Abstract
Microsomal triglyceride transfer protein (MTP) is essential for the assembly of neutral lipid rich apolipoprotein B (apoB)-lipoproteins. Previously we reported that the Drosophila MTP transfers phospholipids but does not transfer triglycerides. In contrast, human MTP transfers both lipids. To explore the acquisition of triglyceride transfer activity by MTP, we evaluated amino acid sequences, protein structures, as well as the biochemical and cellular properties of various MTP orthologs obtained from species that diverged during evolution. All MTP orthologs shared similar secondary and tertiary structures, associated with protein disulfide isomerase, localized to the endoplasmic reticulum, and supported apoB secretion. While vertebrate MTPs transferred triglyceride invertebrate MTPs lacked this activity. Thus, triglyceride transfer activity was acquired during the transition from invertebrates to vertebrates. Within vertebrates, fish, amphibians, and birds displayed 27%, 40% and 100% triglyceride transfer activity compared to mammals. We conclude that MTP triglyceride transfer activity first appeared in fish and speculate that the acquisition of triglyceride transfer activity by MTP provided for a significant advantage in the evolution of larger and more complex organisms.
Keywords: Lipoproteins, apoB, MTP, triglycerides, evolution, phospholipids, vertebrates, invertebrates
Distinct extracellular lipid transport systems that utilize lipoproteins evolved more than 900 million years ago. These include the apolipophorins circulating through the hemolymph of insects (1; 2), vitellogenins of oviparous animals (3; 4), and the apoB-lipoproteins secreted by vertebrates (5; 6). In insects, hemolymph contains two lipid-containing particles, lipophorins, which exist as high density and low density particles, and lipid transfer particle (LTP). Both these particles are synthesized and secreted by cells; however, biochemical mechanisms involved in their biosynthesis have not been elaborated. Lipophorins acts as re-useable shuttle whereas the LTP loads and unloads lipids, mainly diacylglycerols, onto these particles. High density lipophorins contain two proteins lipophorin I (Mr ~250 kDa) and II (Mr ~70 kDa) that arise from proteolytic cleavage of a precursor protein. The low density lipophorin, in addition, contains lipophorin III (Mr ~18 kDa) that is acquired during the loading of lipids to cover surface of the particles (2; 4; 7). LTP is a very high-density lipoprotein consisting of 14% lipid and three apolipoproteins, apoLTP-1, -II. –III of ~ 350, 85, and 60 kDa. Vitellogenins are female-specific lipoproteins synthesized intracellularly. The protein associated with these lipoproteins is a large molecule of ~ 210 kDa (4). In contrast to the reusable lipophorins, vitellogenins deliver lipids to oocytes via receptor-mediated endocytosis. In the oocytes, these particles undergo proteolytic cleavage and are referred to as lipovitellins and consist of dimeric vitellogenins (3; 4).
The assembly of apoB-lipoproteins in mammalian hepatic and intestinal cells occurs in the endoplasmic reticulum (ER). Microsomal triglyceride transfer protein (MTP) is acknowledged to play a critical role during the assembly and secretion of these particles (8–11). Besides its role in the assembly of apoB-lipoproteins, recent reports suggest that MTP may be vital to the assembly and secretion of vitellogenins as well as lipophorins (12; 13), in the biosynthesis of a lipid antigen presenting molecule CD1d (14–16), and in the development of NKT cells (16). Wetterau and Zilversmit first identified bovine MTP as a heterodimer of MTP and protein disulfide isomerase (PDI) subunits that transfers lipids but prefers to use triacylglycerols, cholesteryl esters and phospholipids as substrates when this activity is measured in vitro (17–19). Antagonists to this activity increase the intracellular degradation of apoB (20) and reduce the secretion of these particles both in cell culture as well as animal models (21; 22). In humans, the absence of MTP activity results in abetalipoproteinemia, a disease characterized by the deficiency of plasma apoB and severely reduced lipid levels (23). Thus, the MTP lipid transfer activity is essential for the formation of apoB-lipoproteins.
In order to study the importance of different lipid transfer activities of MTP, we cloned a Drosophila ortholog (24; 25). The Drosophila MTP transferred phospholipids but did not transfer triacylglycerols. Even though it lacked neutral lipid transfer activity, the Drosophila MTP assisted the secretion of human apoB-lipoproteins. We hypothesized that the phospholipid transfer activity was the most ancient activity associated with MTP and that the neutral lipid transfer activity was acquired during evolution. To test this hypothesis, we compared sequences as well as the various biochemical and cellular properties of MTP from different organisms to establish the period during evolution that MTP acquired neutral lipid transfer activity. This comparison revealed that fish was the first of the MTP to acquire triglyceride transfer activity during evolution. These studies demonstrate that protein structure-function relationships can be studied by exploring the evolutionary changes proteins undergo over periods of time.
Materials and Methods
Protein alignments and structural analyses
MTP amino acid sequences were acquired by executing an iterative protein-protein BLAST (26) against all non-redundant GenBank CDS translations + RefSeq Proteins + PDB + SwissProt + PIR + PRF protein databases using human MTP (CAA58142) as the query. Full-length proteins that produced significant alignments (E threshold value = 0.0) included: Equus caballus (horse, XP_001498540), Bos taurus (bovine, CAA55310), Mus musculus (mouse, NP_032668), Mesocricetus auratus (hamster, AAA53143), Gallus gallus (chicken, XP_420662), Canis familiaris (dog, XP_544995), Sus scrofa (pig, NP_999350), Rattus norvegicus (rat, XP_227765), Pan troglodytes (chimpanzee, XP_526779), Monodelphis domestica (gray short-tailed opossum, XP_001369612), Danio rerio (zebrafish, NP_998135), Strongylocentrotus purpuratus (sea urchin, XP_001192053), Drosophila melanogaster (fruit fly, NP_610075), Drosophila pseudoobscura (fruit fly, EAL33909), Apis mellifera (honeybee, XP_623644), Tribolium castaneum (red flour beetle, XP_973610), Anopheles gambiae (mosquito, EAA13951), and Caenorhabditis elegans (nematode, AAR27937), and Caenorhabditis briggsae (nematode, CAE67922). An incomplete sequence (693 amino acids) for Tetraodon nigroviridis (green spotted pufferfish, CAG03740, E value = 0.0) was also acquired. The Fugu rubripes (Japanese pufferfish) MTP protein sequence has been reported (24). A partial Oryzia latipes (sea squirt) MTP was assembled using protein ESTs (UniGene accession numbers: BJ014235, BJ000420, BJ499123, and BJ735768) (27). Xenopus tropicalis (frog) MTP was acquired from Xenbase (www.xenbase.org). Protein alignments were performed using default settings, thus avoiding the introduction of bias into selection processes, and phylogenetic trees were generated using CLUSTAL W and DRAWTREE programs in Biology Workbench (28). Secondary and three dimensional protein structures were resolved using PELE (Biology Workbench) and PHYRE (www.sbg.bio.ic.ac.uk/~phyre, Protein Homology Analogy Recognition Engine, Imperial College, London), respectively. MTP structural domains N-terminal β-barrel (βN), central α-helical domain (α), C-terminal β-strands (βC and βA) were compared with the corresponding human MTP amino acid sequence (9).
Expression plasmids
Expression vectors containing human MTP, Drosophila MTP (24; 25), and apoB48 (29; 30) have been described. For the expression of zebrafish and C. elegans MTPs, full-length cDNA clones were acquired from the Open Biosystems and the National Institute of Genetics, Mahima, Japan, respectively, amplified by PCR, and subcloned into the mammalian expression vector pCDNA3.1 (Invitrogen). Forward and reverse primers used were 5′CGGGGTACCGACCCCAAACATGATGCCGG3′ and 5′CGGGGTACCCCAGGCCGGCTCAAAGACCTTC3′ as well as 5′CGGGGTACCACCAGAGATGTTCTCATCACG3′ and 5′CGGGGTACCCAACTACAATCTAAACTGCTCC3′ for zebrafish and C. elegans MTP, respectively. In order to generate MTP containing C-terminal FLAG epitope tags, the 3′ antisense primers were made to encode the FLAG sequence (DYKDDDDK) followed by an in frame termination codon.
Cell culture and apoB secretion
COS-7 cells were grown in DMEM (CellGrow) supplemented with 10% fetal bovine serum (FBS), L-glutamine and antibiotic/antimycotic mixture. Cells were plated in 6 well plates at a density of 400,000 cells per well 24 h prior to DNA transfections. DNA was introduced to cells using Polyfect reagent (Qiagen) according to the manufacturer’s instructions. After 48 h, the media were aspirated and 1 ml of lipid-containing media (DMEM, 0.4 mM oleic acid: 1.5% BSA complex, and 1 mM glycerol) was added. Following additional 18 h incubation, the media were collected, protease inhibitor cocktail (Sigma) added, and centrifuged (2,500 rpm, 4°C) to pellet cell debris. The presence of apoB was measured using ELISA (29; 31). Particle density was determined by subjecting conditioned media to a KBr gradient as previously described (25). Briefly, 4 ml of media were brought to a density of 1.30 g/ml by adding KBr and overlaid with 2 ml each of 1.24, 1.15, and 1.063 g/ml density solutions, followed by 1 ml each of 1.019 and 1.006 g/ml. After ultracentrifugation (SW41 rotor, 40,000 rpm, 17 h, 15°C), 1 ml fractions were collected and apoB content determined. The density of each fraction was measured using a refractometer (Fisher Scientific).
Immunofluorescence
COS cells transfected with MTP-FLAG expression plasmids were grown on coverslips in 24-well tissue culture dishes. After 48 h, the cells were fixed and permeabilized in methanol for 15 minutes at −20°C. Fixed cells were blocked with phosphate buffered saline (PBS) containing 1 mM MgCl2, 0.5 mM CaCl2, 3% BSA, and 1% goat serum. Immunofluorescence was performed as described (25). Primary and secondary antibodies were diluted 1:100 in the same buffer used for blocking: M2 anti-FLAG (Sigma), anti-calnexin (Stressgen), Alexa Fluor 488 and Alexa Fluor 594 antibodies (Molecular Probes). The cover slips were mounted in PBS containing 10% glycerol and 12% triethyldiamine (Sigma) to prevent fluorescent bleaching and visualized using a Biorad Radiance 2000 confocal microscope.
Affinity purification of MTP-FLAG
The purification of MTP-FLAG chimeras from COS cell lysates that transiently expressed MTP was performed using M2-anti-FLAG agarose (Sigma) as described (25). Briefly, cell monolayers were washed with PBS, incubated for 2 minutes in hypotonic buffer (1 mM Tris-Cl, pH 7.4, 1 mM MgCl2, and 1 mM EGTA), scraped and passaged 20 times through a 25 Gauge needle. Homogenates were then centrifuged (50,000 rpm, SW55 rotor, 4° C, 1 h) and the supernatant transferred to M2-agarose beads (Sigma). Following incubation (3–5 h at 4°C), M2-bound proteins were eluted with 250 ng/μl FLAG peptide (10 mM Tris-Cl, pH 7.4, 150 mM NaCl, and protease inhibitors). M2 agarose beads were pelleted by centrifugation (10,000 rpm, 30 sec, 4°C) and the supernatant was collected and stored at 4°C. SDS-PAGE followed by western blotting using M2 anti-FLAG antibody (Sigma) was performed to determine the amounts of MTP-FLAG.
Measuring lipid transfer activity
MTP lipid transfer activity was assayed using donor vesicles containing phosphatidylcholine and 1,2 dioleoyl 3-nitrobenzoaxadiazole-labeled triacylglycerols (Chylos Inc.) according to published protocols (32; 33). Cell lysates were obtained by hypotonic lysis followed by ultracentrifugation (SW55 rotor, 50,000 rpm, 4°C, 1 h) to pellet cell debris. To prepare liver homogenates, 100–200 mg of tissues were rinsed with PBS and homogenized in hypotonic buffer containing protease inhibitor cocktail (Sigma) using a Polytron homogenizer (1 minute pulse, setting 5). The homogenates were passed through a 20 Gauge needle followed by a 25 Gauge needle (10–15 times each), centrifuged (SW55 rotor, 50,000 rpm, 1h, 4°C), supernatants were collected, and protein concentrations were determined using Coomassie reagent (34). Equal amounts of soluble proteins were used to assay triacylglycerol transfer activity by MTP. To study the inhibition of triacylglycerol transfer activity, the antagonist CP-346086 (13), kindly provided by Dr. James Harwood of Pfizer, was added to the assay mixture to obtain the appropriate concentration prior to the addition of the MTP source. The final concentration of DMSO did not exceed 0.25%.
Results
Identification of MTP orthologs
Homologs are sequences with common origins that may or may not have same activity. Orthologs are homologous proteins expressed in different species. They represent genes with similar function that were derived from a common ancestor and diverged during evolution. To identify human MTP orthologs, we searched non-redundant protein databases using the full-length human MTP sequence. Initially, 83 proteins were found to share homology with human MTP. These included 16 known MTP proteins from mammals, birds, fish and insects, as well as the previously reported homologous proteins apoB, apolipophorin, and vitellogenin (35). In addition 3 sequences from F. rubripes, O. latipes, and X. tropicalis were obtained as described in Materials and Methods. All 19 MTP sequences were aligned using CLUSTAL W and two conserved regions were identified. The first was located in the N-terminus (Fig 1A). This conserved region is also present in apoB, vitellogenin, as well as apolipophorin (36), and is therefore not specific to MTP. In fact, this region has been used to define the large lipid transfer protein family (35; 37). A second region of conserved residues was identified in the C-terminus (Fig. 1B). Unlike the N-terminal sequence, this region was only recognized in MTP proteins and was not conserved in apoB, apolipophorin, or vitellogenin. Furthermore, it was not present in phospholipid transfer protein or any other lipid transfer protein. We therefore suggest that the C-terminal sequence (QLRPVTFFNGYSDLMSKMLSASGDPISVVKGDHSQELQLQSGLKANIEVQGGLAIDISGAMEFSLW) is specific to MTP. In contrast to the 83 proteins recognized by the full-length human MTP, only 36 sequences were found to share homology with the MTP specific sequence. Of these, 13 represented redundant sequences and two sequences were from the nematode Caenorhabditis. The first was previously described as the dsc-4 gene product in C. elegans (38), while the second corresponded to a hypothetical protein from C. briggsae. The nematode MTP proteins shared homology with both the N- and C-terminal highly conserved regions (Fig 1A and 1B). Thus, only a subset of proteins homologous to MTP contains the MTP specific sequence.
Figure 1. Conserved N- and C-terminal MTP sequences.
(A) The MTP sequences from vertebrates and insects were aligned using CLUSTAL W and color-coded using BOXSHADE. The conserved N-terminal sequence is shown. Identical amino acids are shaded black. The conserved cysteine residues (asterix) known to form a disulfide linkage in lipovitellin (36) are also present in vitellogenin, apolipophorin and apoB. The conserved region identified in vertebrates and insects was present in nematodes. (B) An alignment of C-terminal MTP sequences from different species derived by CLUSTAL W is shown. This sequence contains the MTP specific sequence. Identical residues are shaded black. This region was also present in nematodes but not in apoB and apolipophorin.
In an attempt to understand evolutionary relationship amongst different MTP protein sequences, we generated a phylogenetic tree (Fig 2). The MTP orthologs segregated into vertebrate and invertebrate clusters that could be further divided into four main groups. These corresponded to mammals (group 1) and fish (group 2), as well as insects (group 3) and nematodes (group 4) in the vertebrate and invertebrate clusters, respectively. This division provided a useful framework to investigate the biochemical as well as the functional similarities and differences amongst MTP orthologs.
Figure 2. Phylogram of the MTP proteins.
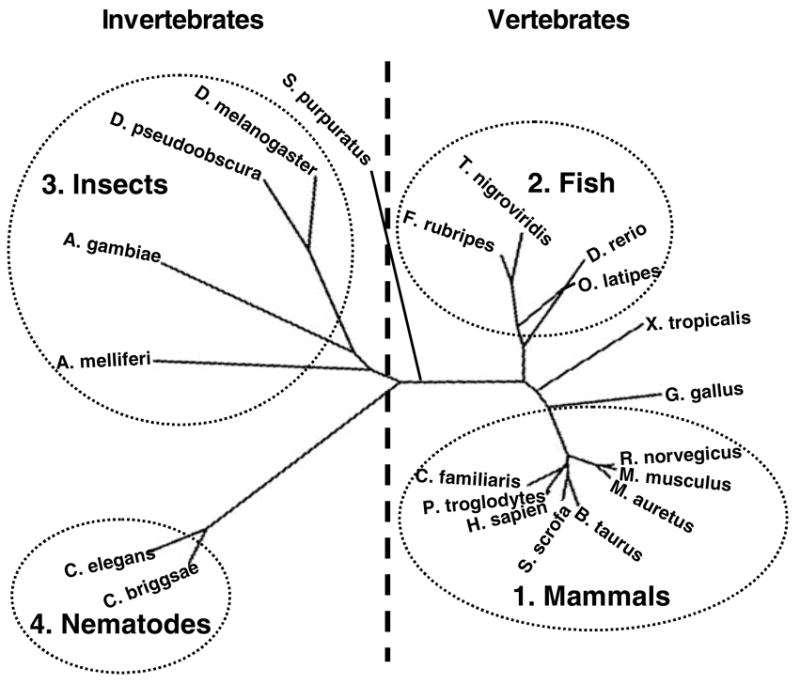
Full and partial MTP sequences were used to perform a phylogenetic comparison. MTP protein sequences acquired from BLAST analysis and databank searches (Table 1) were used to generate an un-rooted tree using the CLUSTAL W and DRAWTREE programs in Biology Workbench. Sequences belonging to mammals (group 1), fish (group 2), insects (group 3), and nematodes (group 4) are shown.
Characterization of MTP orthologs
We previously reported that Drosophila MTP (group 3), like its human ortholog (group 1), was present in the endoplasmic reticulum and Golgi apparatus (25). Here, we investigated the subcellular localization of FLAG-tagged zebrafish (group 2) and C. elegans (group 4) MTP proteins in COS cells using indirect immunofluorescence microscopy (Fig 3A). A reticular fluorescent pattern was detected for both zebrafish and C. elegans MTPs (green) (Fig 3A; a, d). Calnexin staining (red) was used to illustrate the location of the ER (Fig 3A; b, e). Upon merging the images, a significant overlap (yellow) between the MTP and calnexin signals was observed (Fig 3A; c, f). These results demonstrate that both zebrafish and C. elegans MTP proteins reside in the ER similar to the intracellular localization of the mammalian and insect MTP orthologs.
Figure 3. Subcellular localization and interaction with protein disulfide isomerase of MTP orthologs.

(A) The zebrafish and C. elegans sequences were cloned as FLAG-tagged proteins and expressed in COS cells grown on cover slips. After 48 h, the cells were fixed and treated as described in Materials and Methods. Fixed cells were stained with M2 anti-FLAG antibodies (a, d) to identify MTP and anti-calnexin antibodies (b, e) to illustrate the ER. Co-localization of MTP and calnexin is shown in the merged images (c, f) as a yellow color. (B) MTP was purified from rat liver as described by Wetterau et al. (48) and subjected to SDS-PAGE followed by staining with Coomassie Blue. The bands at ~100 and 55 kDa represents the M and P subunits, respectively. (C) COS cells transiently expressing either human (a, b, c), zebrafish (d, e, f), Drosophila (g, h, i), or C. elegans (j, k, l) MTP were fixed and treated with M2 anti-FLAG antibodies to demonstrate MTP (a, d, g, j) while protein disulfide isomerase was visualized using α-PDI antibodies (b, e, h, k). Merged images (c, f, i, l) reveal the co-localization of MTP and PDI as a yellow color. (D) Homogenates from COS cells transiently expressing human, zebrafish, Drosophila, and C. elegans MTP were incubated with M2 (anti-FLAG) agarose beads. Bound proteins were eluted using FLAG peptide and analyzed by Western blotting using antibodies against MTP–FLAG (α-FLAG) as well as protein disulfide isomerase (α-PDI).
Bovine MTP is known to be a lumenal protein that forms a heterodimeric complex with PDI (18; 39). Purified rat MTP also contains two subunits corresponding to 110 and 55 kDa (Fig 3B) indicating that mammalian MTP consist of two subunits that interact in vivo. We have previously demonstrated that Drosophila MTP interacts with PDI (25). To determine if the nematode and fish MTP orthologs also form a soluble heterodimeric complex with mammalian PDI, we first studied the intracellular distribution of zebrafish and C. elegans MTPs and endogenous PDI in COS cells (Fig 3C). All MTP-FLAG proteins and PDI exhibited punctate staining. There was significant overlap between these signals as illustrated by the yellow color in the merged images (Fig 3C; c, f, i, and l representing human, zebrafish, Drosophila, and C. elegans, respectively) indicating extensive co-localization. We next determined whether these MTP orthologs physically associate with PDI. FLAG-chimeras were purified by affinity chromatography from COS cells, and the presence of both the MTP and PDI subunits was studied by Western blotting (Fig 3D). M2 anti-FLAG antibody recognized a band migrating at ~100 kDa while the α-PDI antibody revealed a lower molecular weight protein that migrated at ~55 kDa. Therefore, all the MTP orthologs form a heterodimeric complex with mammalian PDI.
MTP orthologs support apoB secretion
The major function of human MTP is to support apoB-lipoprotein assembly and secretion. We have previously shown that Drosophila MTP assists in the secretion of human apoB (24; 25). To determine if the MTP belonging to groups 2 (fish) and 4 (nematodes) also support apoB secretion, COS cells were co-transfected with different MTP orthologs and apoB48 expression plasmids (Fig 4). Cells were incubated with media supplemented or not with oleic acid (Fig 4A). As expected apoB48 was secreted by cells transfected with human MTP in the absence of oleic acid, and the secretion was enhanced upon the addition of oleic acid to the media. Similarly, Zebrafish, Drosophila and C. elegans MTP orthologs also supported apoB48 secretion that was further augmented when cells were incubated with oleic acid. In a separate experiment, COS cells transfected with human apoB48 and different MTP orthologs were incubated with oleic acid containing media. The conditioned media were subjected to density gradient ultracentrifugation to determine whether apoB48 was secreted as a lipoprotein particle (Fig 4B). Although the total secretion of apoB was greater in cells expressing the human or zebrafish MTPs compared to either the Drosophila or C. elegans MTPs (area under the curves), the density of the secreted particles were similar and ranged between 1.1 – 1.25 g/ml. These studies show that MTP orthologs from fish and nematodes also support the secretion of human apoB48 as a primordial lipoprotein particle similar to that of human MTP.
Figure 4. Zebrafish and C. elegans MTP support the secretion of human apoB.
COS cells were transfected with plasmids expressing different MTP orthologs and apoB48. (A) After 48 h, cells were provided with media supplemented with or without glycerol and oleic acid/BSA complex (− or + OA) as described in Materials and Methods. After 24 h, conditioned media were used to measure apoB secretion. (B) Cells were provided with media containing glycerol and oleic acid/BSA complex as described in Materials and Methods. Conditioned media obtained after 24 h were subjected to density gradient ultracentrifugation and apoB was quantified in each fraction. The density in each fraction is shown as the dashed line.
Vertebrate, but not invertebrate, MTP transfer triacylglycerols
We previously showed that while human MTP (group 1) transfers both phospholipids and triacylglycerols, the Drosophila MTP (group 3) only transfers phospholipids (25). Therefore, we asked whether MTP from zebrafish (group 2) and C. elegans (group 4) transfer triacylglycerols (Fig 5A). Human MTP demonstrated rapid and significant transfer of triacylglycerols in the presence of synthetic lipid donor and acceptor vesicles. In contrast, this activity was absent in lysates prepared from cells expressing Drosophila or C. elegans MTP. The triacylglycerol transfer activity of zebrafish MTP was less than that of human MTP even though greater amounts were used in the in vitro assays (Fig 5A, inset). As we have shown that MTP orthologs assist the secretion of human apoB-lipoproteins, we next considered whether the Drosophila, C. elegans, and zebrafish MTP might transfer triacylglycerols in the presence of an acceptor particle that contains apoB. To assess this possibility, apoB-containing low-density lipoproteins (LDL) were used as acceptors. Cell lysates containing human and zebrafish MTP transferred triacylglycerols in the presence of LDL but those expressing Drosophila and C. elegans MTP were once more deficient in measurable triglycerol transfer activity (Fig 5B). These data show that while vertebrate MTPs (human and zebrafish) transfer triacylglycerols, the invertebrate MTP (Drosophila and C. elegans) are deficient in this activity. Since the differences in triacylglycerol transfer were independent of apoB, we suggest that these differences are inherent properties of MTP orthologs.
Figure 5. Zebrafish, but not C. elegans, MTP transfers triacylglycerols.
(A) COS cells expressing human, zebrafish, Drosophila, and C. elegans MTP were hypotonically lysed, centrifuged to obtain lumenal proteins and used to measure triacylglycerol transfer activity (30 μg protein/assay for human, zebrafish, and Drosophila MTP, and 50 μg protein/assay for C. elegans) using donor and acceptor vesicles (32) as elaborated in Materials and Methods. The amounts of MTP protein present in these samples were compared by Western blotting (inset, H, human; Z, zebrafish; D, Drosophila; C, C.elegans). (B) Lumenal proteins were also used to measure triacylglycerol transfer activity in the presence of donor vesicles and human LDL (3μg protein) as described in Materials and Methods.
Zebrafish MTP is less efficient in transferring triacylglycerols and is less sensitive to inhibition by MTP antagonists
We next measured triacylglycerol transfer activity using similar amounts of human and zebrafish MTP (Fig 6A). Again, even though the amount of protein used in the assay were similar (Fig 6A, inset), zebrafish MTP was less efficient than human MTP in transferring triacylglycerols. On average, the zebrafish MTP was 27 ± 10 % as efficient as the human MTP in this activity (Fig 6B). To compare further the activities between these orthologs, we studied the effect of human MTP antagonist on zebrafish MTP (Fig 6C). CP-346086 inhibited human MTP by ~80% at 10 nM concentration. However, zebrafish MTP was not inhibited at this concentration and only partial inhibition was achieved at higher concentrations. Next, we studied the effect of this inhibitor on the secretion of apoB supported by different MTP orthologs (Fig 6D). At 10 nM concentration, CP-346086 significantly inhibited apoB secretion supported by human MTP. On the other hand, CP-346086 had very little effect on apoB secretion supported by zebrafish and Drosophila MTP (Fig 6D). These data indicate that zebrafish MTP was less efficient in transferring triacylglycerols and this activity was less susceptible to inhibition by antagonists. Thus, there exists a correlation between the efficiency of triacylglycerol transfer activity and its inhibition by antagonist. MTP orthologs demonstrating lower triglyceride transfer activity are less susceptible to inhibition by CP-346086.
Figure 6. Zebrafish MTP is less efficient in transferring triacylglycerols than the human MTP.
(A) To evaluate the activities associated with the human and zebrafish MTP orthologs, equal amounts of MTP-FLAG protein determined by western blotting (inset), were used. Triacylglycerol transfer assays were performed using donor and acceptor vesicles. (B) The transfer activities for human and zebrafish MTPs were normalized to the amount of MTP protein present in the assays performed in triplicate. The human MTP activity was normalized to 100% and relative zebrafish activity was calculated. Values are means ± standard deviations, n = 3. (C) The effect of different concentrations of CP-346086 on human and zebrafish MTPs was studied using lysates (30 μg protein) from COS cells transiently expressing these proteins in the presence of donor and acceptor vesicles. (D) COS cells were transfected with plasmids expressing apoB48 and different MTP orthologs and incubated with different indicated concentrations of the MTP inhibitor (CP-346086) and the amounts of apoB secreted were quantified by ELISA.
Evolution of triacylglycerol transfer activity within vertebrates
Since there were significant differences in the specific activities of human and zebrafish MTPs, we evaluated the triacylglycerol transfer activities of different distinct vertebrate MTPs. Homogenates prepared from the livers of frog, chicken, mouse, rat, and rhesus monkey contained measurable lipid transfer activity (Fig 7A) but Xenopus muscle lysates did not (Fig 7A, control). The specific activity of triacylglycerol transfer in the Xenopus liver was significantly less than that observed in the liver homogenates prepared from chicken, mice, rats, or monkeys (Fig 7B). The frog MTP was ~40% as efficient as the bird or mammalian MTP in transferring triacylglycerols. The reduced activity could be due to low expression of protein or its reduced efficiency of triacylglycerol transfer. As antibodies that recognize all orthologs with equal efficiency were not available we could not compare MTP protein in these samples. Thus, we used CP-346086 to obtain more information about the triacylglycerol transfer activity of different MTP orthologs (Fig 7C). The inhibitor reduced transfer activities in a dose dependent manner in each of the samples. The monkey MTP was the most sensitive and the frog hepatic MTP was the least sensitive to inhibition. These studies indicate that frog MTP transfers triacylglycerols but is less efficient in this activity compared to either bird or mammalian MTP. In addition the frog MTP is also less sensitive to a human MTP inhibitor than other vertebrate MTP proteins.
Figure 7. Triacylglycerol transfer activity in liver homogenates obtained from different species.
Liver (100–300 mg tissue) homogenates were prepared in a hypotonic buffer (1 mM Tris-HCl, pH 7.5, 1 mM MgCl2, and 1 mM EGTA) by several passages through a 25 Gauge needle, centrifuged (50,000 rpm, 1h, 4°C), the supernatant collected, and protein concentrations determined. (A) To measure triacylglycerol transfer, 10 μg of protein were added to MTP assay mixtures containing donor and acceptor vesicles. Incubations were performed at 37°C and fluorescence at 550 nm was monitored over time after excitation at 485 nm. Control represents a lysate prepared from Xenopus muscle. (B) The specific activities from each sample were calculated using a 30 min reading from Panel A. Bar graphs and error bars represent mean ± S.D. (* = p < 0.05). (C) The inhibition of triacylglycerol transfer activity was measured by adding different concentrations of a human MTP antagonist, CP-346086, and assaying for 30 min.
Structural comparison of MTP orthologs
Our studies indicate that invertebrate MTPs are deficient in triglyceride transfer activity. We next sought to identify structural elements responsible for these differences. To determine overall sequence conservation in the MTP orthologs, we compared their % identity with respect to human MTP (Table 1). The vertebrate MTP proteins exhibited greater than 50% identity, while the invertebrate MTP proteins from insects and nematodes shared less than 25% identity. Therefore, a considerable difference in amino acid content was present between the vertebrate and invertebrate proteins.
Table 1.
Sequence identity in MTP orthologs and in their structural domains:
| Group | Amino acids | % Identity | % Identity Within domains | ||
|---|---|---|---|---|---|
| βN | α | βC & βA | |||
| Vertebrates | |||||
| Group 1 (mammals) | |||||
| H. sapien | 894 | 100 | 100 | 100 | 100 |
| P. troglodytes# | 881 | 98 | |||
| S. scrofa | 894 | 89 | |||
| E. caballus | 894 | 89 | 90 | 88 | 91 |
| B. Taurus | 887 | 87 | |||
| R. norvegicus | 896 | 86 | |||
| M. musculus | 894 | 86 | |||
| M. auratus | 895 | 86 | 84 | 86 | 90 |
| C. familiaris# | 905 | 85 | |||
| M. domestica | 875 | 79 | 75 | 78 | 88 |
| G. gallus | 893 | 67 | 60 | 73 | 90 |
| X. tropicalis$ | 889 | 62 | 52 | 66 | 71 |
| Group 2 (fish) | |||||
| O. latipes* | 554 | 58 | |||
| F. rubripes | 870 | 56 | 49 | 59 | 66 |
| D. rerio | 884 | 54 | 40 | 59 | 65 |
| T. nigroviridus# * | 693 | 52 | |||
| Invertebrates | |||||
| S. purpuratus# | 900 | 25 | 25 | 28 | 34 |
| Group 3 (insects) | |||||
| T. castaneum | 872 | 24 | 22 | 21 | 30 |
| A. melliferi# | 894 | 23 | 24 | 25 | 27 |
| D. melanogaster | 886 | 20 | 20 | 24 | 21 |
| D. pseudoobscura | 889 | 19 | 17 | 20 | 20 |
| A. gambiae* | 776 | 19 | |||
| Group 4 (nematodes) | |||||
| C. briggsae# | 888 | 15 | 15 | 16 | 16 |
| C. elegans | 892 | 13 | 14 | 14 | 14 |
Sequence acquired from Xenbase
Predicted protein sequence
Partial amino acid sequence
Human MTP is predicted (9) to consist of an N-terminal β-barrel (βN), central α-helical domain (α) and two C-terminal β-sheets (βC and βA). In efforts to establish whether other MTP orthologs were composed of similar domains we compared their secondary and tertiary structures. The predicted secondary structures of different orthologs were similar to those of human MTP (Fig. 8A). All MTP proteins exhibited an overall βN-α-βC-βA arrangement. Tertiary structures were assembled using PHYRE program (Fig. 8B) and modeled according to the crystal structure of lipovitellin (40). These structures consisted of βN, central α-helical domain, and two C-terminal β-sheets. Thus, these analyses indicate that the secondary and tertiary structures of the MTP orthologs have been conserved throughout the protein’s evolution even though there exist extensive variation in the primary protein sequences.
Figure 8. Structural analysis of MTP orthologs.
(A) The secondary structures of each MTP ortholog were predicted using the PELE Protein Structure Prediction algorithm and the PHYRE protein structure prediction program. Each MTP was found to contain a βN (N-terminal β-barrel), α (central α helical domain), βC (C-terminal α-sheet 1), and βA (C-terminal α-sheet 2). (B) The tertiary structure of human MTP has been described (9) and structures for other MTP orthologs were derived using PHYRE. Four structural domains consistent with those reported for the human MTP were detected (9; 36; 49). (C) The central α-helical domain containing helices 2–9 from vertebrate and invertebrate MTP are shown. Barrels depict α-helices and numbers refer to helices in the α-helical region. Shaded residues are conserved. The C-terminal βC (D) and βA (E) domains were aligned using CLUSTAL W and TEXSHADE (Biology Workbench). Barrels and arrows depict α-helices and β-sheets, respectively. Numbers C2–C7 and A2–A7 represent βC and βA sheets, respectively. Numbers AH1 and AH2 refer to helices within the C-terminal domain. Shaded residues are conserved.
In order to detect changes in amino acid sequences within the structural domains of the MTP proteins, we determined the % identity within these regions (Table 1). The identity between the full-length proteins as well as individual domains decreased progressively from humans to other mammals, amphibians, fish, insects, and nematodes (moving vertically through the table). Therefore, greater substitution of amino acids occurred as the evolutionary distance from humans increased. However, when comparing the identities within individual protein domains (moving horizontally through the table), we detected differences between vertebrates and invertebrates. The βN domains of vertebrates were less conserved than the central α-helical and the C-terminal β-sheets. For example, while the βN of zebrafish MTP was 40% identical to the βN domain in human MTP, the α domain was 59% and C-terminal βC and βA sheets were 65% identical. In contrast, there existed less preference for the conservation of either of these domains in insect and nematode proteins.
To further understand why invertebrate MTPs do no transfer TG, we compared the sequences within different structural domains involved in the triglyceride transfer by mammalian MTP. In lipovitellin, the helices 4–6 of the helical structural domain in addition to the C-terminal β-sheet domains create a large, lipid-containing cavity (41). The hypothetical structure of human MTP also contains a cavity composed of α-helices as well as the entire βC and βA domains and is believed to be involved in triglyceride transfer. We identified greater conservation in helices 4–6 than other helices (7–9) within the central domain of vertebrate MTP (Fig 8C). In contrast, helices 4–6 were not well conserved in the invertebrate MTP orthologs. Similar to the helical domain, βC (Fig 8D) and βA (Fig 8E) domains showed a high degree of conservation in vertebrates. Yet, there was only minimal amino acid conservation present in the invertebrate MTP. These data indicate that an evolutionary trend toward the preferential conservation of the α-helical and C-terminal α-sheet domains exists in vertebrate but not in insect and nematode MTPs. Thus, we conclude that the absence of triglyceride transfer activity in invertebrates might be due to significant sequence variations in the TG transfer domain that is highly conserved in vertebrates.
Discussion
We have previously shown that Drosophila MTP shares several biochemical and functional characteristics with human MTP (25). This includes subcellular localization to the ER and Golgi, binding to PDI, phospholipid transfer activity, and its ability to assist in the assembly and secretion of apoB-lipoproteins. The major difference noted was that the Drosophila MTP was unable to transfer triacylglycerols. In the present study, we investigated how the triglyceride transfer activity of MTP was evolved. Fish, but not nematode, MTP transferred triacylglycerols. The activity in fish, amphibians, and birds was ~27% (Fig 6B), 40% (Fig 7B), and 100%, respectively, of that observed in mammals. Thus, we conclude that MTP triacylglycerol transfer activity first appeared in fish, matured in birds, and was conserved in mammals.
By comparing distinct orthologs, MTP proteins could be divided into four main groups that included vertebrates (mammals and fish) as well as invertebrates (insects and nematodes). All the identified orthologs exhibited secondary and tertiary structures consisting of βN-α-βC-βA domains. They associated with PDI when expressed in monkey kidney COS cells and localized to the ER. Furthermore, they assisted in the assembly and secretion of apoB-lipoproteins. The latter observation is intriguing because apoB is not present in invertebrates. Hence, the basic tenets required for the assembly of primordial apoB-lipoproteins were present prior to the emergence of apoB as the primary transporter of neutral lipids. The acquisition of triacylglycerol transfer activity by the vertebrate MTP might have enhanced the efficiency of packaging neutral lipids into apoB-lipoproteins.
Currently, the MTP structure is believed to consist of βN-α-βC-βA domains. Based on comparative sequence analyses, we suggest that the βN is not specific to MTP as it is also conserved in apoB, lipovitellin and apolipophorin. Furthermore, similar structures are found in other intracellular lipid transfer proteins including phosphatidylcholine transfer protein (PCTP) (42), phosphatidylinositol transfer protein (PITP) (43), and fatty-acid binding protein (FABP) (44). Within the β-barrel of PITP, PCTP and FABP resides a single lipid molecule (42–44). It is noteworthy that a molecule of phospholipid was also identified in the β-barrel of the lipovitellin crystal structure (41). Thus, it is possible that the βN domain in MTP may represent the second phospholipid binding site postulated to exist based on kinetic analysis (45). Based on the structural homology it shares with PCTP, PITP and FABP, MTP might have evolved from these cytoplasmic or as yet unidentified lipid transfer proteins. Subsequently, MTP could have acquired the central α-helical domain and C-terminal β-strands and was able to transfer triglycerides. In contrast to the similarity in the βN domain, MTP and the phospholipid transfer proteins share no significant homology in amino acid sequence. Therefore, it appears that there is no shared ancestry between these proteins, or that the proteins have diverged to such an extent that common ancestry can no longer be predicted based on sequence comparisons.
We performed secondary and tertiary structural studies and found that all the orthologs exhibit very similar secondary and tertiary structural properties. Furthermore, the helical domain and C-terminal β-sheets believed to be involved in triglyceride transfer are conserved in different vertebrate MTP orthologs but not in invertebrates. Despite the structural similarities, our novel observation is that these proteins exhibit very different biochemical properties with respect to their ability to transfer triglycerides. Amino acid sequence comparison studies indicate several differences within the triglyceride transfer domain in the invertebrate and vertebrate MTPs (Fig 8C–E). For example, the central α-helical domain and C-terminal β-sheets are more conserved than the N-terminal (βN) domain in vertebrate MTPs. Within the 18 helices of the α-helical domain, helices 4–6 demonstrate more conservation than the surrounding helices (Fig. 8C). These helices create the superior border of the lipid binding cavity in lipovitellin (3; 40). Similarly, βC (Fig 8D) and βA (Fig 8E) are highly conserved in vertebrates but not in invertebrates. Thus, we suggest that the α-helical domain and the C-terminal β-sheets critical for the triglyceride transfer activity are preserved in vertebrate MTPs but the corresponding domains are not retained in invertebrates. Therefore, the acquisition of triglyceride transfer activity does not appear to be the consequence of simple, few amino acid substitutions.
It is known that organisms use different lipoproteins for lipid transport (Fig 9). Nematodes and insects secrete vitellogenins and lipophorins (1; 3), whereas vertebrates utilize apoB-lipoproteins (46) to transport lipids. Although apoB, vitellogenin, and apolipophorin share sequence similarities (35), these proteins are quite distinct from each other in the amount and types of lipids they transport (1; 3; 9; 46). Vitellogenin and lipophorin are phospholipid-rich lipoproteins and carry smaller amounts of neutral lipids. In contrast, apoB-lipoproteins are neutral lipid rich particles and can transport greater than a thousand lipid molecules. ApoB emerged in fish and birds and was retained in mammals as the primary mode of lipid transport (Fig 9). Thus, the acquisition of triacylglycerol transfer activity by vertebrate MTP was co-incident with the utilization of apoB as the primary carrier of extracellular lipids. We speculate that the emergence of apoB and the acquisition of triacylglycerol transfer activity by MTP provided an efficient system to transport greater amounts of dietary neutral lipids reducing the reliance on endogenous sources of lipids.
Figure 9. Co-emergence of triacylglycerol transfer activity of MTP and apoB.
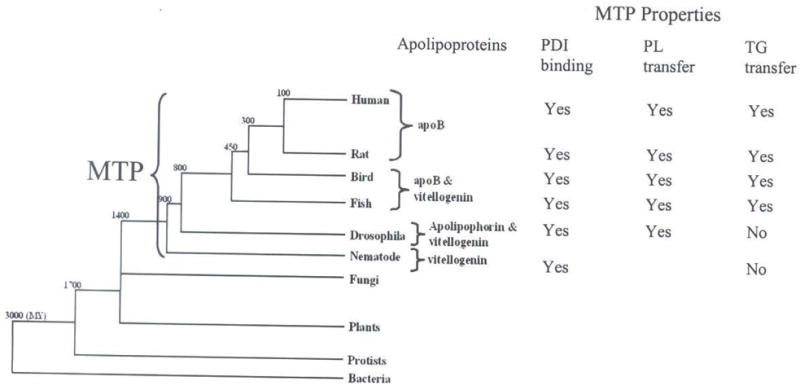
A tree diagram illustrates the evolution of bacteria, yeast, fungi, nematodes, insects, fish, birds, and mammals. The preferred apolipoprotein (apoB, apolipophorin or vitellogenin) utilized by different species as the primary carrier for lipids is indicated. The presence of MTP in different organisms is depicted. Properties of MTP in various orthologs are shown. The numbers located at breakpoints in the tree indicate predicted approximate time of divergence (MY=millions of years ago).
While MTP is required for the assembly of apoB-lipoproteins, it is clear that this protein is not restricted to organisms that utilize apoB to transport extracellular lipids. MTP orthologs have been reported in nematodes (38) and insects (24) as well as in fish (47), birds, and mammals. We were unable to identify MTP orthologs from organisms that diverged earlier than Caenorhabditis suggesting that MTP might have evolved during the emergence of nematodes or that an earlier precursor may share only minimal homology. The conservation of MTP in insects and nematodes suggest that a property other than its triacylglycerol transfer activity must be most ancient and required for its survival. We have shown that insect MTP can transfer phospholipids (25). Thus, the ancient activity evolved in MTP might be the ability to transfer phospholipids and its initial biological role related to phospholipid metabolism.
Usually structure function analysis of proteins is performed using site-directed mutagenesis. Alternative methods include those that identify naturally occurring mutants based on distinct phenotypic characteristics and altered function. In this report, we present an alternate, novel method of comparing the whole protein sequences and then evaluating their biochemical properties using expression systems. This novel approach enabled us to evaluate much larger regions than single amino acids and to speculate on the entire functional domains of MTP. Based on these evolutionary studies, we noted that MTP triglyceride transfer activity evolved with the emergence of apoB as the predominant extracellular lipid carrier. Thus, evolutionary differences can be exploited to gain insights into molecular and functional changes that occurred in proteins and their impact on biological functions.
In conclusion, we have shown that MTP from nematodes and insects do not transfer triacylglycerols. However, fish MTP can transfer triacylglycerols. Appearance of this activity coincides with the progression from relying on the phospholipid-rich lipoproteins (vitellogenin and apolipophorin) to neutral lipid-rich apoB-lipoproteins for extracellular lipid transport. In addition this activity continued to evolve in amphibians and birds and was retained in mammals. We observed that the central α-helical domain and C-terminal β-sheets are preferentially conserved amongst vertebrates but not in invertebrates and suggest that these regions might be critical for the robust triacylglycerol transfer activity associated with vertebrate MTP.
Acknowledgments
This work was supported in part by National Institutes of Health grant HL64272.
Abbreviations used
- ApoB
apolipoprotein B
- BSA
bovine serum albumin
- ER
endoplasmic reticulum
- LTP
lipid transfer particle
- MTP
microsomal triglyceride transfer protein
- PBS
phosphate buffered saline
- PDI
protein disulfide isomerase
- PCTP
phosphatidylcholine transfer protein
- PHYRE
protein homology analogy recognition engine
- PITP
phosphatidylinositol transfer protein
- FABP
fatty-acid binding protein
References
- 1.van der Horst DJ, Van Hoof D, van Marrewijk WJ, Rodenburg KW. Alternative lipid mobilization: the insect shuttle system. Mol Cell Biochem. 2002;239:113–119. [PubMed] [Google Scholar]
- 2.Canavoso LE, Jouni ZE, Karnas KJ, Pennington JE, Wells MA. Fat metabolism in insects. Annu Rev Nutr. 2001;21:23–46. doi: 10.1146/annurev.nutr.21.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Banaszak L, Sharrock W, Timmins P. Structure and function of a lipoprotein: lipovitellin. Annu Rev Biophys Biophys Chem. 1991;20:221–246. doi: 10.1146/annurev.bb.20.060191.001253. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler R, van Antwerpen R. Lipid uptake by insect oocytes. Insect Biochem Mol Biol. 2006;36:264–272. doi: 10.1016/j.ibmb.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Chapman MJ, Forgez P. Lipid transport systems: some recent aspects in swine, cattle and trout during development. Reprod Nutr Dev. 1985;25:217–226. doi: 10.1051/rnd:19850211. [DOI] [PubMed] [Google Scholar]
- 6.Babin PJ, Vernier JM. Plasma lipoproteins in fish. J Lipid Res. 1989;30:467–489. [PubMed] [Google Scholar]
- 7.Ryan RO, van der Horst DJ. Lipid transport biochemistry and its role in energy production. Annu Rev Entomol. 2000;45:233–260. doi: 10.1146/annurev.ento.45.1.233. [DOI] [PubMed] [Google Scholar]
- 8.Shoulders CC, Shelness GS. Current biology of MTP: implications for selective inhibition. Curr Top Med Chem. 2005;5:283–300. doi: 10.2174/1568026053544560. [DOI] [PubMed] [Google Scholar]
- 9.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apolipoprotein B-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 10.Wetterau JR, Lin MCM, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345:136–150. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 11.Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- 12.Shelness GS, Ledford AS. Evolution and mechanism of apolipoprotein B-containing lipoprotein assembly. Curr Opin Lipidol. 2005;16:325–332. doi: 10.1097/01.mol.0000169353.12772.eb. [DOI] [PubMed] [Google Scholar]
- 13.Sellers JA, Hou L, Schoenberg DR, Batistuzzo dM, Sr, Wahli W, Shelness GS. Microsomal triglyceride transfer protein promotes the secretion of Xenopus laevis vitellogenin A1. J Biol Chem. 2005;280:13902–13905. doi: 10.1074/jbc.M500769200. [DOI] [PubMed] [Google Scholar]
- 14.Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, Kaser A, Glickman J, Kuo T, Little A, Morrison J, Corazza N, Kim JY, Colgan SP, Young SG, Exley M, Blumberg RS. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- 15.Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, Kronenberg M, Johnson C, Exley M, Hussain MM, Blumberg RS. Microsomal triglyceride transfer protein: Lipidation and control of CD1d on antigen presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougan SK, Rava P, Hussain MM, Blumberg RS. MTP regulated by an alternate promoter is essential for NKT cell development. J Exp Med. 2007;204:533–545. doi: 10.1084/jem.20062006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetterau JR, Zilversmit DB. A triglyceride and cholesteryl ester transfer protein associated with liver microsomes. J Biol Chem. 1984;259:10863–10866. [PubMed] [Google Scholar]
- 18.Wetterau JR, Combs KA, Spinner SN, Joiner BJ. Protein disulfide isomerase is a component of the microsomal triglyceride transfer protein complex. J Biol Chem. 1990;265:9800 –9807. [PubMed] [Google Scholar]
- 19.Jamil H, Dickson JK, Jr, Chu C-H, Lago MW, Rinehart JK, Biller SA, Gregg RE, Wetterau JR. Microsomal triglyceride transfer protein. Specificity of lipid binding and transport. J Biol Chem. 1995;270:6549–6554. doi: 10.1074/jbc.270.12.6549. [DOI] [PubMed] [Google Scholar]
- 20.Haghpassand M, Wilder D, Moberly JB. Inhibition of apolipoprotein B and triglyceride secretion in human hepatoma cells (HepG2) J Lipid Res. 1996;37:1468–1480. [PubMed] [Google Scholar]
- 21.Chandler CE, Wilder DE, Pettini JL, Savoy YE, Petras SF, Chang G, Vincent J, Harwood HJ., Jr CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans. J Lipid Res. 2003;44:1887–1901. doi: 10.1194/jlr.M300094-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Jamil H, Gordon DA, Eustice DC, Brooks CM, Dickson JK, Jr, Chen Y, Ricci B, Chu C-H, Harrity TW, Ciosek CP, Jr, Biller SA, Gregg RE, Wetterau JR. An inhibitor of the microsomal triglyceride transfer protein inhibits apoB secretion from HepG2 cells. Proc Natl Acad Sci U S A. 1996;93:11991–11995. doi: 10.1073/pnas.93.21.11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- 24.Sellers JA, Hou L, Athar H, Hussain MM, Shelness GS. A drosophila microsomal triglyceride transfer protein homolog promotes the assembly and secretion of human apolipoprotein B: Implications for human and insect lipid transport and metabolism. J Biol Chem. 2003;278:20367–20373. doi: 10.1074/jbc.M300271200. [DOI] [PubMed] [Google Scholar]
- 25.Rava P, Ojakian GK, Shelness GS, Hussain MM. Phospholipid transfer activity of microsomal triacylglycerol transfer protein is sufficient for the assembly and secretion of apolipoprotein B lipoproteins. J Biol Chem. 2006;281:11019–11027. doi: 10.1074/jbc.M512823200. [DOI] [PubMed] [Google Scholar]
- 26.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Church DM, DiCuccio M, Edgar R, Federhen S, Helmberg W, Kenton DL, Khovayko O, Lipman DJ, Madden TL, Maglott DR, Ostell J, Pontius JU, Pruitt KD, Schuler GD, Schriml LM, Sequeira E, Sherry ST, Sirotkin K, Starchenko G, Suzek TO, Tatusov R, Tatusova TA, Wagner L, Yaschenko E. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2005;33:D39–D45. doi: 10.1093/nar/gki062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussain MM, Zhao Y, Kancha RK, Blackhart BD, Yao Z. Characterization of recombinant human apoB-48-containing lipoproteins in rat hepatoma McA-RH7777 cells transfected with apoB48 cDNA: Overexpression of apoB-48 decreases synthesis of endogenous apoB-100. Arterioscler Thromb Vasc Biol. 1995;15:485–494. doi: 10.1161/01.atv.15.4.485. [DOI] [PubMed] [Google Scholar]
- 30.Luchoomun J, Zhou Z, Bakillah A, Jamil H, Hussain MM. Assembly and secretion of VLDL in nondifferentiated Caco-2 cells stably transfected with human recombinant apolipoprotein B48 cDNA. Arterioscler Thromb Vasc Biol. 1997;17:2955–2963. doi: 10.1161/01.atv.17.11.2955. [DOI] [PubMed] [Google Scholar]
- 31.Bakillah A, Zhou Z, Luchoomun J, Hussain MM. Measurement of apolipoprotein B in various cell lines: correlation between intracellular levels and rates of secretion. Lipids. 1997;32:1113–1118. doi: 10.1007/s11745-997-0143-8. [DOI] [PubMed] [Google Scholar]
- 32.Athar H, Iqbal J, Jiang XC, Hussain MM. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J Lipid Res. 2004;45:764–772. doi: 10.1194/jlr.D300026-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Rava P, Athar H, Johnson C, Hussain MM. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J Lipid Res. 2005;46:1779–1785. doi: 10.1194/jlr.D400043-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Babin PJ, Bogerd J, Kooiman FP, van Marrewijk WJ, van der Horst DJ. Apolipophorin II/I, apolipoprotein B, vitellogenin, and microsomal triglyceride transfer protein genes are derived from a common ancestor. J Mol Evol. 1999;49:150–160. doi: 10.1007/pl00006528. [DOI] [PubMed] [Google Scholar]
- 36.Mann CJ, Anderson TA, Read J, Chester SA, Harrison GB, Köchl S, Ritchie PJ, Bradbury P, Hussain FS, Amey J, Vanloo B, Rosseneu M, Infante R, Hancock JM, Levitt DG, Banaszak LJ, Scott J, Shoulders CC. The structure of vitellogenin provides a molecular model for the assembly and secretion of atherogenic lipoproteins. J Mol Biol. 1999;285:391–408. doi: 10.1006/jmbi.1998.2298. [DOI] [PubMed] [Google Scholar]
- 37.Smolenaars MM, Madsen O, Rodenburg KW, van der Horst DJ. Molecular diversity and evolution of the large lipid transfer protein superfamily. J Lipid Res. 2007;48:489–502. doi: 10.1194/jlr.R600028-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Shibata Y, Branicky R, Landaverde IO, Hekimi S. Redox regulation of germline and vulval development in Caenorhabditis elegans. Science. 2003;302:1779–1782. doi: 10.1126/science.1087167. [DOI] [PubMed] [Google Scholar]
- 39.Wetterau JR, Combs KA, McLean LR, Spinner SN, Aggerbeck LP. Protein disulfide isomerase appears necessary to maintain the catalytically active structure of the microsomal triglyceride transfer protein. Biochemistry. 1991;30:9728–9735. doi: 10.1021/bi00104a023. [DOI] [PubMed] [Google Scholar]
- 40.Raag R, Appelt K, Xuong NH, Banaszak L. Structure of the lamprey yolk lipid-protein complex lipovitellin- phosvitin at 2.8 A resolution. J Mol Biol. 1988;200:553–569. doi: 10.1016/0022-2836(88)90542-6. [DOI] [PubMed] [Google Scholar]
- 41.Thompson JR, Banaszak LJ. Lipid-protein interactions in lipovitellin. Biochemistry. 2002;41:9398–9409. doi: 10.1021/bi025674w. [DOI] [PubMed] [Google Scholar]
- 42.Roderick SL, Chan WW, Agate DS, Olsen LR, Vetting MW, Rajashankar KR, Cohen DE. Structure of human phosphatidylcholine transfer protein in complex with its ligand. Nat Struct Biol. 2002;9:507–511. doi: 10.1038/nsb812. [DOI] [PubMed] [Google Scholar]
- 43.Yoder MD, Thomas LM, Tremblay JM, Oliver RL, Yarbrough LR, Helmkamp GM., Jr Structure of a multifunctional protein. Mammalian phosphatidylinositol transfer protein complexed with phosphatidylcholine. J Biol Chem. 2001;276:9246–9252. doi: 10.1074/jbc.M010131200. [DOI] [PubMed] [Google Scholar]
- 44.Zanotti G, Scapin G, Spadon P, Veerkamp JH, Sacchettini JC. Three-dimensional structure of recombinant human muscle fatty acid-binding protein. J Biol Chem. 1992;267:18541–18550. doi: 10.2210/pdb2hmb/pdb. [DOI] [PubMed] [Google Scholar]
- 45.Atzel A, Wetterau JR. Identification of two classes of lipid molecule binding sites on the microsomal triglyceride transfer protein. Biochemistry. 1994;33:15382–15388. doi: 10.1021/bi00255a019. [DOI] [PubMed] [Google Scholar]
- 46.Segrest JP, Jones MK, De Loof H, Dashti N. Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res. 2001;42:1346–1367. [PubMed] [Google Scholar]
- 47.Marza E, Barthe C, Andre M, Villeneuve L, Helou C, Babin PJ. Developmental expression and nutritional regulation of a zebrafish gene homologous to mammalian microsomal triglyceride transfer protein large subunit. Dev Dyn. 2005;232:506–518. doi: 10.1002/dvdy.20251. [DOI] [PubMed] [Google Scholar]
- 48.Wetterau JR, Zilversmit DB. Purification and characterization of microsomal triglyceride and cholesteryl ester transfer protein from bovine liver microsomes. Chem Phys Lipids. 1985;38:205–222. doi: 10.1016/0009-3084(85)90068-4. [DOI] [PubMed] [Google Scholar]
- 49.Read J, Anderson TA, Ritchie PJ, Vanloo B, Amey J, Levitt D, Rosseneu M, Scott J, Shoulders CC. A mechanism of membrane neutral lipid acquisition by the microsomal triglyceride transfer protein. J Biol Chem. 2000;275:30372–30377. doi: 10.1074/jbc.C000364200. [DOI] [PubMed] [Google Scholar]








