick E, et al. Clinical correlates of enuresis in ADHD and non-ADHD children. J Child Psychol Psychiatry. 1995;36:865–77. doi: 10.1111/j.1469-7610.1995.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. A PET study on cortical and subcortical control of pelvic floor musculature in women. J Comp Neurol. 1997;389:535–44. [PubMed] [Google Scholar]
- Blok BF, Sturms LM, Holstege G. Brain activation during micturition in women. Brain. 1998;121(Pt 11):2033–42. doi: 10.1093/brain/121.11.2033. [DOI] [PubMed] [Google Scholar]
- Bonney WW, Gupta S, Hunter DR, Arndt S. Bladder dysfunction in schizophrenia. Schizophr Res. 1997;25:243–9. doi: 10.1016/s0920-9964(97)00021-2. [DOI] [PubMed] [Google Scholar]
- Byrd RS, Weitzman M, Lanphear NE, Auinger P. Bed-wetting in US children: epidemiology and related behavior problems. 1996;98:414–9. [PubMed] [Google Scholar]
- Callicott JH, Ramsey NF, Tallent K, Bertolino A, Knable MB, Coppola R, et al. Functional magnetic resonance imaging brain mapping in psychiatry: methodological issues illustrated in a study of working memory in schizophrenia. Neuropsychopharmacology. 1998;18:186–96. doi: 10.1016/S0893-133X(97)00096-1. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Mattay VS, Hariri AR, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci USA. 2005;102:8627–32. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–8. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Comings DE, Comings BG. A controlled study of Tourette syndrome. VI. Early development, sleep problems, allergies, and handedness. Am J Hum Genet. 1987;41:822–38. [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow TJ, Done DJ, Sacker A. Childhood precursors of psychosis as clues to its evolutionary origins. Eur Arch Psychiatry Clin Neurosci. 1995;245:61–9. doi: 10.1007/BF02190732. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Gscheidle T, Weirich M, Rawlings R, Hyde TM, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- Feehan M, McGee R, Stanton W, Silva PA. A 6 year follow-up of childhood enuresis: prevalence in adolescence and consequences for mental health. J Paediatr Child Health. 1990;26:75–9. doi: 10.1111/j.1440-1754.1990.tb02390.x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ. Nocturnal enuresis and behavioral problems in adolescence: a 15-year longitudinal study. Pediatrics. 1994;94:662–8. [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Shannon FT. Factors related to the age of attainment of nocturnal bladder control: an 8-year longitudinal study. Pediatrics. 1986;78:884–90. [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders – Clinician Verson (SCID-CV). Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- Fish B, Kendler KS. Abnormal infant neurodevelopment predicts schizophrenia spectrum disorders. J Child Adolesc Psychopharmacol. 2005;15:348–61. doi: 10.1089/cap.2005.15.348. [DOI] [PubMed] [Google Scholar]
- Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect. A review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry. 1992;49:221–35. doi: 10.1001/archpsyc.1992.01820030053007. [DOI] [PubMed] [Google Scholar]
- Fish B, Shapiro T, Halpern F, Wile R. The prediction of schizophrenia in infancy: 3 a ten-year follow-up report of neurological and psychological development. Am J Psychiatry. 1965;121:768–75. doi: 10.1176/ajp.121.8.768. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–9. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, et al. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology. 2000;14:353–60. doi: 10.1037//0894-4105.14.3.353. [DOI] [PubMed] [Google Scholar]
- Henin A, Biederman J, Mick E, Hirshfeld-Becker DR, Sachs GS, Wu Y, et al. Childhood antecedent disorders to bipolar disorder in adults: a controlled study. J Affect Disord. 2007;99:51–7. doi: 10.1016/j.jad.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Henriksson KM, McNeil TF. Health and development in the first 4 years of life in offspring of women with schizophrenia and affective psychoses: Well-Baby Clinic information. Schizophr Res. 2004;70:39–48. doi: 10.1016/j.schres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hollis C. Developmental precursors of child- and adolescent-onset schizophrenia and affective psychoses: diagnostic specificity and continuity with symptom dimensions. Br J Psychiatry. 2003;182:37–44. doi: 10.1192/bjp.182.1.37. [DOI] [PubMed] [Google Scholar]
- Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–74. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hublin C, Kaprio J, Partinen M, Koskenvuo M. Nocturnal enuresis in a nationwide twin cohort. Sleep. 1998;21:579–85. doi: 10.1093/sleep/21.6.579. [DOI] [PubMed] [Google Scholar]
- Isohanni M, Jones PB, Moilanen K, Rantakallio P, Veijola J, Oja H, et al. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res. 2001;52:1–19. doi: 10.1016/s0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- Jarvelin MR, Vikevainen-Tervonen L, Moilanen I, Huttunen NP. Enuresis in seven-year-old children. Acta Paediatr Scand. 1988;77:148–53. doi: 10.1111/j.1651-2227.1988.tb10614.x. [DOI] [PubMed] [Google Scholar]
- Johnson M. Nocturnal enuresis. Urol Nurs. 1998;18:259–73. quiz 274–5. [PubMed] [Google Scholar]
- Jones PD, Done DJ. From birth to onset: a developmental perspective of schizophrenia in two national birth cohorts. In: Keshavan MSM, Murray RM, editors. Neurodevelopment and adult psychopathology. Cambridge: Cambridge University Press; 1997. pp. 119–36. [Google Scholar]
- Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–402. doi: 10.1016/s0140-6736(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Dementia praecox. New York: Churchill Livingston: Inc; pp. 1919–71. [Google Scholar]
- Kuhtz-Buschbeck JP, van der Horst C, Pott C, Wolff S, Nabavi A, Jansen O, et al. Cortical representation of the urge to void: a functional magnetic resonance imaging study. J Urol. 2005;174:1477–81. doi: 10.1097/01.ju.0000173007.84102.7c. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun Z. Age of attaining nocturnal bladder control and adolescent suicidal behavior. J Affect Disord. 2005;87:281–9. doi: 10.1016/j.jad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Liu X, Sun Z, Uchiyama M, Li Y, Okawa M. Attaining nocturnal urinary control, nocturnal enuresis, and behavioral problems in Chinese children aged 6 through 16 years. J Am Acad Child Adolesc Psychiatry. 2000;39:1557–64. doi: 10.1097/00004583-200012000-00020. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T. Human brain region response to distention or cold stimulation of the bladder: a positron emission tomography study. J Urol. 2002;168:2035–9. doi: 10.1016/S0022-5347(05)64290-5. [DOI] [PubMed] [Google Scholar]
- Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9:100–110. [Google Scholar]
- Mochizuki H, Saito H. Mesial frontal lobe syndromes: correlations between neurological deficits and radiological localizations. Tohoku J Exp Med. 1990;161(Suppl):231–9. [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc Biol Sci. 1996;263:989–95. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Neveus T, Lackgren G, Tuvemo T, Hetta J, Hjalmas K, Stenberg A. Enuresis—background and treatment. Scand J Urol Nephrol Suppl. 2000:1–44. [PubMed] [Google Scholar]
- Nour S, Svarer C, Kristensen JK, Paulson OB, Law I. Cerebral activation during micturition in normal men. Brain. 2000;123(Pt 4):781–9. doi: 10.1093/brain/123.4.781. [DOI] [PubMed] [Google Scholar]
- Oppel WC, Harper PA, Rider RV. Social, psychological, and neurological factors associated with nocturnal enuresis. Pediatrics. 1968;42:627–41. [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridler K, Veijola JM, Tanskanen P, Miettunen J, Chitnis X, Suckling J, et al. Fronto-cerebellar systems are associated with infant motor and adult executive functions in healthy adults but not in schizophrenia. Proc Natl Acad Sci USA. 2006;103:15651–6. doi: 10.1073/pnas.0602639103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N. Linkage strategies for genetically complex traits. II. The power of affected relative pairs. Am J Hum Genet. 1990;46:229–41. [PMC free article] [PubMed] [Google Scholar]
- Sakakibara R, Fowler CJ, Hattori T. Voiding and MRI analysis of the brain. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:192–9. doi: 10.1007/s001920050044. [DOI] [PubMed] [Google Scholar]
- Sommer IE, Ramsey NF, Mandl RC, van Oel CJ, Kahn RS. Language activation in monozygotic twins discordant for schizophrenia. Br J Psychiatry. 2004;184:128–35. doi: 10.1192/bjp.184.2.128. [DOI] [PubMed] [Google Scholar]
- Stein ZA, Susser MW. Socio-medical study of enuresis among delinquent boys. Br J Prev Soc Med. 1965;19:174–81. doi: 10.1136/jech.19.4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette E, Petit D, Paquet J, Tremblay RE, Boivin M, Montplaisir JY. Bed-wetting and its association with developmental milestones in early childhood. Arch Pediatr Adolesc Med. 2005;159:1129–34. doi: 10.1001/archpedi.159.12.1129. [DOI] [PubMed] [Google Scholar]
- von Gontard A, Schaumburg H, Hollmann E, Eiberg H, Rittig S. The genetics of enuresis: a review. J Urol. 2001;166:2438–43. doi: 10.1097/00005392-200112000-00117. [DOI] [PubMed] [Google Scholar]
- Wang QW, Wen JG, Zhang RL, Yang HY, Su J, Liu K, et al. Family, & segregation studies: 411, Chinese children with primary nocturnal enuresis. Pediatr Int. 2007;49:618–22. doi: 10.1111/j.1442-200X.2007.02406.x. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–24. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50:825–44. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Brinkhoff C, Kremser C, et al. Brain activation patterns during a verbal fluency test-a functional MRI study in healthy volunteers and patients with schizophrenia. Schizophr Res. 2004;70:287–91. doi: 10.1016/j.schres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Weiss EM, Hofer A, Golaszewski S, Siedentopf C, Felber S, Fleischhacker WW. Language lateralization in unmedicated patients during an acute episode of schizophrenia: a functional MRI study. Psychiatry Res. 2006;146:185–90. doi: 10.1016/j.pscychresns.2005.11.003. [DOI] [PubMed] [Google Scholar]