Abstract
Background and Aims
Clinical genetic testing can help direct cancer screening for members of Lynch Syndrome families; however there is limited information about family communication of genetic test results.
Methods
174 probands who had genetic testing for Lynch Syndrome were enrolled through 4 U.S. cancer genetics clinics. Subjects were asked whether they had disclosed their genetic test results to first, second and third-degree relatives. Univariate and multivariate analyses were used to identify clinical and demographic factors associated with informing immediate and extended family of genetic test results.
Results
171/174 probands (98% [95%CI: 95%-100%]) reported they had disclosed their genetic test result to a first-degree relative. Communication of test results to other relatives occurred significantly less often, with only 109 of 162 (67% [95%CI: 59%-74%]) subjects with second or third-degree relatives sharing their results. Individuals with a pathogenic mutation were significantly more likely to inform distant relatives than were subjects with a negative or indeterminate test result (OR 2.49 [95% CI: 1.14-5.40]). Probands' age, gender, and cancer status did not influence communication of genetic test results. Lack of closeness and concerns that relatives would worry or not understand the implications of test results were the primary reasons for not sharing genetic test results.
Conclusions
Most individuals who undergo genetic testing for Lynch syndrome share their test results with first-degree family members; however these results reach more distant relatives significantly less often. Interventions to improve communication of genetic test results to members of the extended family are necessary to provide the optimal cancer prevention care to at-risk families.
Introduction
Genetic testing plays an increasing role in the care of patients at risk for cancer due to hereditary cancer syndromes. Lynch Syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC), is the most common hereditary colorectal cancer syndrome, and accounts for approximately 3-5% of all diagnosed colorectal cancer (CRC) cases (1). Genetic testing is clinically available for mutations in the DNA mismatch repair (MMR) genes MLH1, MSH2, and MSH6, which are the most common causes of Lynch Syndrome. Because of the increased risk for colorectal and extracolonic cancers, individuals at risk for Lynch syndrome require colonoscopy every 1-2 years starting at age 20-25 (2) and women should have screening for endometrial and ovarian cancers or consider prophylactic hysterectomy (2).
Identification of a pathogenic mutation through genetic testing confirms the clinical diagnosis of Lynch syndrome and provides an opportunity to stratify cancer risk for other family members. Individuals who undergo genetic testing for Lynch Syndrome appear to be more likely to adhere to recommended cancer screening guidelines (3). A recent survey of cancer centers in the US indicated that the demand for genetic evaluation services for familial cancer syndromes has rapidly increased over the last decade (4). Although genetic testing is expensive, economic analyses have supported the clinical utility of genetic testing for Lynch syndrome and have demonstrated that cost-effectiveness increases substantially when the benefits of testing are extended to probands' family members (5). Studies have suggested that most relatives of patients with CRC would be interested in genetic testing for cancer predisposition (6); however, there are limited data regarding how information about genetic testing is actually communicated in families undergoing molecular evaluation for Lynch Syndrome.
The objectives of our study were to examine how genetic testing information is communicated in families at risk for Lynch Syndrome, and to identify factors associated with disclosure of genetic test results to close and distant family members.
Methods
We conducted a cross-sectional questionnaire study among individuals with a personal or family history fulfilling clinical criteria for Lynch Syndrome. Subjects were recruited through 4 cancer genetics clinics in the United States: Dana-Farber Cancer Institute (DFCI) (Boston, MA), Massachusetts General Hospital (MGH) (Boston, MA), University of Michigan (UMich) (Ann Arbor, MI) and University of California San Francisco (UCSF) (San Francisco, CA). Eligible subjects included individuals whose personal or family history fulfilled Bethesda Guidelines for Lynch Syndrome (7). All participants were age 18 years or older, and were required to read and write English.
Eligible individuals were identified through visits at one of 4 cancer genetics clinics or through referral by a family member. Potential subjects were invited to enroll in the study either at a clinical visit or by mail. Individuals approached by mail received an initial study packet with an introduction letter and questionnaire, as well as a decline to participate form. Individuals who did not return study materials after two follow-up telephone calls and two mailings were considered non-responders. Subjects who had undergone genetic testing were enrolled at least 3 months after their genetic test result had been disclosed to them. Questionnaire data was scanned and entered into a computerized database. The study was approved by the institutional review board of each participating study site.
Four hundred sixty-six eligible individuals were approached for enrollment. Of these, 270 (58%) completed the study questionnaire, 34 (7%) declined to participate, and 158 (34%) were non-responders. Females and college graduates were more likely to complete the study questionnaires. There were no significant differences between other demographic characteristics (such as age, cancer status) of study responders and non-responders. Of the 270 subjects who completed study questionnaires, 174 (64%) reported that they had had genetic testing for Lynch Syndrome; only these individuals are included in this analysis.
Measures
The study questionnaires collected standard demographic data including age, gender, race, ethnicity, marital status, household income, level of education and type of health insurance. Subjects provided details about personal cancer history and were asked to estimate their own risk for developing cancer and whether they had ever undergone genetic testing for Lynch Syndrome. In a detailed family history section, subjects provided information about numbers of siblings, children, prevalence of cancers among first, second and third-degree relatives as well as history of genetic testing and specific genetic test results for individuals in their immediate and extended family who had undergone genetic testing. A family pedigree was constructed for each subject.
Subjects were asked “Have you shared your genetic test result with any of the following people: mother, father, sisters, brothers, spouse/partner, daughters, sons, aunts/uncles, or cousins?” Subjects were asked to choose among reasons why they had or had not shared genetic test results with each of those family members and were permitted to select more than one response.
Statistical Analysis
For subjects who reported having had genetic testing, each participant's family history and pedigree was reviewed to determine that they had at least 1 living first-degree relative (FDR) and at least 1 living second or third-degree relative (SDR/TDR). Subjects who indicated they had shared their genetic test result with their mother, father, brothers, sisters, or children were classified as having disclosed the result to a first degree relative (FDR). Subjects who indicated they had shared their result with uncles, aunts, or cousins were classified as having disclosed results to second or third degree relatives (SDR/TDR). Subjects without at least 1 living first or second/third degree relative were not included in the corresponding analysis.
The potential effects of clinical and demographic factors on the decision to disclose genetic test results were explored using univariate tests of association (Fisher's Exact and t-tests). Factors which were found to be significant on univariate analysis or which were believed to have empiric clinical relevance were included in multivariable logistic regression models to identify variables associated with sharing genetic test results with first and second/third degree relatives. Generalized estimating equations were used to account for potential clustering of results among members of the same family. Analyses were performed using SAS software. All p-values are two-sided and a p-value of <0.05 was considered significant.
Results
Subject Characteristics
Most of the 174 subjects who reported having had genetic testing for Lynch Syndrome were female (70%), of white race (91%), college graduates (69%) and married (76%). The mean age of participants was 46.7 years (range 18-79 years). More than half of study participants (61%) had a cancer diagnosis, and 104 (60%) individuals had a confirmed positive genetic mutation associated with Lynch Syndrome. (Table 1)
Table 1. Characteristics of the Study Population (N=174) and Univariate Analysis of Factors Predicting Disclosure of Genetic Test Results to Any Family Members Beyond 1st Degree.
| All Subjects who had Genetic Testing
(N=174) |
Subject Disclosure to Family Members | |||
|---|---|---|---|---|
| Told Beyond 1st Degree
(N=109)* |
Did Not Tell Beyond 1st Degree
(N=53)* |
|||
| Frequency (%) | Frequency (%) | Frequency (%) | p-value† | |
| Mean Age (Range) | 46.7 (18-79) | 45.96 | 45.26 | 0.73 |
| Gender | 0.20 | |||
| Male | 52 (29.9) | 28 (59.6) | 19 (40.4) | |
| Female | 122 (70.1) | 81 (70.4) | 34 (29.6) | |
| Race | 0.15 | |||
| White | 157 (90.8) | 96 (65.3) | 51 (34.7) | |
| Non-white | 16 (9.3) | 13 (86.7) | 2 (13.3) | |
| Unknown/missing | 1 (--) | |||
| Education | 0.72 | |||
| Less than college grad | 53 (31.0) | 34 (69.4) | 15 (30.6) | |
| At least college grad | 118 (69.0) | 73 (65.8) | 38 (34.2) | |
| Unknown/missing | 3 (--) | 2 (--) | -- (--) | |
| Marital Status | 1.00 | |||
| Married | 132 (75.9) | 81 (66.9) | 40 (33.1) | |
| Not Married | 38 (21.8) | 26 (68.4) | 12 (31.6) | |
| Unknown/missing | 4 (2.3) | 2 (--) | 1 (--) | |
| Cancer Diagnosis | 0.50 | |||
| Yes | 106 (60.9) | 66 (69.5) | 29 (30.5) | |
| No | 68 (39.1) | 43 (64.2) | 24 (35.8) | |
| Test Results | 0.03 | |||
| Positive | 104 (59.8) | 73 (75.3) | 24 (24.7) | |
| Indeterminate | 47 (27.0) | 24 (57.1) | 18 (42.9) | |
| True negative | 23 (13.2) | 12 (52.2) | 11 (47.8) | |
| Cancer Worry | 0.55 | |||
| Low | 58 (33.5) | 35 (66.0) | 18 (34.0) | |
| Moderate | 65 (37.6) | 44 (72.1) | 17 (27.9) | |
| High | 50 (28.9) | 30 (62.5) | 18 (37.5) | |
| Unknown/missing | 1 (--) | |||
| Prior Genetic Testing in Family | 1.00 | |||
| Yes | 128 (73.6) | 82 (67.2) | 40 (32.8) | |
| No | 39 (22.4) | 24 (68.6) | 11 (31.4) | |
| Don't Know | 7 (4.0) | 3 (--) | 2 (--) | |
| Mutation Previously identified in Family | 0.48 | |||
| Yes | 113 (64.9) | 75 (69.4) | 33 (30.6) | |
| No | 61 (35.1) | 34 (63.0) | 20 (37.0) | |
| History of Lynch Syndrome cancer in 1 or more relatives | 1.00 | |||
| Yes | 155 (89.1) | 98 (67.1) | 48 (32.9) | |
| No | 19 (10.9) | 11 (68.8) | 5 (31.2) | |
| Mean number of relatives with Lynch Syndrome Cancers | 3.71 [+/- 2.0] | 4.04 [+/- 2.1] | 3.38 [+/- 1.8] | 0.05 |
| Subjects With Children | 0.72 | |||
| Yes | 122 (70.1) | 76 (68.5) | 35 (31.5) | |
| No | 52 (29.9) | 33 (64.7) | 18 (35.3) | |
| Ever evaluated in a genetics/ high risk clinic | 0.78 | |||
| Yes | 154 (89.0) | 98 (67.6) | 47 (32.4) | |
| No | 19 (11.0) | 10 (62.5) | 6 (37.5) | |
| Unknown/missing | 1 (--) | 1 (--) | -- (--) | |
162 Subjects who indicated they have at least 1 SDR or TDR to tell
Comparison between subjects who did and did not tell relatives beyond 1st degree, Fisher's Exact Test used for all categorical variables; t-test used for all continuous variables
Disclosure of Genetic Test Results to First Degree Family Members
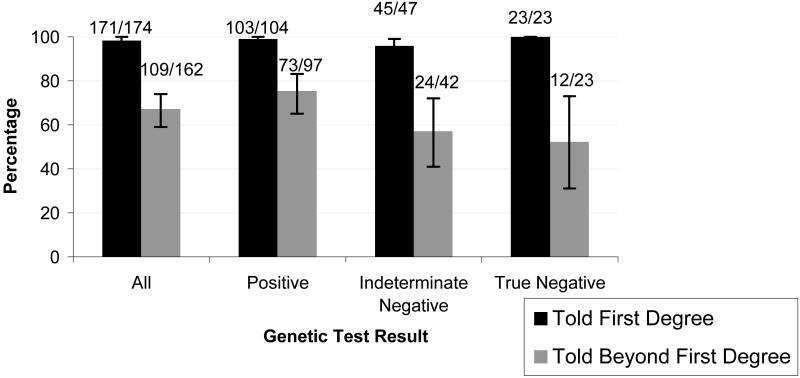
Overall, 171/174 (98% [95%CI: 95-100]) subjects reported that they had shared their genetic test results with at least one first-degree relative (FDR)(Figure 1). Only 5 subjects with a living parent reported that they had not disclosed their genetic test results to their mother or father, 4 had not disclosed their result to a sister, and 2 had not informed a brother. Nearly 90% of subjects with children informed their sons or daughters of their test results, and most of the others indicated they would wait until their children were older before discussing the testing. There were no observed differences in rates of disclosure to FDRs by probands' genetic test results or gender.
Figure 1.
Percentage of Participants Disclosing Test Results to Family Members, by Test Result*
*Error bars represent 95% Exact Confidence Intervals
Disclosure of Genetic Test Results to More Distant Relatives
Overall, 109 of 162 (67%) subjects with living second or third degree family members reported that they had shared their genetic test result with one or more of these SDR/TDRs (Figure 1). Of the 97 individuals whose genetic test result demonstrated a pathogenic mutation (positive test), 73 (75%) disclosed their test result to a relative beyond their nuclear family. Rates of disclosure to SDR/TDRs were significantly lower among subjects with indeterminate or true negative results, with only 24/42 (57%) and 12/23 (52%) indicating they had shared test results with relatives beyond first degree (p=0.03) (Table 1). In univariate analysis, subjects who had more relatives diagnosed with cancers associated with Lynch Syndrome appeared more likely to share their genetic test result with family members beyond FDRs (p=0.05)(Table 1). Aside from genetic test result, there were no other significant associations between disclosure of genetic test results to a SDR/TDR and probands' sociodemographic characteristics such as gender, age, level of education, race, marital status, having children and personal history of cancer. Similarly, individuals with higher level of cancer worry, previous history of genetic testing in the family, or prior evaluation at a high-risk/genetics clinic were not any more likely to disclose their results to SDR/TDRs (Table 1). There were no differences in rates of disclosure of genetic test results among subjects enrolled from any of the 4 study sites.
In multivariate analysis controlling for subjects' gender and personal and family history of cancer, having a genetic test result which revealed a pathogenic mutation was the only significant predictor of disclosing the test result to one or more SDR/TDRs (OR: 2.49 [95% CI 1.14 – 5.40]) (Table 2).
Table 2. Multivariate analysis1 of factors predicting disclosure to any family members beyond 1st degree (N = 159)2.
| Characteristic | OR (95% CI) | p value |
|---|---|---|
| Personal History of Cancer | ||
| Yes | 1.23 (0.61 – 2.51) | 0.57 |
| No | --- | |
| Gender | ||
| Female | 1.68 (0.82 – 3.44) | 0.17 |
| Male | --- | |
| Positive mutation carrier | ||
| Yes | 2.49 (1.14 – 5.40) | 0.02 |
| No | --- | |
| Mean Number of relatives with Lynch Syndrome cancers | 0.133 | 0.21 |
using Generalized Estimating Equation to control for family effects, with exchangeable working correlation = -0.02
Includes individuals who had complete data on all variables
Parameter Estimate
Reasons for Disclosing/Not Disclosing Genetic Test Results
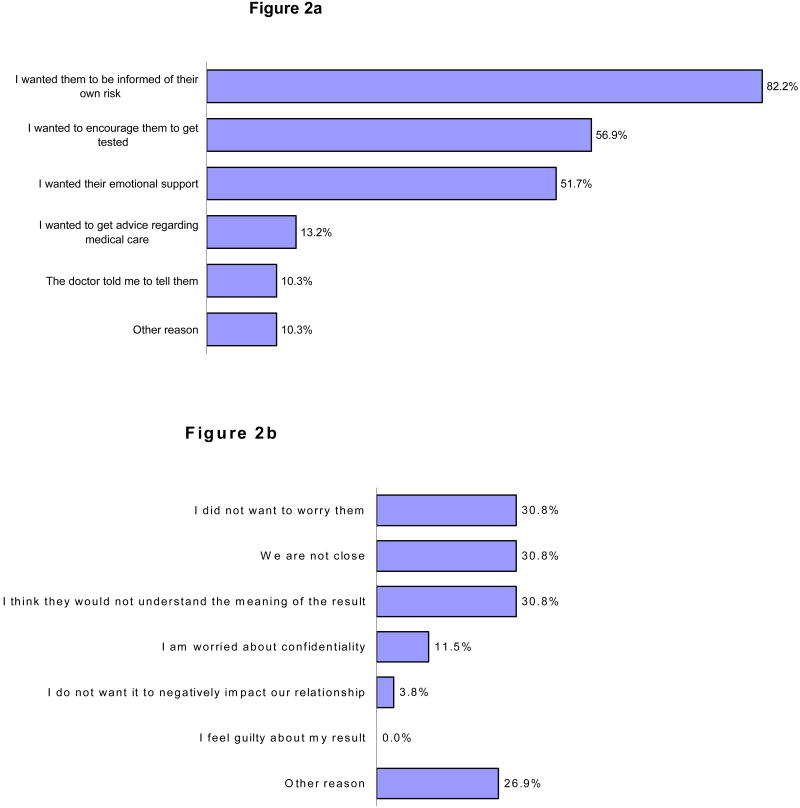
Subjects were asked to choose from a list of reasons why they had or had not disclosed genetic test results to one or more family members (Figure 2). The most frequently cited reasons for sharing a genetic test result with family members were 1) to inform them of their risk, 2) to encourage testing, and 3) to obtain emotional support. Only 1 in 10 respondents reported that they informed their family members of their genetic test result because their physician told them to.
Figure 2.
Figure 2a. Reasons for Disclosing Results to Family Members (174 subjects' reponses)*
Figure 2b. Reasons for Not Disclosing Results to Family Members (26 subjects' responses)*
* Subjects were permitted to select more than 1 option
The most frequently cited reasons for not sharing genetic test results were 1) they were not close to their family members, 2) concern that family members would not understand the test result, and 3) they did not want relatives to worry. Only three individuals listed concerns about confidentiality as a reason why they did not share their test result. None listed guilt about their result as a reason for not sharing information about their testing. Among other reasons were wanting to wait until young children were older before informing them about results of genetic tests, inability to contact specific family members, and concern that the information would be too distressing. Although 53 of 162 (32.7%) individuals with living SDR/TDRs said they did not share genetic test results beyond their immediate family, only 26 (15%) reported that they had deliberately withheld their genetic test results from any family member.
Discussion
In a multi-center study of 174 individuals who had undergone genetic testing for Lynch Syndrome, we found that nearly all (98%) had disclosed their test results to first-degree relatives; and two-thirds had shared these results with more distant family members such as cousins, aunts, or uncles. Seventy-three of 97 (75%) subjects whose testing identified a gene-mutation communicated this result to one or more SDR/TDRs. Having a “true positive” genetic test result was the only clinical or demographic factor significantly associated with disclosure of genetic test results to relatives beyond the immediate family.
To date, most of the research examining family communication about genetic testing has focused on individuals undergoing evaluation for hereditary breast and ovarian cancer syndrome (HBOS). These studies have shown that most patients tested for BRCA 1 and 2 mutations inform their first-degree relatives of their genetic test results; however communication to more distant relatives occurs less frequently (8). In the predominantly female BRCA 1 and 2 cohorts, patients were more likely to disclose genetic test results to other female family members rather than to male relatives also at-risk (8, 9) and positive results were more likely to be shared, as compared with negative or uninformative test results (8, 9). Previous studies on disclosure of genetic test results in Lynch syndrome families, each of which reported on fewer than 40 subjects, also found that genetic test results are disclosed less frequently to at-risk relatives outside the nuclear family (10-12). One study suggested that male probands were less likely than females to communicate genetic test results to family members (11) and proposed that male patients might require additional counseling resources to ensure appropriate communication of results.
Our findings, from a much larger Lynch Syndrome cohort, demonstrate that probands' gender, race, cancer history, and concerns about privacy and confidentiality do not appear to influence most patients' decisions about sharing genetic test results with family; however the genetic test result is still a significant factor. Most subjects who did not disclose test results said they did not intentionally withhold this information. The fact that disclosure was nearly universal among FDRs, but occurred less frequently among more distant relatives suggests that subjects 1) may not be fully aware of the potential impact of their genetic test result on health care of SDR/TDRs and/or 2) may encounter other barriers to communicating results to more distant relatives.
Why is sharing genetic test results important for other relatives in a family with Lynch syndrome? In the case of a “true positive” test, identifying a clearly pathogenic gene mutation in a proband confirms the diagnosis and allows unaffected family members to ascertain their cancer risk through informative (and less expensive) mutation-specific testing. A true positive genetic test result affects clinical management for FDR/SDR/and TDRs, who, through testing, would learn whether they require high risk cancer screening. By comparison, a “true negative” genetic test result (which occurs when an individual tests negative for a mutation previously identified in the family) changes clinical management for only the tested individual and his/her progeny (children/grandchildren), providing reassurance that they did not inherit the increased cancer risk. Since a “true negative” test result does not change management for other relatives that are not direct descendants, lower rates of disclosure of “true negative” test results to SDR/TDRs might be expected.
In the case of an “indeterminate/uninformative” genetic test result (which occurs when an individual's genetic test result does not reveal a mutation and there has not been a mutation previously identified in the family), the diagnosis of Lynch syndrome can neither be confirmed nor rejected and the importance of disclosing this test result is less obvious. However, an indeterminate/uninformative result may still have clinical relevance. Discussion of the genetic testing provides an opportunity to share information about Lynch Syndrome with other family members who may be at-risk and may benefit from specialized cancer surveillance. In our cohort more than half of the subjects with an indeterminate/uninformative genetic test result met Amsterdam Criteria and would still be considered at-risk for Lynch Syndrome.
Overall, our results demonstrate that in most cases information about genetic test results does reach members of the immediate and extended family. In 75% of cases in which a pathogenic mutation was identified, this information was shared with one or more SDR/TDRs. If this information led other family members to get tested, this would support models of cost effectiveness for genetic testing, suggesting benefits are likely to extend to family members beyond the proband and his/her immediate family. With regard to individuals with indeterminate genetic test results, our finding that rates of disclosure to SDR/TDRs are significantly lower (57%) may reflect the difficulty in interpreting the clinical significance of these uninformative results and conveying this information in a way patients and their families can understand. Some prior studies have suggested that individuals with indeterminate genetic test results may be falsely reassured that their cancer risk is now lower because there was no mutation identified, and may not feel that information about the genetic test result is important to disseminate (8).
One of the major challenges in improving the effectiveness of genetic testing for cancer prevention is to develop ways to distribute this information to family members and health care providers. At present, most genetic testing is conducted in specialized centers where patients meet with genetic counselors before and after disclosure of genetic test results. It is generally accepted that health care providers have an obligation to inform probands of implications of the genetic diagnosis for other family members and encourage them to share their test results with relatives (13). In the United States, privacy laws prevent physicians from disseminating this information without a patient's consent. However, simply telling patients that they need to inform family members of their genetic test result may not be enough. Our findings suggest that even under ideal conditions, some patients do not inform relatives of their genetic test results, in many cases because of concerns their family members will worry, or will not understand, or because they are “not close.” Providing patients with a detailed letter describing the implications of the test result and giving them an annotated copy of the family tree indicating which family members should receive genetic testing information may help ensure that this information is shared with others who may benefit.
There are several limitations to consider in this study. Subjects who were willing to spend 30 minutes completing study questionnaires may have been more motivated and thus more likely to communicate test results to family members, or more likely to report that they had. We asked subjects whether they had shared their genetic test result with specific types of relatives (sisters, aunts, cousins, etc); however we did not have total counts of each proband's living relatives, so it was not possible to calculate the proportion of at-risk relatives informed of genetic test results. We did not have any way to confirm that subjects actually shared genetic testing information with relatives who were at-risk, nor could we assess the quality of the communication or the accuracy of the information transmitted to family members. Consequently, our findings may overestimate the true rates of genetic test disclosure. Finally, our study did not collect information about outcomes of disclosure of genetic testing information to relatives; therefore we could not ascertain whether clinical care of family members changed as result of genetic testing.
Despite these limitations, our report provides useful data about patterns of communication about genetic testing in Lynch Syndrome families. Our study of patients from 4 U.S. cancer centers demonstrates that genetic test results are often shared with members of the immediate family, but are less often communicated to more distant at-risk relatives. As it is expected that a greater share of genetic testing will move from specialized cancer centers to physician's private offices, it is important to develop and implement strategies that will help patients communicate with family members about genetic testing. Specific interventions, such as providing patients with documentation of genetic test results and screening recommendations and providing them with strategies for disseminating test results to at-risk family members, may help remove barriers to family communication and to improve effectiveness of genetic testing for cancer prevention.
Acknowledgments
Research Support: American College of Gastroenterology Junior Faculty Award (2004—Dr. Stoffel), GlaxoSmithKline GIDH Clinical Research Award (2004—Dr. Stoffel), K24 NCI CA 113433 (Dr. Syngal)
Grant support NCI K07 CA 120448-01-A1 (Elena M. Stoffel MD)
Footnotes
Conflicts of Interest: Drs. Syngal, Chung, and Terdiman disclose that they have consultant/advisory relationships with Myriad Genetic Laboratories
References
- 1.Lynch HT, Watson P, Shaw TG, et al. Clinical impact of molecular genetic diagnosis, genetic counseling, and management of hereditary cancer. Part II: Hereditary nonpolyposis colorectal carcinoma as a model. Cancer. 1999;86 11:2457–63. doi: 10.1002/(sici)1097-0142(19991201)86:11+<2457::aid-cncr2>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121(1):198–213. doi: 10.1053/gast.2001.25581. [DOI] [PubMed] [Google Scholar]
- 3.Wagner A, van Kessel I, Kriege MG, et al. Long term follow-up of HNPCC gene mutation carriers: compliance with screening and satisfaction with counseling and screening procedures. Fam Cancer. 2005;4(4):295–300. doi: 10.1007/s10689-005-0658-9. [DOI] [PubMed] [Google Scholar]
- 4.Epplein M, Koon KP, Ramsey SD, Potter JD. Genetic services for familial cancer patients: a follow-up survey of National Cancer Institute Cancer Centers. J Clin Oncol. 2005;23(21):4713–8. doi: 10.1200/JCO.2005.00.133. [DOI] [PubMed] [Google Scholar]
- 5.Ramsey SD, Clarke L, Etzioni R, Higashi M, Berry K, Urban N. Cost-effectiveness of microsatellite instability screening as a method for detecting hereditary nonpolyposis colorectal cancer. Ann Intern Med. 2001;135(8 Pt 1):577–88. doi: 10.7326/0003-4819-135-8_part_1-200110160-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kinney AY, Choi YA, DeVellis B, Kobetz E, Millikan RC, Sandler RS. Interest in genetic testing among first-degree relatives of colorectal cancer patients. Am J Prev Med. 2000;18(3):249–52. doi: 10.1016/s0749-3797(99)00162-2. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Bigas MA, Boland CR, Hamilton SR, et al. A National Cancer Institute Workshop on Hereditary Nonpolyposis Colorectal Cancer Syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89(23):1758–62. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 8.Claes E, Evers-Kiebooms G, Boogaerts A, Decruyenaere M, Denayer L, Legius E. Communication with close and distant relatives in the context of genetic testing for hereditary breast and ovarian cancer in cancer patients. Am J Med Genet A. 2003;116(1):11–9. doi: 10.1002/ajmg.a.10868. [DOI] [PubMed] [Google Scholar]
- 9.Patenaude AF, Dorval M, DiGianni LS, Schneider KA, Chittenden A, Garber JE. Sharing BRCA1/2 test results with first-degree relatives: factors predicting who women tell. J Clin Oncol. 2006;24(4):700–6. doi: 10.1200/JCO.2005.01.7541. [DOI] [PubMed] [Google Scholar]
- 10.Peterson SK, Watts BG, Koehly LM, et al. How families communicate about HNPCC genetic testing: findings from a qualitative study. Am J Med Genet C Semin Med Genet. 2003;119(1):78–86. doi: 10.1002/ajmg.c.10010. [DOI] [PubMed] [Google Scholar]
- 11.Gaff CL, Collins V, Symes T, Halliday J. Facilitating family communication about predictive genetic testing: probands' perceptions. J Genet Couns. 2005;14(2):133–40. doi: 10.1007/s10897-005-0412-3. [DOI] [PubMed] [Google Scholar]
- 12.Mesters I, Ausems M, Eichhorn S, Vasen H. Informing one's family about genetic testing for hereditary non-polyposis colorectal cancer (HNPCC): a retrospective exploratory study. Fam Cancer. 2005;4(2):163–7. doi: 10.1007/s10689-004-7992-1. [DOI] [PubMed] [Google Scholar]
- 13.Offit K, Groeger E, Turner S, Wadsworth EA, Weiser MA. The “duty to warn” a patient's family members about hereditary disease risks. Jama. 2004;292(12):1469–73. doi: 10.1001/jama.292.12.1469. [DOI] [PubMed] [Google Scholar]