Abstract
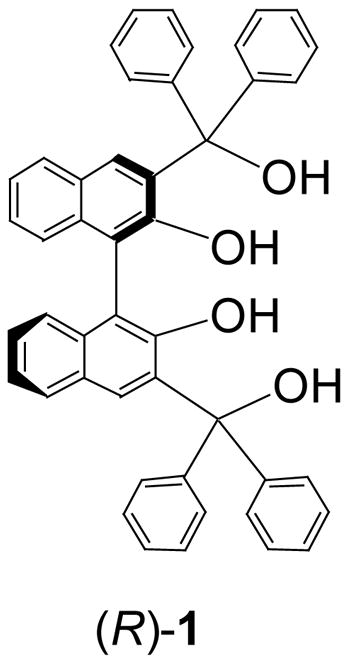
The tetrahydroxyl derivative of BINOL, (S)- or (R)-1, and its analogs are synthesized. (S)- or (R)-1 can be used to conduct the enantioselective recognition of chiral amino alcohols. In comparison with BINOL, the two additional hydroxyl groups of (S)- or (R)-1 have increased the binding of this compound with the amino alcohols and significantly improved the fluorescence quenching efficiency. The fluorescence responses of (S)- or (R)-1 towards amino alcohols are compared with those of its analogs (R)-4 and (R)-6. It shows that the interaction of the central naphthyl hydroxyl groups of (S)- or (R)-1 with the substrates is responsible for the observed fluorescence quenching and the two additional alkyl hydroxyl groups increase the quenching efficiency.
Introduction
1,1′-Bi-2-naphthol (BINOL) and its derivatives have been extensively used in chiral recognition and asymmetric catalysis.1 Because of the fluorescence properties of the

naphthalene groups in these compounds, their fluorescence responses toward various chiral molecules have also been investigated.2,3 For example, in 1992, Iwanek and Mattay reported that the fluorescence of BINOL could be quenched by chiral amines with a small degree of enantioselectivity for certain substrates.2d In recent years, there is growing interest in developing enantioselective fluorescent sensors for organic compounds since these sensors can potentially provide a real time analytical tool for rapid chiral assay.2–4 In order to improve the fluorescent recognition of BINOL for amino alcohols and amines, we have attempted to increase the intermolecular hydrogen binding capability of BINOL by introducing additional hydroxyl groups adjacent to the central diols. For this purpose, compound (R)-1 and its derivatives have been studied and a significantly improved efficiency in the fluorescent recognition of chiral amino alcohols has been observed. Herein, these results are reported.
Results and Discussion
1. Synthesis and 1H NMR Analyses of (R)-1, (R)-4 and (R)-6
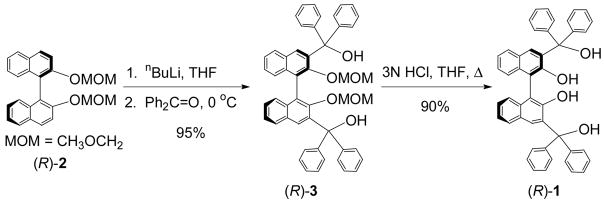
The synthesis of the tetrahydroxyl 1,1′-binaphthyl compound (R)-1 was previously reported by Chen5a and Kozlowski5b but only low yield and low enantiomeric purity were observed from the asymmetric biaryl coupling reactions. We conducted the ortho-metallation of the enantiomerically pure (R)-2 with nBuLi (Scheme 1).6 Addition of benzophenone led to the formation of (R)-3 in 95% yield which upon hydrolysis under acidic condition gave the optically pure tetraol (R)-1 in 90% yield. A similar method to prepare (S)-1 was reported while this work was in progress.7
Scheme 1.
Synthesis of the Tetrahydroxyl 1,1′-Binaphthyl (R)-1.
In order to understand the functions of the two types of hydroxyl groups in (R)-1, that is, the two aryl hydroxyls and the two alkyl hydroxyls, we synthesized compounds (R)-4 and (R)-6 where the hydroxyls are selectively protected with methyl groups (Scheme 2 and 3). Reaction of (R)-1 with methanol in the presence of trifluoroacetic acid gave (R)-4 in 82% yield (Scheme 2). In (R)-4, its alkyl hydroxyls were protected. The ortho-metallation of (R)-2,2′-dimethoxy-1,1′-binaphthalenyl,8 (R)-5, with nBuLi in the presence of TMEDA followed by addition of benzophenone gave (R)-6 in 60% yield (Scheme 3). In this compound, the two aryl hydroxyls are protected.
Scheme 2.
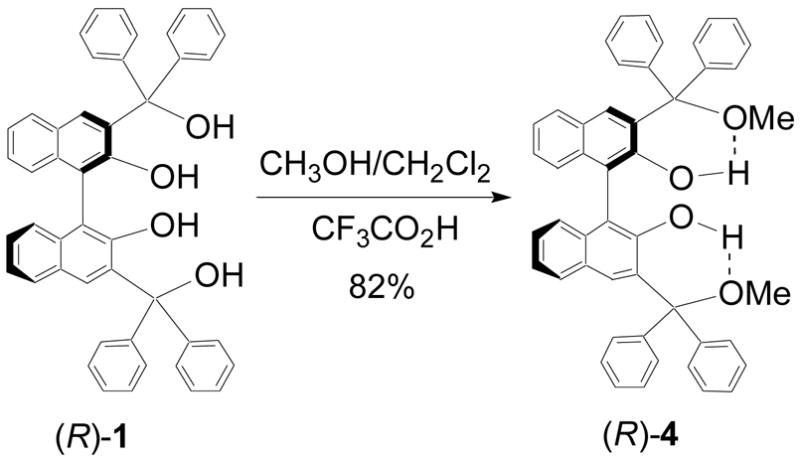
Synthesis of (R)-4.
Scheme 3.

Synthesis of (R)-6.
The 1H NMR spectrum of (R)-1 in CDCl3 was compared with those of (R)-4 and (R)-6. It was found that the hydroxyl protons of (R)-4 showed the most down-field chemical shift at δ 8.49 (s, 2H). (R)-1 gave two hydroxyl proton signals at δ 6.53 (s, 2H) and 4.64 (s, 2H), and (R)-6 gave one hydroxyl signal at δ 5.56 (s, 2H). The strong intramolecular hydrogen bonds of (R)-4 due to the combination of the more acidic aryl hydroxyl protons with the more basic alkyl methoxyl oxygens probably contributed to its most down field hydroxyl proton signal. The less acidic alkyl hydroxyl protons of (R)-6 might not form a strong hydrogen bond with the less basic aryl methoxyl oxygens and a much smaller chemical shift was observed for its hydroxyl protons. (R)-1 contained two types of protons, and the more acidic aryl hydroxyl protons might form intramolecular hydrogen bonds but the less acidic alkyl hydroxyl protons might not.
2. Optical Spectroscopic Studies of (R)-1, (R)-4 and (R)-6
The UV spectra of (R)-1, (R)-4, (R)-6 and (R)-BINOL are compared. The longest absorption band of (R)-4 is significantly red-shifted (λmax = 342 nm) relative to those of the other compounds. This could be attributed to the more polarized O-H bonds of (R)-4 due to the strong intramolecular interaction between its acidic aryl hydroxyl protons and the basic alkyl methoxyl oxygens. The longest wavelength absorption band of (R)-6 is blue-shifted (λmax = 327 nm) because of the methyl protection of the aryl hydroxyl groups. (R)-1 and BINOL have similar UV absorptions at the long wavelengths. At the short wavelengths, (R)-1 shows increased intensity with a slightly red-shifted maximum relative to that of BINOL due to the alkyl substituents at the 3,3′-positions.
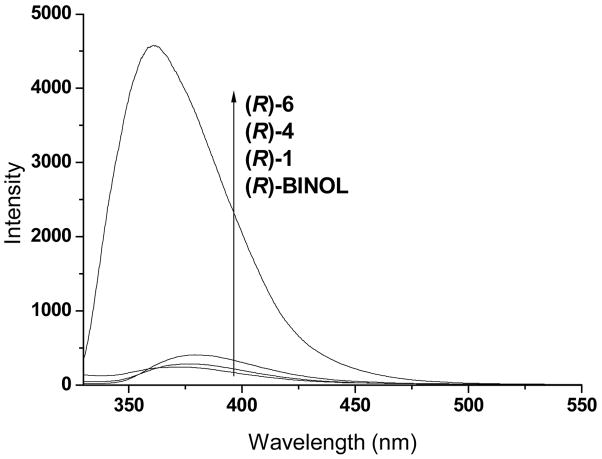
The fluorescence spectra of compounds (R)-1, (R)-4, (R)-6 and (R)-BINOL in methylene chloride were obtained. As shown in Figure 1, the emission maximums of (R)-1 and (R)-4, both at 379 nm, are similar to that of (R)-BINOL, at 372 nm, with small red-shifts. However, (R)-6 exhibits a greatly increased emission signal at λem = 361 nm with over 10 nm blue shifts from those of (R)-1, (R)-4 and (R)-BINOL. This indicates that the acidic aryl hydroxyl groups of (R)- 1, (R)-4 and (R)-BINOL significantly quenched their fluorescence probably due to intra- or intermolecular hydrogen bonds and/or proton transfer at the excited states.
Figure 1.
Fluorescence Spectra of (R)-BINOL, (R)-1, (R)-4 and (R)-6 in CH2Cl2 (8.0 × 10−6 M, λexc at 294, 298, 298 and 302 nm, respectively, emission slits: 5.0 nm).
We also found that addition of hexane to the methylene chloride solution of (R)-1 caused only a small change in its fluorescence. Later, hexane will be added to the methylene chloride solution of this compound to encourage its hydrogen bonding interaction with chiral amino alcohols.
3. Enantioselective Fluorescent Recognition of Chiral Amino Alcohols and a Chiral Diamine Using (R)-1 and Its Analogs.9
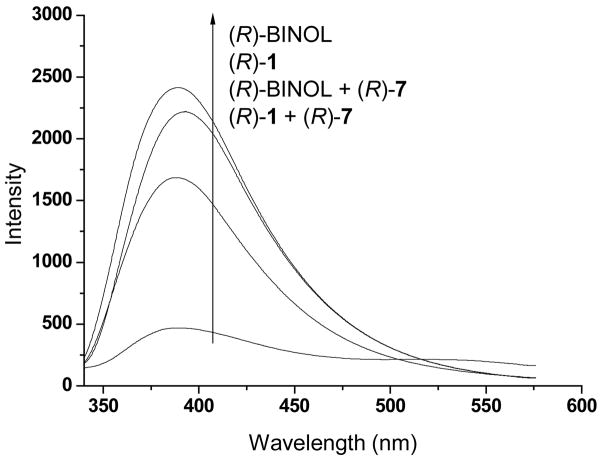
As shown in the above section, introduction of two additional hydroxyl groups in (R)-1 does not significantly change its UV absorption and fluorescence spectroscopic properties from those of (R)-BINOL. We compared their fluorescence responses in the presence of an amino alcohol. Figure 2 gives the fluorescence spectra of (R)-1 and (R)-BINOL before and after they were treated with (R)-phenylalaninol, (R)-7. It shows that the amino alcohol quenched the fluorescence of (R)-1 (I0/I = 4.0) much more efficiently than that of (R)-BINOL (I0/I = 1.4).
Figure 2.
Fluorescence Spectra of (R)-1 and (R)-BINOL (both at 1.0 × 10−5 M) in CH2Cl2/hexane (30:70) with and without (R)-7 (8.0 × 10−3 M) (λexc at 298 and 300 nm respectively).
We further studied the fluorescence responses of (R)-1 in the presence of both enantiomers of 7. It was found that the fluorescence of (R)-1 at 1.0 × 10−5 M was quenched by the optically pure (R)- and (S)-7 with significantly different efficiency. In the concentration range studied, the fluorescence quenching follows the Stern-Völmer equation:10
where I0 is the fluorescence intensity in the absence of a quencher and I the fluorescence intensity in the presence of a quencher. [Q] is the quencher concentration. KSV is the Stern-Völmer constant, which measures the efficiency of quenching. Figure 3 shows the Stern-Völmer plot of (R)-1 (1.0 × 10−5 M) in the presence of (R)- and (S)-7 (2.0 × 10−3 − 6.0 × 10−3 M) in a mixed solvent of methylene chloride and hexane (30:70). The error bars in the plot are in the range of -13% - +15%. The Stern-Völmer constant of (R)-1 is 389 M−1 (KSVR,R) in the presence of (R)-7 and 309 M−1 (KSVR,S) in the presence of (S)-7. Thus, (R)-7 quenches the fluorescence of (R)-1 more efficiently than (S)-7 with KSVRR/KSVRS = 1.26.
Figure 3.
Stern-Völmer Plot of (R)-1 (1.0 × 10−5 M) in CH2Cl2/hexane (30:70) in the presence of (R)- and (S)-7 (λexc = 298 nm).
We also prepared (S)-1, the enantiomer of (R)-1, and studied its interaction with (R)- and (S)-7 which showed the opposite enantioselectivity. That is, (S)-7 quenched the fluorescence of (S)-1 more efficiently than (R)-7. This confirms that the observed differences in the fluorescence quenching are due to chiral discrimination.
The Stern-Völmer constants of (R)-BINOL in the presence of (R)- and (S)-7 were found to be 106 M−1 and 91 M−1 respectively under the same conditions as the use of (R)-1. The ratio KSVR,R/KSVR,S is 1.16. Similar to (R)-1, the R enantiomer of the amino alcohol quenched the fluorescence of (R)-BINOL more efficiently than the S enantiomer does.
The amine-induced fluorescence quenching of (R)- and (S)-BINOL was attributed to both ground-state hydrogen bonding as well as excited-state deprotonation of individual binaphthol molecules.2d We expect that the fluorescence quenching of (R)- and (S)-1 by the amino alcohol should involve similar mechanisms. Thus, the additional hydroxyl groups of (R)-1 might have increased the interaction of this compound with the amino alcohol both at the ground state and the excited state, leading to the much increased fluorescence quenching. As the concentration of the amino alcohol increased, a new emission signal also appeared at above 500 nm probably contributed by the deprotonated (R)- or (S)-1 at the excited state.
We also studied the fluorescence of (S)-1 in the presence of both enantiomers of chiral phenylglycinol (8), leucinol (9) and valinol (10). All these molecules were found to quench the fluorescence of (S)-1 efficiently with certain enantioselectivity. The Stern-Völmer constants, the errors and enantioselective factors of (S)-1 in the presence of the chiral amino alcohols 7 – 10 are summarized in Table 1. In all cases, the (S)-amino alcohols quenched the fluorescence of (S)-1 more efficiently than the (R)-amino alcohols did. This indicates that the interaction of (S)-1 with the (S)-amino alcohols is better than it with the (R)-amino alcohols.
Table 1.
Stern-Völmer Constants and Enantioselective Factors of (S)-1 in the presence of the Chiral Amino Alcohols 7 – 10.
| Amino Alcohol | (S)-7 | (R)-7 | (S)-8 | (R)-8 | (S)-9 | (R)-9 | (S)-10 | (R)-10 |
|---|---|---|---|---|---|---|---|---|
| KSV (M−1) | 320 | 258 | 83 | 68 | 279 | 226 | 312 | 285 |
| Error | -10% - +5% | -3% - +4% | -14% - +28% | -9% - +15% | ||||
| Factor | 1.24 | 1.22 | 1.24 | 1.09 | ||||

The fluorescence responses of (R)-4 and (R)-6 in the presence of the amino alcohols were investigated. As shown in Figure 1, the fluorescence intensity of (R)-6 is much greater than that of (R)-1. When (R)-6 was treated with 7, no fluorescence quenching was observed. This demonstrates that the interaction of the central hydroxyl protons with the amino alcohol is essential for the fluorescence quenching of (R)-1. When (R)-4 was interacted with the amino alcohols 7 and 9, nonmonotonic fluorescence enhancements were observed. It is not clear why the amino alcohols cause the fluorescence enhancement of (R)-4, but the strong intramolecular hydrogen bonds in (R)-4 as indicated by its 1H NMR spectrum should have contributed to this. For example, the intramolecular hydrogen bonds of (R)-4 plus its intermolecular hydrogen bonds with the amino alcohols could lead to the formation of a structurally more rigid complex that could have enhanced fluorescence. This process would be in competition with the amine-induced fluorescence quenching at the central BINOL unit.
However, when (S)-1 and (R)-4 were treated with the chiral diamine (R,R)- or (S,S)-11, efficient fluorescence quenching was observed for both fluorophores. Thus, the two basic amine groups of (R,R)- or (S,S)-11 might have overcome the effect of the intramolecular hydrogen bonds in (R)-4, leading to the expected fluorescence quenching. Figure 4 is the Stern-Völmer plots of (S)-1 in the presence of (R,R)- and (S,S)-11 in CH2Cl2. The Stern-Völmer constants of (S)-1 in the presence of (R,R)- and (S,S)-11 are 1175 M−1 and 795 M−1 respectively, and those of (R)-4 are 807 M−1 and 553 M−1 respectively. Both (S)-1 and (R)-4 are very sensitive and enantioselective in the fluorescent recognition of the chiral diamine. The fluorescence quenching of (S)-1 by the chirality matched (R,R)-11 is more efficient than that of (R)-4 by the chirality-matched (S,S)-11. Therefore, the four hydroxyl groups of (S)-1 should have provided better binding with the diamine than the two hydroxyl groups of (R)-4. The Stern-Völmer constants of (R)-1 in the presence of the diamine are also much greater than those in the presence of the amino alcohols 7 – 10. This demonstrates that there is a much stronger interaction for (R)-1 with the diamine than with the amino alcohols.
Figure 4.
Stern-Völmer Plot of (S)-1 (1.0 × 10−5 M) in CH2Cl2 in the presence of (R,R)- and (S,S)-11 (λexc = 255 nm).

In summary, we have demonstrated that with the introduction of two additional hydroxyl groups to BINOL, the resulting compounds (S)- or (R)-1 have exhibited a significantly improved fluorescent sensitivity towards amino alcohols. Through a selective methylation of the two different types of the hydroxyl groups of (S)- or (R)-1, the roles of these hydroxyl groups in the enantioselective fluorescent recognition of amino alcohols have been identified. The interaction of the central naphthyl hydroxyl groups of (S)- or (R)-1 with the substrates is responsible for the observed fluorescence quenching and the two alkyl hydroxyl groups of (S)- or (R)-1 increase the efficiency of the fluorescence quenching.
Experimental Section
Preparation and Characterization of (R)-3,3′-Bis(diphenylhydroxymethyl)-2,2′-di-hydroxy[1,1′]binaphthalenyl, (R)-1
(a) Preparation of (R)-3.
Under nitrogen, (R)-2 (5.0 mmol, 1.87 g) was dissolved in THF (100 mL, dried with sodium/benzophenone) in a flask equipped with a side arm and a magnetic stirring bar. The solution was cooled to 0 °C and nBuLi (25.0 mmol, 2.5 M in hexane, 10 mL) was added dropwise. The reaction mixture was stirred for 3 h at 0 °C and benzophenone (15.0 mmol, 2.73 g) was added as a solid. The reaction was allowed to warm to room temperature overnight and quenched with saturated aqueous NH4Cl solution. THF was removed in vacuo and the aqueous solution was extracted with CH2Cl2. The organic phase was dried with MgSO4 and evaporated to dryness. The residue was recrystallized from benzene and methanol to afford (R)-3 as a white solid in 95% yield (3.51 g). (S)-3 was prepared from (S)-2 with the same procedure. 1H NMR (400 MHz, CDCl3) δ7.63 (d, J = 8.0 Hz, 2H), 7.44 (d, J = 8.8 Hz, 4H), 7.37-7.26 (m, 20H), 7.19 (d, J = 8.4 Hz, 2H), 7.15 (s, 2H), 5.80 (s, 2H), 3.81 (d, J = 4.8 Hz, 2H), 3.77 (d, J = 4.8, 4H), 2.82 (s, 6H). ESI-MS m/z: 761.4 (M + Na+, 100).
(b) Preparation of (R)-1
Under nitrogen, (R)-3 (10.9 mmol, 8.08 g) was dissolved in THF (160 mL) to which 3N HCl (30 mL) was added. After the mixture was heated at reflux (80 °C) for 6 h, 10% NaHCO3 (100 mL) was added to quench the reaction. The mixture was extracted with ethyl acetate and the organic phase was washed with water and dried with MgSO4. After evaporation, the residue was dissolved in diethyl ether, and precipitated with petroleum ether. The precipitate was recrystallized from acetone to afford (R)-1 as a white solid in 90% yield (6.40 g). (S)-1 was also prepared from (S)-3 with the same procedure. The optical purity of (R)-1 was 95% ee as determined by using HPLC-Chiralcel OD column (Solvent: Hexane/iPrOH = 20/80, Flow rate: 0.3 mL/min). The specific optical rotation of (R)-1, [α]D20, is +113.4 (c = 1.00, CHCl3), and that of (S)-1, [α]D20, is -109.5 (c = 1.00, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.60 (d, J = 9.2 Hz, 2H), 7.35-7.22 (m, 24H), 7.13 (s, 2H), 7.09 (d, J = 8.8 Hz, 2H), 6.57 (s, 2H), 4.65 (s, 2H). 13C NMR (50 MHz, CDCl3) δ 151.3, 145.4, 145.2, 133.9, 133.4, 131.0, 129.0, 128.2, 128.1, 128.0, 127.6, 127.3, 124.2, 124.1, 114.4, 83.0. ESI-MS: m/z 649.3 (M-H+, 100). HRMS calcd for C46H34Na1O4 (M+Na): 673.2349. Found: 673.2338. Anal. Calcd for C46H36O5 ((R)-1+H2O): C, 82.61; H, 5.43. Found: C, 82.50; H, 5.62.
Preparation and Characterization of (R)-3,3′-Bis(diphenylmethylmethoxyl)-2,2′-dihydroxy[1,1′]binaphthalenyl, (R)-4
Under nitrogen, (R)-1 (2.4 mmol, 1.52 g) was dissolved in CH2Cl2 (35 mL) and CH3OH (120 mL). CF3CO2H (1 mL) was added at 0°C. The mixture was gradually warmed up to room temperature. The reaction progress was monitored by conducting thin layer chromatography on silica gel. In about 8 h, there was a white precipitate in the mixture. CH2Cl2 (35 mL) was added to dissolve the precipitate, and the mixture was neutralized to pH 6 – 7 with 3% NaHCO3. It was extracted with CH2Cl2 and the organic phase was washed with water and dried with MgSO4. After removal of the volatile solvent under reduced pressure, the residue was passed through a silica gel column eluted with EtOAc/petroleum ether (1/9) to afford (R)-4 as a light yellow solid in 82% yield (1.31 g). 1H NMR (400 MHz, CDCl3) δ 8.51 (s, 2H), 7.65 (d, J = 9.2 Hz, 4H), 7.51-7.45 (m, 8H), 7.40-7.10 (m, 16H), 6.97 (d, J = 8.0 Hz, 2H), 3.30 (s, 6H). 13C NMR (50 MHz, CDCl3) δ 152.0, 141.7, 140.8, 133.8, 130.3, 130.2, 129.6, 128.5, 128.1, 127.9, 127.8, 127.6, 126.7, 124.5, 123.2, 117.6, 90.3, 53.1. [α]D20 = +192.1 (c = 1.01, CHCl3). HRMS calcd for C48H38Na1O4 (M+Na): 701.2662. Found: 701.2654. Anal. Calcd for C48H38O4: C, 84.93; H, 5.64. Found: C, 84.72; H, 5.58.
Preparation and Characterization of (R)-3,3′-Bis(diphenylhydroxymethyl)-2,2′-dimethoxy[1,1′]binaphthalenyl, (R)-6
Under nitrogen, diethyl ether (100 mL, dried) and TMEDA (15.0 mmol, 1.75 g) were placed in a 250 mL three-necked round-bottomed flask equipped with a side arm and a magnetic stirring bar. To this solution was added nBuLi (20.0 mmol, 2.5 M in hexane, 8 mL) dropwise. It was then stirred at room temperature for 30 min. (R)-2,2′-Dimethoxy-1,1′-binaphthalenyl, (R)-5 (5.0 mmol, 1.55 g), was added in one portion, and the reaction mixture was stirred for 3 h. Benzophenone (15.0 mmol, 2.73 g) in diethyl ether (10 mL) was added to the resulting light brown suspension via syringe over 10 min. The reaction mixture was stirred overnight and quenched with saturated aqueous NH4Cl solution. The volatile solvent was removed in vacuum and the remaining aqueous solution was extracted with CH2Cl2. The organic phase was dried over MgSO4 and evaporated to dryness. The residue was passed through a silica gel column eluted with CH2Cl2/petroleum ether/EtOAc (1/1/0.001) to afford (R)-6 (2.01 g) as a white solid in 60% yield. 1H NMR (400 MHz, CDCl3) δ 7.63 (d, J = 8.0 Hz, 2H), 7.44-7.20 (m, 26H), 7.14 (s, 2H), 5.56 (s, 2H), 2.41 (s, 6H). 13C NMR (50 MHz, CDCl3) δ 155.6, 147.4, 145.5, 140.7, 133.8, 130.0, 129.3, 128.7, 128.5, 128.3, 127.9, 127.8, 127.5, 127.3, 127.1, 126.9, 126.5, 125.2, 125.1, 124.1, 82.2, 60.1. [α]D20 = +84.2 (c =5.02, CHCl3). HRMS calcd for C48H38Na1O4 (M+Na): 701.2662. Found 701.2675. Anal. Calcd for C48H40O5 ((R)-5+H2O): C, 82.73; H, 5.79. Found: C, 82.37; H, 5.60.
Preparation of Samples for Fluorescence Measurement
All solutions were prepared using volumetric syringes, pipettes and volumetric flasks. The stock solutions of fluorophores and quenchers were freshly prepared and used for each measurement. For the fluorescence quenching study, a fluorophore solution was mixed with the amino alcohol and amine solutions in a 10 mL volumetric flask and diluted to the desired concentration. The resulting solution was allowed to stand at room temperature for 3 – 4 h before the fluorescence measurement in order to allow the interaction to reach equilibrium. For each series of experiments, three independent fluorescence measurements were carried out. The average value of the Stern-Völmer constant was given in all the plots.
Supplementary Material
Additional UV, fluorescence and NMR spectra of the chiral 1,1′-binaphthyl compounds and Stern-Völmer Plots. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 20328203, 20272039), Program for New Century Excellent Talents in University and Scientific Fund of Sichuan Province for Outstanding Young scientist. LP thanks the partial support of this work from the US National Institute of Health (R01GM58454/R01EB002037-05) and Changjiang Scholarship of the Educational Ministry of China.
References
- 1.(a) Pu L. Chem Rev. 1998;98:2405–2494. doi: 10.1021/cr970463w. [DOI] [PubMed] [Google Scholar]; (b) Kocovsky P, Vyskocil S, Smrcina M. Chem Rev. 2003;103:3213–3245. doi: 10.1021/cr9900230. [DOI] [PubMed] [Google Scholar]; (c) Walsh PJ, Lurain AE, Balsells J. Chem Rev. 2003;103:3297–3344. doi: 10.1021/cr0000630. [DOI] [PubMed] [Google Scholar]; (d) Brunel JM. Chem Rev. 2005;105:857–897. doi: 10.1021/cr040079g. [DOI] [PubMed] [Google Scholar]
- 2.(a) Irie M, Yorozu T, Hayashi K. J Am Chem Soc. 1978;100:2236–2237. [Google Scholar]; (b) Yorozu T, Hayashi K, Irie M. J Am Chem Soc. 1981;103:5480–5484. [Google Scholar]; (c) Avnir D, Wellner E, Ottolenghi M. J Am Chem Soc. 1989;111:2001–2003. [Google Scholar]; (d) Iwanek W, Mattay J. J Photochem Photobiol A: Chem. 1992;67:209–226. [Google Scholar]; (e) James TD, Sandanayake KRAS, Shinkai S. Nature. 1995;374:345–347. [Google Scholar]; (f) James TD, Sandanayake KRAS, Iguchi R, Shinkai S. J Am Chem Soc. 1995;117:8982–8987. [Google Scholar]; (g) Parker KS, Townshend A, Bale SJ. Anal Proc. 1995;32:329–332. [Google Scholar]; (h) Kubo Y. Synlett. 1999;2:161–174. [Google Scholar]; (i) Beer G, Rurack K, Daub J. J Chem Soc, Chem Commun. 2001:1138–1139. [Google Scholar]
- 3.A review on the enantioselective fluorescent recognition of chiral organic molecules: Pu L. Chem Rev. 2004;104:1687–1716. doi: 10.1021/cr030052h.Pugh V, Hu QS, Pu L. Angew Chem, Int Ed. 2000;39:3638–3641.Lin J, Hu QS, Xu MH, Pu L. J Am Chem Soc. 2002;124:2088–2089. doi: 10.1021/ja011971x.Xu MH, Lin J, Hu QS, Pu L. J Am Chem Soc. 2002;124:14239–14246. doi: 10.1021/ja020989k.Li ZB, Lin J, Pu L. Angew Chem, Int Ed. 2005;44:1690–1693. doi: 10.1002/anie.200462471.
- 4.(a) Mei X, Wolf C. J Am Chem Soc. 2004;126:14736–14737. doi: 10.1021/ja0459781. [DOI] [PubMed] [Google Scholar]; (b) Reetz MT, Sostmann S. Tetrahedron. 2001;57:2515–2520. [Google Scholar]; (c) Xu YF, McCarroll ME. J Phys Chem A. 2004;108:6929–6932. [Google Scholar]
- 5.(a) Barhate NB, Chen C. Org Lett. 2002;4:2529–2532. doi: 10.1021/ol026156g. [DOI] [PubMed] [Google Scholar]; (b) Li XL, Hewgley B, Mulrooney CA, Yang J, Kozlowski MC. J Org Chem. 2003;68:5500–5511. doi: 10.1021/jo0340206. [DOI] [PubMed] [Google Scholar]
- 6.Cox PJ, Wang W, Snieckus V. Tetrahedron Lett. 1992;33:2253–2256. [Google Scholar]
- 7.Dixon DI, Tillman AL. Synlett. 2005;17:2635–2638. [Google Scholar]
- 8.Lingenfelter DS, Helgeson RG, Gram DJ. J Org Chem. 1981;46:393–406. [Google Scholar]
- 9.Selected recent work on the chiral recognition of amino alcohols: Tsubaki K, Tanima D, Nuruzzaman M, Kusumoto T, Fuji K, Kawabata T. J Org Chem. 2005;70:4609–4616. doi: 10.1021/jo050387u.Kim KM, Park H, Kim H-J, Chin J, Nam W. Org Lett. 2005;7:3525–3527. doi: 10.1021/ol051267b.Dai ZH, Xu XD, Canary JW. Chirality. 2005;17:S227–S233. doi: 10.1002/chir.20130.Lee SL, Lin WB. J Am Chem Soc. 2002;124:4554–4555. doi: 10.1021/ja0256257.Liu Y, Li B, Wada T, Inoue Y. Tetrahedron. 2001;57:7153–7161.Reetz MT, Sostmann S. Tetrahedron. 2001;57:2515–2520.
- 10.Lakowicz JR. Principles of Fluorescence Spectroscopy. 1. Plenum Press; New York: 1983. pp. 257–301. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional UV, fluorescence and NMR spectra of the chiral 1,1′-binaphthyl compounds and Stern-Völmer Plots. This material is available free of charge via the Internet at http://pubs.acs.org.