We report herein a new class of small-molecule inhibitors of the anti-apoptotic Bcl-2 proteins. The most potent compound 7 binds to Bcl-2, Bcl-xL and Mcl-1 proteins with Ki values of 110, 638 and 150 nM, respectively. Compound 7 is highly effective in induction of cell death in breast cancer cells with high levels of Bcl-2, Bcl-xL and Mcl-1 proteins, and represents a promising lead compound for the design of new anticancer drugs.
Apoptosis, or programmed cell death, is a critical cell process in normal development and homeostasis of multicellular organisms. Inappropriate regulation of apoptosis has been implicated in many human diseases, including cancer.1–3 Targeting critical apoptosis regulators is an attractive therapeutic approach for the development of new classes of therapies for the treatment of cancer and other human diseases.1
The Bcl-2 family proteins are a class of central arbiters of apoptosis and are comprised of anti-apoptotic members such as Bcl-2, Bcl-xL and Mcl-1 and pro-apoptotic members such as Bim, Bid, Bak and Bax.4–7 The anti-apoptotic proteins in the Bcl-2 family are overexpressed in many cancer cell lines and human cancer tissues. This overexpression protects cancer cells from the induction of apoptosis by current anticancer therapies and plays a role in the failure of conventional anticancer drugs.4–7 Consequently, these anti-death Bcl-2 proteins are considered to be promising molecular targets for the design of novel anticancer drugs.
Although the precise mechanism by which Bcl-2 proteins regulate apoptosis in cells is still under intense investigation,8 it is very clear that these anti-apoptotic Bcl-2 proteins effectively inhibit apoptosis, at least in part, by directly binding to pro-apoptotic Bcl-2 proteins such as Bim, Bid, Bak and Bax and blocking their pro-apoptotic activity. Experimentally determined three- dimensional structures of Bcl-2, Bcl-xL and Mcl-1 by either NMR or x-ray crystallography showed that the BH1 (Bcl-2 homology domain 1), BH2 and BH3 domains in these proteins form a well-defined, hydrophobic surface binding groove, known as the BH3 binding groove, into which Bad, Bid and Bim bind.9–12 Hence, small-molecules that are designed to target the BH3 binding groove in these anti-apoptotic Bcl-2 proteins are predicted to promote apoptosis in cancer cells by antagonizing their anti-apoptotic function. Design of non-peptide, cell-permeable, small-molecule inhibitors that bind to the BH3 binding groove in these anti-death Bcl-2 proteins is being intensely pursued as a new anticancer therapeutic strategy.13–22
Design of non-peptidic, small-molecule inhibitors to target protein-protein interactions (PPIs) is considered one of the most challenge tasks in modern drug discovery and medicinal chemistry. Nevertheless, significant progress has been made in the last few years in the design of small-molecule inhibitors to target the Bcl-2 PPIs13–22 and a number of classes of potent small-molecule inhibitors, shown in Figure 1, have been reported. Among them, compound 1 binds to Bcl-2, Bcl-xL and Bcl-w proteins with a very high affinity but does not bind to Mcl-1.18 Compound 2, a natural product isolated from cotton seeds, concurrently targets Bcl-2, Bcl-xL and Mcl-1 proteins with similar affinities,21 and is currently in clinical trials as an orally administered agent for the treatment of multiple forms of human cancer.22 Using a structure-based strategy, we have recently reported the design of compound 3 as a new class of potent, cell-permeable small-molecule inhibitor of Bcl-2, Bcl-xL and Mcl-1 proteins.21
Figure 1.
Representative small-molecule inhibitors of Bcl-2 proteins.
Although Bcl-2 and Bcl-xL proteins have been the primary focus for the design of small-molecule inhibitors to target these proteins,13–22 recent studies have demonstrated that the Mcl-1 protein plays a crucial role in protecting cancer cells from induction of apoptosis by a variety of anticancer agents. Compound 1 had potent activity only in cancer cells with low levels of Mcl-1 protein but a much weaker activity in cancer cells with high levels of Mcl-1.23 Knocking down Mcl-1 using siRNA in cancer cells greatly sensitizes the activity of 1. Hence, small-molecule inhibitors that target not only Bcl-2 and Bcl-xL but also Mcl-1 could be highly effective in induction of cell death in cancer cells with high levels of these proteins. Herein, we report the structure-based design, synthesis, initial evaluations of pyrogallol based compounds as novel small-molecule inhibitors of Bcl-2, Bcl-xL and Mcl-1.
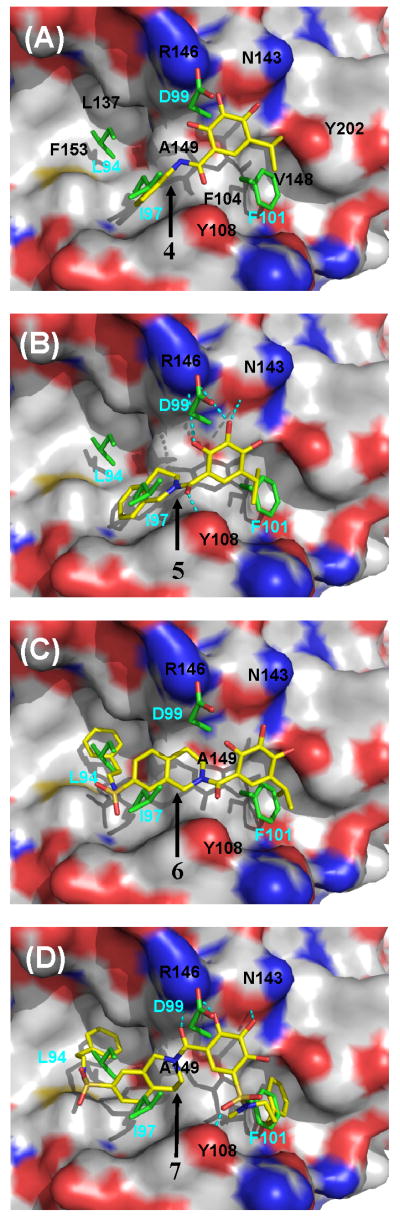
In our previous study,21 compound 4 was designed as an initial lead starting from compound 2 (Figure 2). In our fluorescence polarization (FP)-based binding assay, 4 binds to Bcl-2 with a Ki value of 31.9 μM (Figure 3). Analysis of its binding model to Bcl-2 (Figure 4 and Supporting Information) showed that two of the hydroxyl groups in its phenyl ring form hydrogen bonds with R146 and N143 in Bcl-2, mimicking the key residue D99 in the Bim BH3 peptide. Its phenyl ring mimics I97 and its isopropyl group partially fills the hydrophobic pocket occupied by F101 in the Bim peptide. The predicted binding model for compound 4 in the complex with Bcl-2 forms the structural basis for our current design and optimization.
Figure 2.
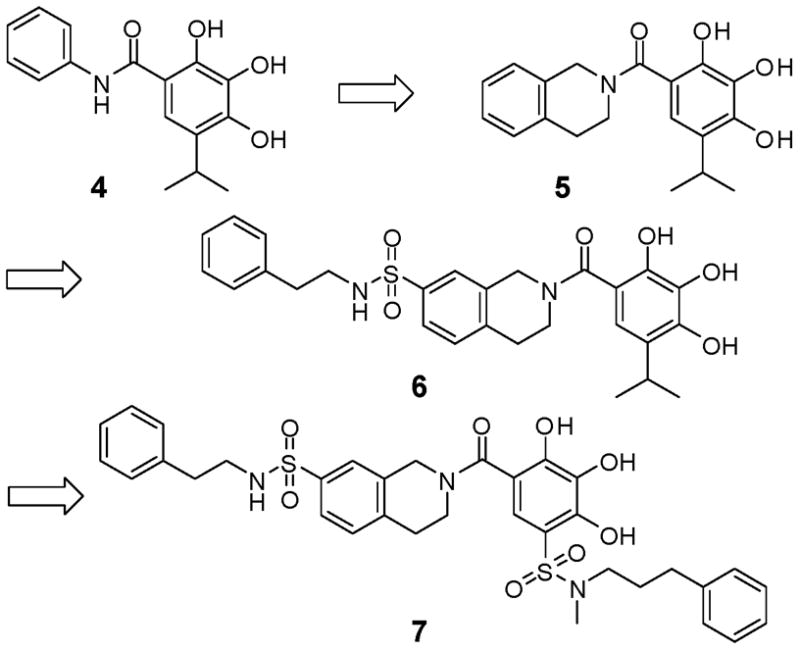
Structure-based design of novel small-molecule inhibitors of Bcl-2 proteins.
Figure 3.
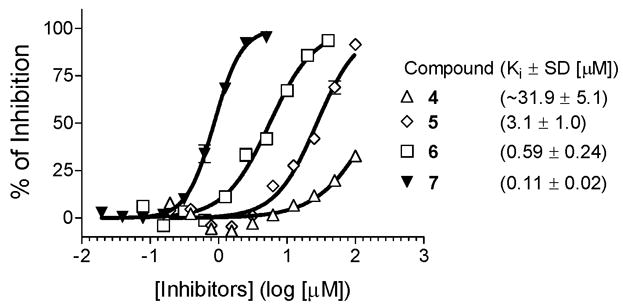
Competitive binding curves of designed small-molecule inhibitors to Bcl-2 as determined using a fluorescence-polarization based binding assay. Recombinant human Bcl-2 protein and fluorescently tagged Bid BH3 peptide were employed in this competitive binding assay.
Figure 4.
Predicted binding models of designed small-molecule inhibitors 4, 5, 6 and 7 to Bcl-2. For inhibitors, carbon atoms are shown in yellow, oxygen atoms in red, nitrogen atoms in blue and sulfur atoms in yellow. For protein, carbon atoms are shown in yellow, oxygen atoms in red, nitrogen atoms in blue. Side chain of crucial residue L94, I97, D99 and F101 in the Bim BH3 peptide are displayed with carbon atoms shown in green and oxygen atoms in red.
Based upon the binding model for compound 4, we designed compound 5 (Figure 2), in which a fused, conformationally constrained bicyclic system was used to replace the phenyl ring in compound 4. Our modeling showed that compound 5 maintains all the hydrogen bonding observed for compound 4 binding to Bcl-2 but has enhanced hydrophobic contacts mediated by the tetrahydroisoquinoline ring (Figure 4). Modeling predicted that compound 5 may bind to Bcl-2 with a higher affinity than 4 (SI). Compound 5 was synthesized (SI) and was determined to bind to Bcl-2 with a Ki value of 3.1 μM (Figure 3). Compound 5 is thus 10-times more potent than 4, supporting our modeling prediction.
Comparison of the predicted binding models for 5 and the Bim BH3 peptide suggested that the hydrophobic pocket occupied by I94 in the Bim peptide is not utilized by 5 (Figure 4). Taking advantage of this key hydrophobic interaction should further improve the binding affinity. We have thus designed compound 6 (Figure 2), in which a phenethyl group is attached to the 7-position of the fused bicyclic system through a sulfonamide linker. This facilitates the synthesis and enhances the solubility of the resulting compound. Modeling predicted that the phenethyl group in compound 6 is inserted into the hydrophobic pocket in Bcl-2 occupied by the I94 residue in the Bim peptide (Figure 4). Compound 6 was synthesized (SI) and determined to bind to Bcl-2 with a Ki value of 590 nM (Figure 3). It is thus 5-times more potent than 5, providing further support for our design strategy.
Analysis of the predicted binding model for 6 in complex with Bcl-2 showed that its isopropyl group inserts into the hydrophobic pocket occupied by F101 (Figure 4). It is clear however that this hydrophobic pocket can accommodate a larger hydrophobic group. Although one could simply replace the isopropyl group with a larger hydrophobic group, the resulting compounds would become too hydrophobic and insoluble. To address this problem, we replaced the isopropyl group with phenylpropyl sulfonamide, as in compound 7. Modeling predicted that the phenylpropyl group in 7 should significantly improve the hydrophobic interactions with Bcl-2 as compared to the isopropyl group in 6. Importantly, the use of a sulfonamide as the linker leads to a compound with a much improved solubility. Interestingly, our modeling also suggested that the sulfonyl group in 7 forms a hydrogen bond with F108 (Figure 4).
Compound 7 was synthesized (Scheme I) and was found to bind to Bcl-2 with a Ki value of 110 nM (Figure 3). It is therefore 5-times more potent than compound 6 and 290-times more potent than the initial lead compound 4.
Scheme I. Synthesis of designed inhibitor 7.

Reagents and conditions: (a) ClSO3H, rt, 91%; (b) 3-phenylpropylamine, NEt3, CH2Cl2, 97%; (c) (i). MeI, K2CO3, acetone, reflux, overnight; (ii). KOH, MeOH, 60 °C, 3hr, 92%; (d) substituted tetrahydroisoquinoline, EDAC, HOBt, DiPEA, CH2Cl2, 85%; (e) BBr3 (15 eq.), CH2Cl2, −78 °C to −20 °C, 70%.
Since FP-based assays can be influenced by autofluorescence of the tested inhibitors, we have developed and validated an enzyme-linked immunosorbent assay (ELISA) for Bcl-2 (SI). Using this ELISA assay, we have determined the binding of our designed inhibitors to Bcl-2. The IC50 values for compounds 5, 6 and 7 are 38.2 ± 6.2, 6.6 ± 2.7 and 0.33 ± 0.05 μM, respectively, obtained from three independent experiments. Hence, the relative binding affinities for these inhibitors, obtained from the ELISA assay are consistent with those obtained from the FP-based assay.
We next evaluated compound 7 for its binding affinities to Bcl-xL and Mcl-1 proteins using our established FP-based binding assays (SI). Compound 7 has Ki values of 638 and 150 nM to Bcl-xL and Mcl-1 proteins, respectively.
To further confirm the binding affinity of compound 7 to Mcl-1, we have developed and validated an enzyme-linked immunosorbent (ELISA) assay using recombinant Mcl-1 protein and a biotinylated Bim peptide (SI). In this assay, compound 7 has an IC50 value of 39 ± 10 nM to Mcl-1 from three independent experiments (Figure 5). Hence, both the FP and the ELISA-based assay showed that compound 7 binds to Mcl-1 protein with a high affinity.
Figure 5.
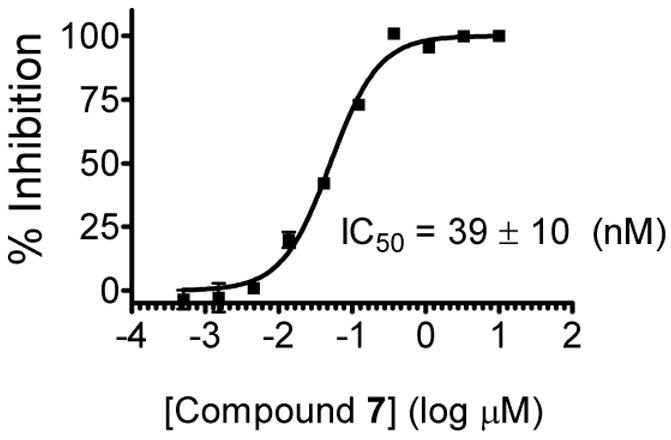
Binding curve of compound 7 to Mcl-1 protein in competition with a biotinylated Bim BH3 peptide as determined using an ELISA assay.
Potent, cell-permeable, small-molecule inhibitors that concurrently target Bcl-2, Bcl-xL and Mcl-1 proteins are predicted to be highly effective in inducing cell death in cancer cells.23 We evaluated these compounds for their ability to induce cell death in the MDA-MB-231 (2LMP) human breast cancer cell line, which has high levels of Bcl-2, Bcl-xL and Mcl-1 proteins (SI). Each inhibitor induces cancer cells to undergo cell death in a dose-dependent manner (Figure 6). Compound 7 effectively induces cell death at concentrations as low as 10 nM and is much more potent than other compounds. For example, compound 7 at 100 nM is as effective as compound 6 at 1000 nM and is much more effective than compounds 4 and 5 at 1000 nM. In direct comparison, compound 1, which does not bind to Mcl-1,18 is 100-times less effective than compound 7 in cell death induction in the MDA-MB-231 cell line (Figure 6).
Figure 6.
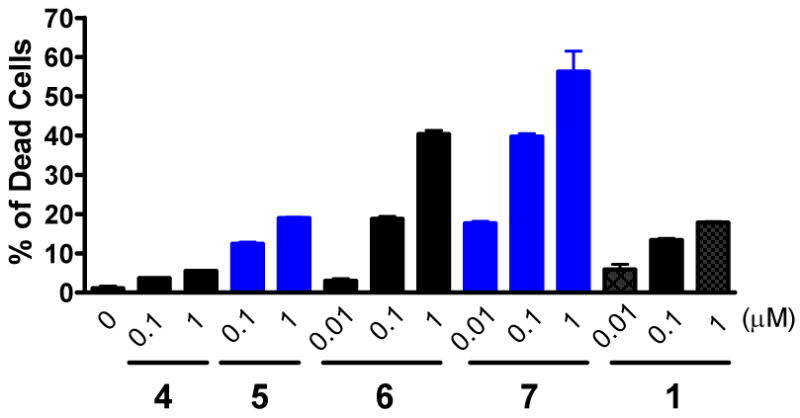
Induction of cell death in the MDA-MB-231 (2LMP) human breast cancer cell line by designed small-molecule inhibitors. Cells were treated with each inhibitor for 96 hours and cell viability was determined using Trypan blue exclusion assay.
In summary, using a structure-based design strategy, we have successfully designed a new class of potent small-molecule inhibitors targeting Bcl-2, Bcl-xL and Mcl-1 proteins. Our most potent inhibitor, compound 7, which was named TM-1206, binds to Bcl-2, Bcl-xL and Mcl-1 proteins with Ki values of 110, 639, and 150 nM, respectively. The binding of compound 7 to Bcl-2 and Mcl-1 proteins have further been confirmed using ELISA-based assays. Compound 7 is effective in induction of cell death in the MDA-MB-231 cancer cells with high levels of Bcl-2, Bcl-xL and Mcl-1 proteins in a dose-dependent manner. Our present study indicates that concurrently targeting Bcl-2, Bcl-xL and Mcl-1 proteins using a small-molecule inhibitor is potentially a promising therapeutic strategy for the design of novel anticancer drugs.
Supplementary Material
An experimental section including the information on the chemical data for compounds 5–7, details of the fluorescence polarization-based and ELISA binding assays, molecular modeling and the cell viability assays is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We are grateful for the financial support from the National Cancer Institute, National Institutes of Health (U19CA113317), the Department of Defense Breast Cancer Program (BC0009140), the Department of Defense Prostate Cancer Program (PC040537), the Prostate Cancer Foundation, the Breast Cancer Research Foundation, the Susan G. Komen Foundation, Ascenta Therapeutics, Inc. and the Intramural Research Program of the National Cancer Institute, Center for Cancer Research.
Footnotes
Abbreviations: Bcl-2, (B-cell leukemia/lymphoma 2), Mcl-1, (myeloid cell leukemia sequence 1), Bid, (BH3 interacting domain death agonist), Bim, (Bcl-2 interacting mediator of cell death), Bax (Bcl-2–associated X protein), Bak (Bcl-2 homologous antagonist/killer), SI (Supporting Information)
References
- 1.Nicholson DW. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- 2.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 3.Ponder BA. Cancer genetics. Nature. 2001;411:336–341. doi: 10.1038/35077207. [DOI] [PubMed] [Google Scholar]
- 4.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 5.Reed JC. Bcl-2 family proteins: strategies for overcoming chemoresistance in cancer. Advances in Pharmacology. 1997;41:501–553. doi: 10.1016/s1054-3589(08)61070-4. [DOI] [PubMed] [Google Scholar]
- 6.Chao DT, Korsmeyer SJ. Bcl-2 family: regulators of cell death. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 7.Minn AJ, Swain RE, Ma A, Thompson CB. Recent progress on the regulation of apoptosis by bcl-2 family members. Advances in Immunology. 1998;70:245–279. doi: 10.1016/s0065-2776(08)60388-0. [DOI] [PubMed] [Google Scholar]
- 8.Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang D. C Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315:856–9. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 9.Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, Matayoshi ED, Oltersdorf T, Fesik SW. Solution structure of the antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA. 2001;98:3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 11.Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, Fesik SW. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day CL, Chen L, Richardson SJ, Harrison PJ, Huang DC, Hinds MG. J Biol Chem. 2005;280:4738–44. doi: 10.1074/jbc.M411434200. [DOI] [PubMed] [Google Scholar]
- 13.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci U S A. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Degterev A, Lugovskoy A, Cardone M, Mulley B, Wagner G, Mitchison T, Yuan J. Identification of small-molecule inhibitors of interaction between the BH3 domain and Bcl-xL. Nat Cell Biol. 2001;3:173–182. doi: 10.1038/35055085. [DOI] [PubMed] [Google Scholar]
- 15.Tzung SP, Kim KM, Basanez G, Giedt CD, Simon J, Zimmerberg J, Zhang KY, Hockenbery DM. Antimycin A mimics a cell-death-inducing Bcl-2 homology domain 3. Nat Cell Biol. 2001;3:183–91. doi: 10.1038/35055095. [DOI] [PubMed] [Google Scholar]
- 16.Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, Guo R, Li B, Zhu X, Huang Y, Long YQ, Roller PP, Yang D, Wang S. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem. 2001;44:4313–4324. doi: 10.1021/jm010016f. [DOI] [PubMed] [Google Scholar]
- 17.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-Cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–4264. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 18.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 19.Petros AM, Dinges J, Augeri DJ, Baumeister SA, Betebenner DA, Bures MG, Elmore SW, Hajduk PJ, Joseph MK, Landis SK, Nettesheim DG, Rosenberg SH, Shen W, Thomas S, Wang X, Zanze I, Zhang H, Fesik SW. Discovery of a potent inhibitor of the antiapoptotic protein Bcl-xL from NMR and parallel synthesis, Discovery of a potent inhibitor of the antiapoptotic protein Bcl-xL from NMR and parallel synthesis. J Med Chem. 2006;49:656–63. doi: 10.1021/jm0507532. [DOI] [PubMed] [Google Scholar]
- 20.Wang S, Yang D. US patent application series # 20030008924. Small Molecule Antagonists of Bcl-2 family proteins. May 30, 2002
- 21.Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, Shangary S, Qiu S, Gao W, Yang D, Meagher J, Stuckey J, Krajewski K, Jiang S, Roller PP, Abaan HO, Tomita Y, Wang S. Structure-Based Design of Potent Small-Molecule Inhibitors of Anti-Apoptotic Bcl-2 Proteins. J Med Chem. 2006;49:6139–6142. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 22.Saleh M, Pitot H, Hartung J, Holmlund J, Albert LoBuglio A, Forero A. Phase I trial of AT-101, an orally bioavailable inhibitor of Bcl-2, in patients with advanced malignancies. The 2005 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics: Discovery, Biology, and Clinical Applications, Abstract #C89; November 14–18, 2005; Philadelphia, Pennsylvania. [Google Scholar]
- 23.van Delft MF, Wei AH, Mason KD, Vandenberg CJ, Chen L, Czabotar PE, Willis SN, Scott CL, Day CL, Cory S, Adams JM, Roberts AW, Huang DC. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
An experimental section including the information on the chemical data for compounds 5–7, details of the fluorescence polarization-based and ELISA binding assays, molecular modeling and the cell viability assays is available free of charge via the Internet at http://pubs.acs.org.