Abstract
Duchenne Muscular Dystrophy (DMD) is the most common and severe form of muscular dystrophy in humans. The goal of myogenic stem cell transplant therapy for DMD is to increase dystrophin expression in existing muscle fibers and to provide a source of stem cells for future muscle generation. Although syngeneic myogenic stem cell transplants have been successful in mice, allogeneic transplants of myogenic stem cells were ineffective in several human trials. To determine whether allogeneic muscle progenitor cells can be successfully transplanted in an immune-tolerant recipient, we induced immune tolerance in two DMD-affected (cxmd) dogs through hematopoietic cell transplantation (HCT). Injection of freshly isolated muscle-derived cells from the HCT donor into either fully or partially chimeric xmd recipients restored dystrophin expression up to 6.48% of wild-type levels, reduced the number of centrally located nuclei, and improved muscle structure. Dystrophin expression was maintained for at least 24 weeks. Taken together, these data indicate that immune tolerance to donor myoblasts provides an important platform from which to further improve myoblast transplantation, with the goal of restoring dystrophin expression to patients with DMD.
Introduction
Duchenne Muscular Dystrophy (DMD) is the most common and severe form of muscular dystrophy in humans, affecting 1 in 3,500 live births.1 , 2 It is an X-linked recessive disorder caused by mutations in the gene for dystrophin, the largest gene identified in the human genome. Mutations are present at birth, yet symptoms do not manifest themselves until 3–5 years of age. Progressive muscle weakness and wasting forces affected individuals to become wheelchair-bound by 12 years of age, and to succumb to the disease in the third decade of life as a result of respiratory and/or cardiac failure.
Transplantation of myogenic stem cells possesses great potential for long-term repair of dystrophic muscle. Indeed, intramuscular injection of adult satellite cell–derived myoblasts from a normal syngeneic donor into mdx mice results in the formation of dystrophin-positive muscle fibers.3 , 4 , 5 , 6 However, small-scale human clinical trials reveal that intramuscular injection of donor myoblasts results in transient expression of dystrophin in a small number of recipient muscle fibers.7 , 8 , 9 , 10 , 11 , 12 In the absence of immunosuppression, injection of donor myoblasts triggers cellular immune responses that destroy newly formed donor myotubes.13
Immunosuppressive drugs, such as cyclosporine (CSP) and FK506, inhibit calcineurin activity and prevent graft rejection. Yet, patients treated with CSP do not display an increased number of dystrophin-positive myofibers relative to patients receiving placebo.10 It has been established that CSP alone is not potent enough to prevent graft rejection; additionally, CSP may negatively affect myoblast engraftment, as calcineurin activity is essential for myogenic differentiation in vitro and in vivo.14 , 15 More important, chronic systemic immunosuppression is associated with serious side effects and considerable risk. Taken together, these observations indicate that myogenic stem cell transplant therapy requires an alternative to traditional immunosuppression.
Nonmyeloablative hematopoietic cell transplantation (HCT) results in mixed chimerism, defined as the coexistence of donor and recipient hematopoietic cells.16 In the clinic, nonmyeloablative transplantation is an outpatient procedure that has been well tolerated in >1,200 patients with malignant and nonmalignant blood disorders.17 Mixed chimerism in both rodents and large animals has successfully induced tolerance to donor-derived kidney, liver, small bowel, heart, lung, and pancreatic islet cells, without the need for immunosuppression.18 , 19 , 20 , 21 , 22 , 23 Specifically, donor kidneys transplanted into mixed chimeric canine recipients were fully functional and remained intact for at least 5 years, even when only 10% of lymphocytes within the recipient were of donor origin.
Therefore, we sought to determine whether HCT could be used to establish an immune-tolerant, random-bred, large animal model of DMD for preclinical myogenic stem cell transplantation studies. The canine model of DMD (cxmd) is characterized by a point mutation in the consensus splice acceptor site in intron 6 of the dystrophin gene, introducing a stop codon within the modified reading frame, resulting in a near complete absence of dystrophin protein.24 , 25 , 26 The dystrophic phenotype of the cxmd canine faithfully recapitulates the human disease, making this an ideal model for investigating potential therapies. HCT alone is unable to restore dystrophin expression to cxmd canines.27 Therefore, we specifically asked whether myeloablative and nonmyeloablative HCT in cxmd canines would permit donor-derived myogenic stem cells to stably engraft and restore dystrophin expression.
Results
HCT and muscle-derived cell transplantation protocol
Bone marrow and granulocyte colony-stimulating factor mobilized peripheral blood mononuclear cells were harvested from two normal donors and transplanted into two irradiated leukocyte antigen–identical cxmd recipients (Figure 1a , Materials and Methods). The hematopoietic cells of one cxmd recipient were all donor derived (Table 1 ; G289; full chimera), whereas the second recipient had a mix of donor and recipient hematopoietic cells (Table 1; G604; mixed chimera). Skeletal muscle–derived mononuclear cells isolated from the same donors were processed for injection immediately after isolation or cultured for 14 days to specifically expand myogenic cells before injection (Figure 1b, Materials and Methods). G289 underwent the myeloablative HCT at 5.5 months of age, followed by muscle-derived cell transplantation at 32 months of age. G604 underwent the nonmyeloablative HCT at 7 months of age, and muscle-derived cell transplantation at 18 months of age.
Figure 1.
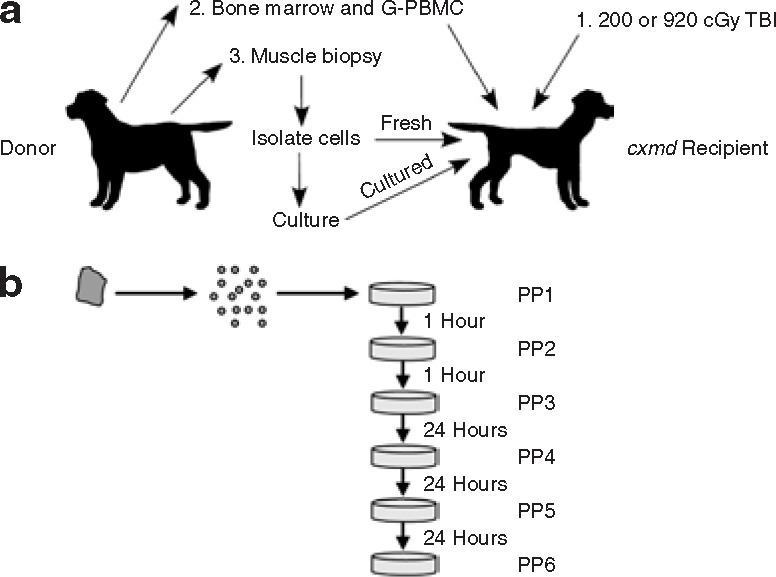
Outline of hematopoietic cell transplantation, donor muscle cell transplant, and cell culture protocols. (a) Myoblast transplantation in an immune-tolerant cxmd canine. Wild-type donor and cxmd recipient dogs were matched by intrafamilial histocompatibility typing. Total body irradiation (TBI; 200 or 920 cGy) of the recipient was followed by intravenous infusion of donor bone marrow (BM), and peripheral blood mononuclear cells were harvested after G-CSF mobilization of hematopoietic stem cells from the BM (G-PBMC). The recipient was ready for donor myoblast transplantation once stable hematopoietic chimerism was reached. For each experiment, the wild-type donor provided two skeletal muscle biopsies, normally harvested from the biceps femoris muscle. The first skeletal muscle biopsy was used to isolate mononuclear cells for culture before injection. The second skeletal muscle biopsy was used to isolate cells that were to be directly injected into the recipient. (b) Cultured cells were separated into distinct cell populations based on differential adherence. Donor muscle–derived cells were plated on culture dishes. After 1 hour, non-adherent cells were transferred to a new plate, and the adherent cells of the original plate were refed with fresh medium. This was repeated at the time intervals indicated until six distinct populations were obtained—PP1 through PP6. Cells were cultured for 14 days and maintained at, or below, 70% confluence. G-CSF, granulocyte colony-stimulating factor; PP, preplate.
Table 1.
Chimeric cxmd recipients used for muscle cell transplantation
| Date of |
% Donor chimerisma |
|||||
|---|---|---|---|---|---|---|
| Recipient dog ID number | Birth | HCT | TBI dose (cGy) | Lymphocyte | Granulocyte | Date of MSC injection |
| G289 | 11/24/2002 | 05/15/2003 | 920 | 100 | 100 | 07/21/2005 |
| G604 | 03/18/2005 | 10/18/2005 | 200 | 80 | 96 | 09/11/2006 |
Abbreviations: HCT, hematopoietic cell transplant; MSC, myogenic stem cell; TBI, total body irradiation. aThe values for donor chimerism represent the last measurement before muscle cell injection.
Injection of donor muscle–derived cells restored dystrophin expression in chimeric cxmd canines
Enzymatic digestion of a skeletal muscle biopsy from the HCT donor released a mixed population of mononuclear cells that included, but was not limited to, satellite cells and fibroblasts. Freshly isolated muscle-derived cells were injected intramuscularly at several sites in the biceps femoris muscle of chimeric recipients. Cryosections from biopsies taken between 4 and 24 weeks after injection were analyzed for dystrophin expression. It was expected that if cells injected into the skeletal muscle of the cxmd chimeric dog were differentiation and fusion competent, then dystrophin expression would be restored.
Indeed, intramuscular injection of 1 × 106 fresh donor muscle–derived cells into the fully chimeric cxmd recipient, G289, restored dystrophin expression to muscle fibers in muscle biopsies taken at 5 and 10 weeks after injection (Figure 2a ). Dystrophin expression was observed throughout the biopsy, extending ∼0.5 cm from the site of injection (data not shown). Similarly, injection of 4.5 × 106 freshly isolated donor muscle–derived cells directly into the muscle of the mixed chimeric cxmd canine, G604, resulted in clusters of dystrophin-expressing fibers, grouped near the sites of injection in biopsies at 4, 8, and 24 weeks after injection (Figure 2b). Expression of dystrophin appeared to be maintained over at least 1,000 μm of fiber length, as assessed by examination of serial sections (data not shown).
Figure 2.
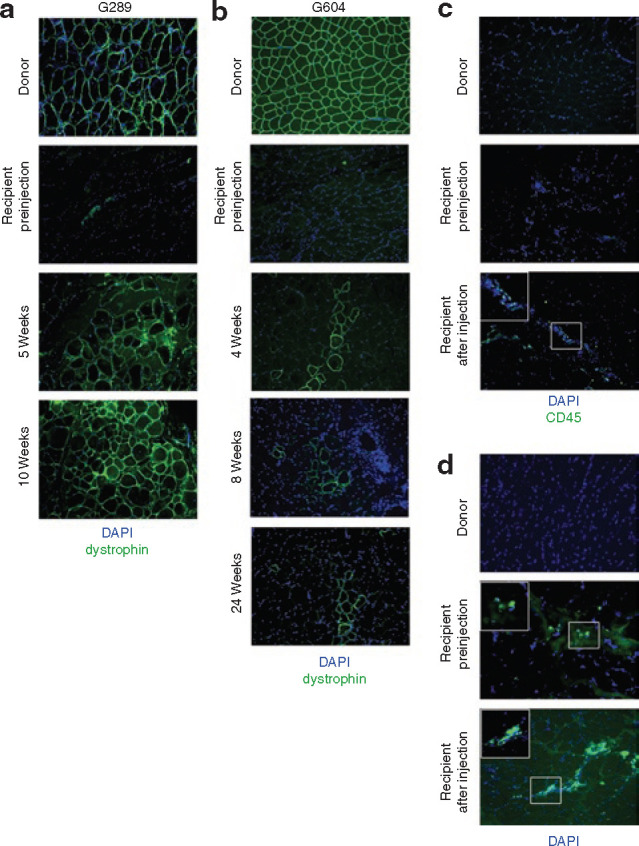
Donor muscle-derived cells engraft into cxmd muscle. (a) Dystrophin expression is restored in full-chimeric cxmd canine. Myeloablative bone marrow transplantation in an cxmd recipient (G289) was followed by intramuscular injection of muscle-derived cells from the marrow donor. Cryosections from donor muscle, recipient muscle preinjection, and recipient muscle 5 and 10 weeks after injection of 1 × 106 freshly isolated cells, were stained using an antibody specific for dystrophin (green). 4′,6-Diamidino-2-phenylindole (DAPI) was used to visualize nuclei (blue). (b) Dystrophin expression is restored in mixed chimeric cxmd canine. Nonmyeloablative bone marrow transplantation in an cxmd recipient (G604) was followed by intramuscular injection of muscle-derived cells from the marrow donor. Cryosections from donor muscle, recipient muscle preinjection, and recipient muscle 4, 8, and 24 weeks after injection of 4.5 × 106 freshly isolated cells were stained using an antibody specific for dystrophin (green). DAPI was used to visualize nuclei (blue). (c,d) Lack of immune cell infiltration in cxmd recipient muscle injected with donor muscle–derived cells. Cryosections from donor muscle, recipient muscle preinjection, and recipient muscle 10 weeks after injection of fresh or cultured cells were stained with antibodies specific to CD45 (hematopoietic cells; green, c) or CD14 (macrophages; green, d). DAPI (blue) was used to visualize nuclei.
Quantitation of donor contribution in injected cxmd recipient muscle
The amount of donor-derived wild-type dystrophin messenger RNA (mRNA) was assessed by real-time reverse transcriptase-PCR (RT-PCR) using a probe that specifically recognizes the wild-type dystrophin splice junction and not the abnormal spliced form in cxmd muscle. The level of dystrophin mRNA observed in wild-type donor muscle was arbitrarily set to 100 and resulted in a relative level of dystrophin mRNA of 0.00035% in the fully chimeric cxmd recipient (G289) before muscle-derived cell transplantation. The level of dystrophin in G289 increased significantly to 6.48% (P = 0.0007) 10 weeks after injection of freshly isolated cells.
The relative level of skeletal muscle dystrophin mRNA in G604 was 0.0019% before muscle-derived cell transplantation (Table 2 ). This increased to 1.29% (P = 0.003) 4 weeks after injection of fresh cells, and remained constant at 24 weeks after injection at 1.32% (P = 0.01). Therefore, in both G289 and G604, the values for dystrophin expression were significantly higher after muscle-derived cell transplantation. Because mononuclear satellite cells and muscle stem cells do not express dystrophin, this indicates that the donor muscle–derived cells differentiated into dystrophin-expressing myotubes.
Table 2.
Quantitation of wild-type dystrophin transcript in cxmd recipient muscle injected with fresh cellsa
| Sample | Relative level of dystrophin expression | P value |
|---|---|---|
| G289 | ||
| Preinjection | 0.00035 ± 0.00009 | — |
| 10 Weeks | 6.48 ± 0.34 | 0.00069 |
| G604 | ||
| Preinjection | 0.0019 ± 0.0001 | — |
| 4 Weeks | 1.29 ± 0.25 | 0.0031 |
| 24 Weeks | 1.32 ± 0.46 | 0.010 |
| WT | 100 | — |
RNA isolated from muscle biopsy cryosections was analyzed by quantitative reverse transcriptase-PCR. Values represent the average level of wild-type dystrophin transcript present in muscle, relative to normal donor, and normalized to β-actin expression (±SD). The level of dystrophin expression in the injected samples was compared to the preinjection sample using the Student's t-test to obtain the P value.
Genomic DNA (gDNA) isolated from groups of cryosections serially cut from biopsies of injected muscle and analyzed using variable number of tandem repeat–PCR confirmed the persistence of significant amounts of donor-derived cell DNA at the latest time points of analysis. The fully chimeric cxmd dog, G289, displayed an average of 13.9% donor gDNA before injection, representing donor blood cell nuclei present as the result of HCT (Table 3 ). Ten weeks after injection of freshly isolated cells, an average of 20.2% of gDNA was donor derived (P = 0.0017), and the highest value of donor contribution to gDNA was 23.1%. The mixed chimeric cxmd dog, G604, displayed an average of 5.0% donor gDNA before injection of muscle-derived cells. Twenty-four weeks after injection of freshly isolated cells, an average of 9.3% was donor derived (P = 0.007), and the highest value of donor contribution to gDNA was 12.3%.
Table 3.
Genomic DNA (gDNA) was isolated from groups of serial cryosections from skeletal muscle biopsies and analyzed using variable number of tandem repeat-PCR
| Sample | % Average donor gDNAa | % Highest value donor gDNA | P value |
|---|---|---|---|
| G289 | |||
| Preinjection | 13.9 | 16 | — |
| 10 Weeks | 20.2 | 23.1 | 0.0017 |
| G604 | |||
| Preinjection | 5.0 | 5.1 | — |
| 24 Weeks | 9.3 | 12.3 | 0.007 |
The average donor DNA represents a minimum of four groups of cryosections. The percent donor gDNA in recipient muscle was compared before and after cell injection using the Student's t-test, and calculated P values are shown.
Injection of donor muscle–derived cells did not elicit an immune response in HCT-transplanted recipients
To determine whether injection of donor muscle–derived cells induced an immune response and infiltration of host immune cells in the recipient, cryosections were immunostained using antibodies directed against CD45, a hematopoietic cell marker; CD3, a T-cell specific marker; and CD14, a monocyte/macrophage-specific marker. Minimal hematopoietic cell infiltration was observed in donor and dystrophic recipient skeletal muscle before transplantation, as evidenced by occasional CD45-expressing cells (Figure 2c—donor and recipient preinjection). There was an absence of CD3-positive T cells (data not shown), yet CD14-positive macrophages were clearly observed in small patches within the recipient DMD-affected muscle before injection (Figure 2d—recipient preinjection), confirming the mild inflammatory process associated with DMD.
At 10 weeks after cell transplantation, a persistent minimal lymphocytic infiltration was observed (Figure 2c—CD45). Careful examination of immunostained cryosections uncovered no CD3-positive cells (data not shown); however, a small region of CD14-positive cells was detected, indicative of monocytes/macrophages, which normally infiltrate degenerating skeletal muscle (Figure 2d—recipient after injection). Therefore, injection of donor muscle–derived cells into cxmd muscle did not induce immune cell infiltration.
Intramuscular injection of donor muscle–derived cells restored muscle structure and reduced regeneration in chimeric cxmd canine
For stem cell therapy to be effective and clinically relevant, function must be restored to skeletal muscle. However, the localized injections used in this study precluded testing for functional improvement. Therefore, we evaluated improvement in muscle structure and stability through histological examination. Hematoxylin and eosin staining of cryosections from the G289 recipient muscle before transplantation revealed a striking loss of muscle structure, considerable variation in muscle fiber size, and increased fatty and connective tissue as compared to normal donor muscle (Figure 3a —donor and recipient preinjection). Injection of freshly isolated muscle-derived cells markedly improved muscle structure at 10 weeks after transplantation and resulted in less connective and fatty tissue infiltration and reduced size variation in fiber diameter when compared to the precell injection biopsy (Figure 3a).
Figure 3.
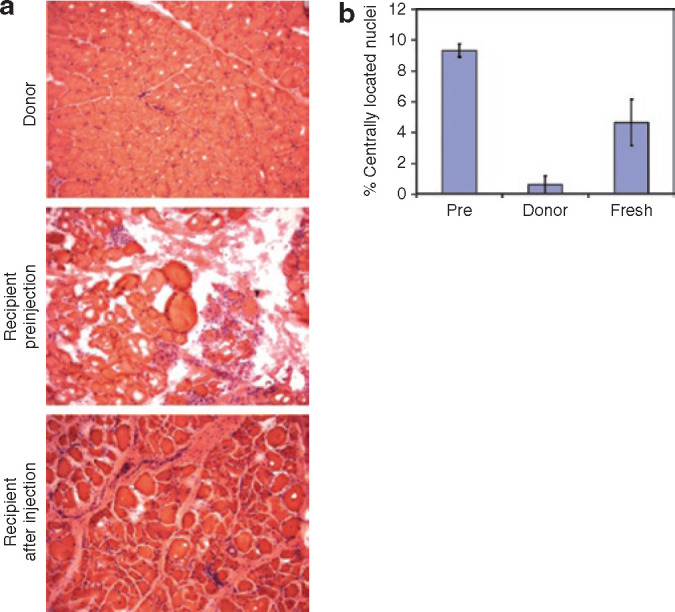
Donor muscle-derived cells restore structure to cxmd muscle. (a) Muscle structure is restored in cxmd recipient muscle injected with donor muscle–derived cells. Cryosections from donor muscle, recipient muscle preinjection, and recipient muscle 10 weeks after injection of freshly isolated cells were stained with hematoxylin and eosin. (b) The number of centrally located nuclei is reduced in cxmd recipient muscle injected with donor muscle–derived cells. The number of centrally located nuclei was counted in four randomly selected fields of view from serial stained sections in a. Bars represent the average percentage of centrally located nuclei in a field of view. Error bars represent SD. The percentage of centrally located nuclei after injection was compared to the percentage preinjection using the Student's t-test (*P < 0.01).
Newly formed skeletal muscle fibers or fibers that have acquired differentiating myocytes as a result of regeneration are characterized by nuclei located in the center of the muscle fiber. In donor wild-type muscle, <1% of muscle nuclei were centrally located. However, ∼10% of muscle nuclei in the G289 DMD-affected recipient were centrally located, indicating that a portion of dystrophic muscle was regenerating (Figure 3b). Injection of fresh muscle-derived cells reduced the number of centrally located myonuclei in the G289 DMD-affected recipient muscle to <5%, which was statistically significant as compared to the recipient before injection (P < 0.01). Together, these findings suggested that engraftment of donor muscle–derived cells and restoration of dystrophin expression stabilized cxmd muscle, preventing repeated attempts to regenerate.
Cultured donor muscle–derived cells restore dystrophin expression
Our data demonstrate that direct injection of freshly isolated donor-derived muscle cells engraft without immune rejection in chimeric recipients. However, many muscle stem cell transplant procedures require expansion of cells in culture, which might alter the immunogenic status of the donor cells. Therefore, to determine whether similar engraftment could be obtained by cells expanded in culture, donor muscle–derived cells were cultured before injection into biceps femoris muscle of the chimeric cxmd recipients. We used a standard serial preplating technique to enrich for myoblasts and analyzed cells from preplates 2 (PP2) and 6 (PP6) (Figure 1b). In order to ensure that donor muscle–derived mononuclear cells were myogenic, adherent PP2 and PP6 cells were fixed in proliferation (Supplementary Figure S1a) and differentiation (Supplementary Figure S1b) conditions. Immunostaining with antibodies specific to desmin and Pax7, two markers of myoblasts in culture, established that >90% of proliferating cultured cells were myogenic.
To assess differentiation potential in vitro, PP2 and PP6 cells were cultured until confluent and fixed after an additional 72 hours. The PP2 population differentiated to form multinucleated myotubes that expressed myogenin and myosin heavy chain (MyHC), two markers of myogenic differentiation (Supplementary Figure S1b—PP2). The PP6 population also expressed myogenin and myosin heavy chain, yet remained primarily mononuclear (Supplementary Figure 1b—PP6). Mouse muscle-derived cells cultured in a comparable manner exhibited similar results.28 , 29
Similar to direct injection of freshly isolated muscle-derived cells, injection of cultured PP6 cells did not induce immune cell infiltration in the injected muscle (data not shown). In addition, the injection of cultured PP6 cells restored dystrophin expression to skeletal muscle of chimeric cxmd recipients (Figure 4a and b). The level of dystrophin reached a maximum of 1.45% of wild-type levels in the fully chimeric dog, G289, and 0.05% in the mixed chimeric dog, G604 (Table 4 ). Although these were significantly higher relative to the recipient levels before muscle cell transplantation (P = 0.0026 and P = 0.009, respectively), the maximum levels of wild-type dystrophin was less than that reached after injection of freshly isolated cells (Table 2).
Figure 4.
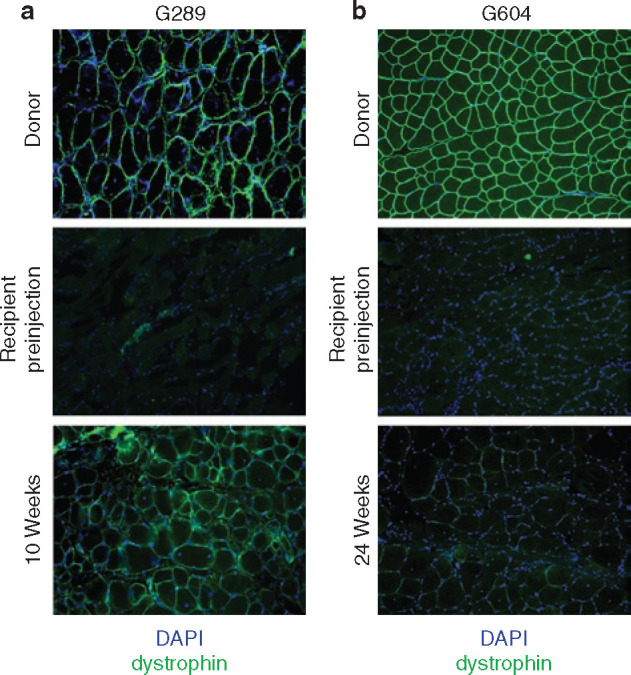
Cultured donor muscle-derived cells engraft into cxmd muscle. (a) Injection of cultured cells into full-chimeric cxmd canine. Myeloablative bone marrow transplantation in an cxmd recipient (G289) was followed by intramuscular injection of muscle-derived cells from the marrow donor. Cryosections from donor muscle, recipient muscle preinjection, and recipient muscle 10 weeks after injection of 5 × 105 cultured cells were stained using an antibody specific for dystrophin (green). 4′,6-Diamidino-2-phenylindole (DAPI) was used to visualize nuclei (blue). (b) Injection of cultured cells into mixed chimeric cxmd canine. Nonmyeloablative bone marrow transplantation in an cxmd recipient (G604) was followed by intramuscular injection of muscle-derived cells from the marrow donor. Cryosections from donor muscle, recipient muscle preinjection, and recipient muscle 24 weeks after injection of 3.0 × 106 cultured cells were stained using an antibody specific for dystrophin (green). DAPI was used to visualize nuclei (blue).
Table 4.
Quantitation of wild-type dystrophin transcript in cxmd recipient muscle injected with cultured cellsa
| Sample | Relative level of dystrophin expression | P value |
|---|---|---|
| G289 | ||
| Preinjection | 0.00035 ± 0.00009 | — |
| 10 Weeks | 1.45 ± 0.15 | 0.0026 |
| G604 | ||
| Preinjection | 0.0019 ± 0.0001 | — |
| 4 Weeks | 0.05 ± 0.02 | 0.009 |
| 24 Weeks | 0.02 ± 0.009 | 0.16 |
| WT | 100 | — |
RNA isolated from muscle biopsy cryosections was analyzed by quantitative reverse transcriptase-PCR. Values represent the average level of wild-type dystrophin transcript present in muscle, relative to normal donor, and normalized to β-actin expression (±SD). The level of dystrophin expression in the injected samples was compared to the preinjection sample using the Student's t-test to obtain the P value.
Discussion
Finding a clinically relevant means of restoring long-term expression of dystrophin to skeletal muscle has been a continuing challenge in the field of DMD research. Transplantation of normal myoblasts into human patients was unsuccessful because of immune rejection of donor cells. While long-term immunosuppression might lengthen muscle cell survival, it is toxic to many organs, including kidneys, liver, muscle, bone, and the central nervous system, and leaves patients vulnerable to opportunistic infections. In this study, we demonstrated that HCT established immune tolerance in cxmd canines, permitting stable engraftment of donor muscle–derived cells in the absence of pharmacological immunosuppression. Importantly, donor muscle–derived cells restored dystrophin expression for at least 24 weeks following transplantation.
Although dystrophin protein was detected extensively throughout cryosections from muscle biopsies obtained after cell injection, the level of dystrophin transcript was relatively low. Notably, it has been suggested in exon-skipping studies that in-frame dystrophin mRNA needs to achieve only 10% of total dystrophin levels to revert affected muscle to a mild Becker phenotype.30 However, it is possible that the level of mRNA observed may not accurately reflect the level of protein present.31 Although dystrophin protein is easily detected by antibody staining of muscle cryosections, dystrophin is estimated to represent <0.01% of cytoplasmic RNA present in adult skeletal muscle,32 suggesting that the muscle fiber may produce only limiting amounts of dystrophin transcript, yet translate the message efficiently into protein. As such, measuring the level of dystrophin transcript may not accurately represent the level of functional dystrophin protein.
Recent studies of allogeneic transplantation of 107 cultured donor myoblasts into DMD patients using a “high-density injection” protocol resulted in 3.5–26% of dystrophin-positive fibers within the injection site area at 4 weeks after transplantation,33 and in one patient some muscle regions had 34.5% positive fibers 18 months after transplantation.34 This protocol relied on the use of FK506 to prevent immune rejection. In our study, we demonstrate that injection of 10- to 20-fold fewer freshly isolated cells resulted in dystrophin-expressing fibers that encompassed ∼30–70% of fibers within the region of injection. Importantly, sustained engraftment did not depend on immunosuppression. This is a crucial distinction, as side effects of FK506 treatment include tremors, hypertension, renal dysfunction, hypomagnesemia, neurological toxicity, and long-term toxicity to liver and kidneys.
Although only two dogs were included in our study, it is important to note that results from separate injection sites within the same dog consistently showed a similar pattern of dystrophin expression. Furthermore, dystrophin expression was clearly restored in both chimeric dogs. Given that HCT induces tolerance to other organs, such as the kidney, lung, and small intestine, the small number of animals in this study nonetheless represent a definitive demonstration of allogeneic tolerance to donor muscle-derived cells and provides an important model from which to further improve dystrophin expression after myogenic cell transplantation.
Achieving greater dystrophin expression requires improving donor muscle–derived cell engraftment. Chimeric cxmd canines provide a model in which to optimize donor muscle–derived cell isolation methods and test molecules that stimulate donor cell proliferation or reprogram recipient muscle to enhance fusion. More than 40 years of success in translating results from canines to humans in the clinic highlights that HCT is a clinically relevant platform from which to study and improve myoblast transplantation.
Materials and Methods
HCT. The Institutional Animal Care and Use Committee at the Fred Hutchinson Cancer Research Center, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, approved this study. Elevated enclosed runs were used for housing, and dogs were maintained in social groups wherever possible. All dogs were enrolled in a veterinary preventative medicine program that included routine anthelmintics and a standard immunization series against canine distemper, parvovirus, adenovirus type 2, parainfluenza virus, coronavirus, and rabies.
Mixed breed cxmd canines were maintained as a colony at the Fred Hutchinson Cancer Research Center, as previously described.27 Littermates composed of healthy wild-type donors, and cxmd recipient dogs were matched by intrafamilial histocompatibility typing using two polymorphic satellite markers—one located in the class I region and one located in the class II region of the major histocompatibility complex.35 , 36 Matching was confirmed in all cases by DRB1 gene sequencing.37
The HCT protocol for cxmd canines was described previously.27 The day of HCT was designated as day 0. Donor marrow cells were aspirated 30 days before transplant, under general anesthesia through needles inserted into the humeri and femora, and cryopreserved. Subcutaneous injections of recombinant canine granulocyte colony-stimulating factor (5 mg/kg b.i.d.; gift from Amgen, Thousand Oaks, CA) into the donor from days −5 to −1 were used to mobilize hematopoietic stem cells from the bone marrow to the peripheral blood. Leukaphereses for collection of granulocyte colony-stimulating factor–“mobilized” peripheral blood mononuclear cells were performed on day 0 using a percutaneous central dual-lumen catheter38 and a continuous flow blood separator (Cobe 2997; Cobe BCT, Lakewood, CO39).
On day 0, each recipient received total body irradiation at a single dose delivered at 7 cGy/min from a linear accelerator (Varian CLINAC 4, Palo Alto, CA).40 One recipient received 200 cGy, and the other received 920 cGy. Within 4 hours of total body irradiation, thawed donor bone marrow cells and freshly isolated granulocyte colony-stimulating factor–“mobilized” peripheral blood mononuclear cells were infused intravenously. Postgrafting immunosuppression consisted of oral CSP, 10 mg/kg twice daily, from days −2 to 35.41 All dogs were given standard supportive care that included subcutaneous fluids with electrolytes, and systemic antibiotics.
Chimerism and fluorescent variable number of tandem repeat–PCR. Hematopoietic chimerism was monitored weekly for the first 8 weeks, biweekly for the next 10 weeks, and monthly thereafter. Donor chimerism was considered stable 28 weeks after HCT. Peripheral blood samples were separated into lymphocytes, granulocytes, and red blood cells using Ficoll-Hypaque gradient fractionation. gDNA was isolated from lymphocytes and granulocytes using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA). gDNA was also isolated from serial cryosections from frozen muscle biopsies using the same QIAamp DNA Mini Kit (Qiagen) and analyzed in the same manner as for hematopoietic chimerism.
Specifically, chimerism was determined using fluorescent variable number of tandem repeats–PCR using ABI Prism 310 Genetic Analyzer and Gene Scan 3.1 software (Applied Biosystems, Foster City, CA). Primers specific for canine microsatellite polymorphisms were used that distinguish donor from recipient.42 , 43
Muscle cell isolation, culture, and injection. For each experiment, the wild-type donor provided two skeletal muscle biopsies, normally harvested from the biceps femoris muscle. The first skeletal muscle biopsy was digested with 400 U/ml collagenase type I (Sigma-Aldrich, St. Louis, MO) in Hank's Balanced Salt Solution (Invitrogen, Carlsbad, CA) or Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with 5 mmol/l CaCl2. Mononuclear cells released from the muscle were plated on culture dishes and maintained in growth medium (Dulbecco's modified Eagle medium, 20% fetal bovine serum, 2.5 ng/ml basic fibroblast growth factor, penicillin and streptomycin, and l-glutamine). A preplating technique similar to the one described by Qu-Petersen et al. 28 (Figure 1b) was used to separate cell populations based on differential adherence. Cells were cultured for 14 days, and maintained at, or below, 70% confluence.
A second skeletal muscle biopsy was removed from the donor on the same day as cells were to be injected into the recipient. Again, mononuclear cells were isolated using collagenase type I; however, cells were washed twice in phosphate-buffered saline (PBS), resuspended in 250 μl of PBS, and prepared for direct injection (fresh cells). A small aliquot was counted and assayed for viability using trypan blue exclusion.
Adherent cells from PP6 of the cultured cells described earlier were harvested using a nonenzymatic cell dissociation buffer, washed twice in PBS, the pellet resuspended in 250 μl of PBS, and prepared for injection. A small aliquot was counted and assayed for viability using trypan blue exclusion.
A 6-cm incision was made in the skin overlaying the muscle of the immune-tolerant cxmd recipient dog to be injected, and the fascia was gently opened to reveal the body of the muscle. Nondissolvable sutures were placed directly in the muscle, and five injections of ∼50 μl each were administered into the muscle surrounding suture, covering ∼1 cm3. The skin was sutured and monitored daily during healing.
For biopsies of injected sites, another incision was made in the skin, and the muscle surrounding each nondissolvable suture was removed. The muscle biopsies were immediately frozen in optimal cutting temperature using liquid nitrogen–cooled isopentane and stored at −80 °C.
Immunostaining. Cryosections were cut (8–10 μm) from frozen muscle biopsies using a Leica CM1850 cryostat, and adhered to Superfrost slides (Fisher Scientific, Pittsburgh, PA). Before staining, sections were fixed in acetone at −20 °C for 10 minutes, allowed to dry, and washed in PBS. Sections were blocked in blocking buffer (2% goat serum, 1% bovine serum albumin, 0.1% cold fish skin gelatin, 0.05% sodium azide, and 0.01 mol/l PBS) and incubated in primary antibody diluted in primary antibody dilution buffer (1% bovine serum albumin, 0.1% cold fish skin gelatin, 0.05% sodium azide, 0.01 mol/l PBS). Primary antibodies against dystrophin included Dy8/6C5 and Dy4/6D3 (Vector Laboratories, Burlington, CA). Monoclonal antibodies MANDYS102 (7D2), MANDYS107 (4H8), and MANEX1A (4C7), specific for dystrophin, were developed by Glenn Morris and obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA. Other primary antibodies used were anti-CD45 (FHCRC Biologics, Seattle, WA) and anti-CD14 (Carpinteria, CA). Fluorescein isothiocyanate–conjugated anti-mouse secondary antibody (Jackson Immunoresearch, West Grove, PA) was diluted in 1× PBS. Sections were mounted using Vectashield containing 4′,6-diamidino-2-phenylindole.
Cultured cells were fixed in 4% paraformaldehyde and permeabilized with 0.3% Triton X-100. Cells were blocked in 10% goat serum and incubated with primary antibody diluted in primary antibody dilution buffer. Primary antibodies specific for Pax7 (Atsushi Kawakami), myogenin (F5D—Woodring E. Wright), and myosin heavy chain (MF20—Donald A. Fischman) were obtained from the Developmental Studies Hybridoma Bank. Monoclonal antibody D33 specific for desmin was purchased from DAKO (Carpinteria, CA). Fluorescein isothiocyanate–conjugated anti-mouse secondary antibody (Jackson Immunoresearch) was diluted in 1× PBS. Sections were mounted using Vectashield containing 4′,6-diamidino-2-phenylindole.
RNA isolation and real-time RT-PCR. RNA was isolated from groups of serial cryosections cut from skeletal muscle biopsies. The cryosections were immediately cut, placed in microcentrifuge tubes kept on dry ice, and stored at −80 °C until processing. Cryosections were incubated in RNALater on ice for 1 minute before RNA isolation. RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA) according to the tissue isolation protocol.
Isolated RNA was quantitated using RiboGreen (Invitrogen, Carlsbad, CA) and a fluorometer, and 100 μg was reverse transcribed into complementary DNA using random hexamers and the High Capacity Complementary DNA Reverse Transcription kit (Applied Biosystems, Foster City, CA), or gene-specific primers and SuperScriptIII (Invitrogen, Carlsbad, CA). The complementary DNA was amplified using the BioRad iQ5 system, with primers and TaqMan probes specific for dystrophin or β-actin as previously described.27 C t values were converted to relative expression levels using the ΔΔC t method.
Supplementary Material
Supplementary Figure S1. Cultured donor muscle-derived cells are myogenic.
Acknowledgments
We thank Szczepan Baran for helping with intramuscular injections and biopsies, and Michael Harkey for chimerism analysis. This work was supported by a Research Development Grant from the Muscular Dystrophy Association (MDA4332, M.H.P.), a Senator Paul D. Wellstone Muscular Dystrophy Cooperative Research Center Grant HD47175, National Institutes of Health grants P01-NS046788, CA15704, and CA78902, and Core Center of Excellence Grant DK56465. The authors have no financial conflicts of interest related to the submitted manuscript.
Footnotes
published online 27 May 2008
Contributor Information
Stephen J Tapscott, Email: stapscot@fhcrc.org.
Rainer Storb, Email: rstorb@fhcrc.org.
References
- 1.Chakkalakal JV, Thompson J, Parks RJ, Jasmin BJ. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies (review) FASEB J. 2005;19:880–891. doi: 10.1096/fj.04-1956rev. [DOI] [PubMed] [Google Scholar]
- 2.Nowak KJ, Davies KE. Duchenne muscular dystrophy and dystrophin: pathogenesis and opportunities for treatment (Review) EMBO Rep. 2004;5:872–876. doi: 10.1038/sj.embor.7400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karpati G, Pouliot Y, Zubrzycka-Gaarn E, Carpenter S, Ray PN, Worton RG. Dystrophin is expressed in mdx skeletal muscle fibers after normal myoblast implantation. Am J Pathol. 1989;135:27–32. [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan JE, Hoffman EP, Partridge TA. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol. 1990;111:2437–2449. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 6.Huard J, Labrecque C, Dansereau G, Robitaille L, Tremblay JP. Dystrophin expression in myotubes formed by the fusion of normal and dystrophic myoblasts. Muscle Nerve. 1991;14:178–182. doi: 10.1002/mus.880140213. [DOI] [PubMed] [Google Scholar]
- 7.Gussoni E, Pavlath GK, Lanctot AM, Sharma KR, Miller RG, Steinman L. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- 8.Huard J, Bouchard JP, Roy R, Labrecque C, Dansereau G, Lemieux B. Myoblast transplantation produced dystrophin-positive muscle fibres in a 16-year-old patient with Duchenne muscular dystrophy. Clin Sci (Lond) 1991;81:287–288. doi: 10.1042/cs0810287. [DOI] [PubMed] [Google Scholar]
- 9.Huard J, Bouchard JP, Roy R, Malouin F, Dansereau G, Labrecque C. Human myoblast transplantation: preliminary results of 4 cases. Muscle Nerve. 1992;15:550–560. doi: 10.1002/mus.880150504. [DOI] [PubMed] [Google Scholar]
- 10.Mendell JR, Kissel JT, Amato AA, King W, Signore L, Prior TW. Myoblast transfer in the treatment of Duchenne's muscular dystrophy. N Engl J Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- 11.Miller RG, Sharma KR, Pavlath GK, Gussoni E, Mynhier M, Lanctot AM. Myoblast implantation in Duchenne muscular dystrophy: the San Francisco study. Muscle Nerve. 1997;20:469–478. doi: 10.1002/(sici)1097-4598(199704)20:4<469::aid-mus10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay JP, Malouin F, Roy R, Huard J, Bouchard JP, Satoh A. Results of a triple blind clinical study of myoblast transplantations without immunosuppressive treatment in young boys with Duchenne muscular dystrophy. Cell Transplant. 1993;2:99–112. doi: 10.1177/096368979300200203. [DOI] [PubMed] [Google Scholar]
- 13.Huard J, Roy R, Bouchard JP, Malouin F, Richards CL, Tremblay JP. Human myoblast transplantation between immunohistocompatible donors and recipients produces immune reactions. Transplant Proc. 1992;24:3049–3051. [PubMed] [Google Scholar]
- 14.Friday BB, Horsley V, Pavlath GK. Calcineurin activity is required for the initiation of skeletal muscle differentiation. J Cell Biol. 2000;149:657–666. doi: 10.1083/jcb.149.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature. 1999;400:576–581. doi: 10.1038/23054. [DOI] [PubMed] [Google Scholar]
- 16.Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem HP. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 17.Sandmaier BM, Storb R, Blume KG, Forman SJ, Appelbaum FR. Thomas' Hematopoietic Cell Transplantation. Blackwell Publishing Ltd.; Oxford: 2004. Nonmyeloablative therapy and hematopoietic cell transplantation for hematologic disorders; pp. 1164–1176. 3rd edn. [Google Scholar]
- 18.Kuhr CS, Allen MD, Junghanss C, Zaucha JM, Marsh CL, Yunusov M. Tolerance to vascularized kidney grafts in canine mixed hematopoietic chimeras. Transplantation. 2002;73:1487–1493. doi: 10.1097/00007890-200205150-00020. [DOI] [PubMed] [Google Scholar]
- 19.Yunusov MY, Kuhr C, Georges GE, Sale GE, Spector M, Lesnikova M. Survival of small bowel transplants in canine mixed hematopoietic chimeras: preliminary results. Transplant Proc. 2002;34:3366–3367. doi: 10.1016/s0041-1345(02)03614-x. [DOI] [PubMed] [Google Scholar]
- 20.Colson YL, Xu H, Huang Y, Ildstad ST. Mixed xenogeneic chimerism induces donor-specific humoral and cellular immune tolerance for cardiac xenografts. J Immunol. 2004;173:5827–5834. doi: 10.4049/jimmunol.173.9.5827. [DOI] [PubMed] [Google Scholar]
- 21.Luo B, Nanji SA, Schur CD, Pawlick RL, Anderson CC, Shapiro AM. Robust tolerance to fully allogeneic islet transplants achieved by chimerism with minimal conditioning. Transplantation. 2005;80:370–377. doi: 10.1097/01.tp.0000167724.38038.ae. [DOI] [PubMed] [Google Scholar]
- 22.Kuhr CS, Yunusov M, Sale G, Loretz C, Storb R. Long-term tolerance to kidney allografts in a preclinical canine model. Transplantation. 2007;84:545–547. doi: 10.1097/01.tp.0000270325.84036.52. [DOI] [PubMed] [Google Scholar]
- 23.Kenyon NS, Yu C, Han D, Storb R, Ricordi C. Stable hematopoietic chimerism leads to acceptance of DLA matched allogeneic islets in the absence of immunosuppression. Blood. 2000;96:1623. [Google Scholar]
- 24.Cooper BJ, Winand NJ, Stedman H, Valentine BA, Hoffman EP, Kunkel LM. The homologue of the Duchenne locus is defective in X-linked muscular dystrophy of dogs. Nature. 1988;334:154–156. doi: 10.1038/334154a0. [DOI] [PubMed] [Google Scholar]
- 25.Fletcher S, Ly T, Duff RM, McC HJ, Wilton SD. Cryptic splicing involving the splice site mutation in the canine model of Duchenne muscular dystrophy. Neuromuscul Disord. 2001;11:239–243. doi: 10.1016/s0960-8966(00)00187-5. [DOI] [PubMed] [Google Scholar]
- 26.Sharp NJ, Kornegay JN, Van Camp SD, Herbstreith MH, Secore SL, Kettle S. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992;13:115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- 27.Dell'Agnola C, Wang Z, Storb R, Tapscott SJ, Kuhr CS, Hauschka SD. Hematopoietic stem cell transplantation does not restore dystrophin expression in Duchenne muscular dystrophy dogs. Blood. 2004;104:4311–4318. doi: 10.1182/blood-2004-06-2247. [DOI] [PubMed] [Google Scholar]
- 28.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jankowski RJ, Deasy BM, Cao B, Gates C, Huard J. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci. 2002;115:4361–4374. doi: 10.1242/jcs.00110. [DOI] [PubMed] [Google Scholar]
- 30.Shiga N, Takeshima Y, Sakamoto H, Inoue K, Yokota Y, Yokoyama M. Disruption of the splicing enhancer sequence within exon 27 of the dystrophin gene by a nonsense mutation induces partial skipping of the exon and is responsible for Becker muscular dystrophy. J Clin Invest. 1997;100:2204–2210. doi: 10.1172/JCI119757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen RC, Nap JP, Mlynarova L. Errors in genomics and proteomics. Nat Biotechnol. 2002;20:19. doi: 10.1038/nbt0102-19b. [DOI] [PubMed] [Google Scholar]
- 32.Smith KP, Moen PT, Wydner KL, Coleman JR, Lawrence JB. Processing of endogenous pre-mRNAs in association with SC-35 domains is gene specific. J Cell Biol. 1999;144:617–629. doi: 10.1083/jcb.144.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skuk D, Goulet M, Roy B, Chapdelaine P, Bouchard JP, Roy R. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J Neuropathol Exp Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- 34.Skuk D, Goulet M, Roy B, Piette V, Cote CH, Chapdelaine P. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Burnett RC, Francisco LV, DeRose SA, Storb R, Ostrander EA. Identification and characterization of a highly polymorphic microsatellite marker within the canine MHC class I region. Mamm Genome. 1995;6:684–685. doi: 10.1007/BF00352386. [DOI] [PubMed] [Google Scholar]
- 36.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 37.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 38.Lee R, Storb R, Little MT, Joslyn A, Spector M, Kuhr CS. Percutaneous central dual-lumen catheter for apheresis in the canine. J Invest Surg. 2002;15:337–341. doi: 10.1080/08941930290086155. [DOI] [PubMed] [Google Scholar]
- 39.Sandmaier BM, Storb R, Santos EB, Krizanac-Bengez L, Lian T, McSweeney PA. Allogeneic transplants of canine peripheral blood stem cells mobilized by recombinant canine hematopoietic growth factors. Blood. 1996;87:3508–3513. [PubMed] [Google Scholar]
- 40.Storb R, Raff R, Deeg HJ, Graham T, Appelbaum FR, Schuening FG. Dose rate-dependent sparing of the gastrointestinal tract by fractionated total body irradiation in dogs given marrow autografts. Int J Radiat Oncol Biol Phys. 1998;40:961–966. doi: 10.1016/s0360-3016(97)00913-9. [DOI] [PubMed] [Google Scholar]
- 41.Zaucha JM, Zellmer E, Georges G, Little MT, Storb R, Storer B. G-CSF-mobilized peripheral blood mononuclear cells added to marrow facilitates engraftment in nonmyeloablated canine recipients: CD3 cells are required. Biol Blood Marrow Transplant. 2001;7:613–619. doi: 10.1053/bbmt.2001.v7.pm11760149. [DOI] [PubMed] [Google Scholar]
- 42.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 43.Scharf SJ, Smith AG, Hansen JA, McFarland C, Erlich HA. Quantitative determination of bone marrow transplant engraftment using fluorescent polymerase chain reaction primers for human identity markers. Blood. 1995;85:1954–1963. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1. Cultured donor muscle-derived cells are myogenic.


