Abstract
Pancreatic cancer (PC) remains the fourth most common cause of cancer related death in the United States. Therefore, novel strategies for the prevention and treatment are urgently needed. Numerous dietary and pharmacological agents have been proposed as alternative strategies for the prevention and/or treatment of PC. Isoflavone is a prominent flavonoid found in soy products and has been proposed to be responsible for lowering the incidence of PC in Asians. Similarly, curcumin, an active ingredient of turmeric, that inhibits growth of malignant neoplasms, has a promising role in the prevention and/or treatment of PC. Here we examined whether isoflavone together with curcumin could elicit a greater inhibition of growth of PC cells than either agent alone, and also sought to determine the molecular mechanism of action. We found that the inhibition of cell growth and induction of apoptosis was significantly greater in the combination group than that could be achieved by either agent alone. These changes were associated with decreased Notch-1 expression and DNA binding activity of NF-κB and its target genes such as Cyclin D1, Bcl-2, and Bcl-xL. Moreover, we found that the combination of four natural agents at lower concentration was much more effective. Collectively, our results suggest that diet containing multiple natural products should be preferable over single agents for the prevention and/or treatment of PC. The superior effects of the combinatorial treatment could partly be attributed to the inhibition of constitutive activation of Notch-1 and NF-κB signaling pathways.
Keywords: Notch-1, isoflavone, curcumin, NF-κB
Introduction
Pancreatic cancer (PC) is a highly aggressive malignant disease, which is currently treated with limited success and dismal outcomes using conventional therapeutics including chemotherapy and irradiation. PC is ranked as the fourth most common cause of cancer related mortality in the United States (Jemal et al., 2008). It is estimated that approximately 37,680 new PC cases will be diagnosed with estimated deaths of 34,290 in 2008 (Jemal et al., 2008). Despite therapeutic advances, the prognosis of patients with PC is extremely poor, with a median survival of 6 months (Jemal et al., 2008). This disappointing outcome strongly suggests that the evaluation of novel, targeted therapeutic agents is urgently needed to improve the outcome of patients diagnosed with this deadly disease.
Epidemiological studies have consistently shown that regular consumption of fruits and vegetables is associated with a markedly reduced risk of cancer (Sarkar and Li, 2006). Moreover, people (Asians) who consume a diet high in soy products and a plant Curcuma longa (Linn), which provide curcumin, have a relatively low incidence and mortality of PC, suggesting that a high intake of soy products and curcumin may protect people against pancreatic cancer (Wang et al., 2006b; Wang et al., 2006c). Soy isoflavone, a natural flavonoid found in soybean products, has been proposed to be associated with lower incidence of PC and is believed to function as a chemopreventive agent (Fotsis et al., 1995; Banerjee et al., 2005; Banerjee et al., 2007). It has been found that isoflavone can inhibit the growth of various cancer cell lines both in vitro and in vivo (Banerjee et al., 2005; Banerjee et al., 2007; El-Rayes et al., 2006; Li et al., 2005). Our previous studies have shown that isoflavone can induce apoptosis and inhibit NF-κB activation in PC cells (Wang et al., 2006c). Curcumin (diferuloylmethane), which is a phenolic compound, is a widely used flavoring agent in food and it has shown to have antitumor activity in many cancers, including PC (Goel et al., 2008; Kunnumakkara et al., 2007; Wang et al., 2006c). Plummer et al. reported that curcumin consistently suppressed NF-κB activation by inhibiting IκB kinase, which induces IκBα phosphorylation, leading to the inhibition of NF-κB downstream gene expression in PC cells (Plummer et al., 1999). NF-κB downstream gene over-expression is associated with apoptosis resistance, angiogenesis, enhanced invasion, and metastasis (Sarkar and Li, 2008).
Moreover, NF-κB plays important roles in the control of cell growth, differentiation, and apoptosis (Sarkar and Li, 2008). The activation of NF-κB involves the phosphorylation of IκB, an inhibitory binding partner of NF-κB complex, for ubiquitination and degradation through proteasome degradation pathway. This allows the translocation of NF-κB into the nucleus where it activates transcription of genes that are involved in controlling cell proliferation, differentiation, apoptosis, inflammation, stress response, angiogenesis, tumor promotion and metastasis, and also control many other cellular and physiological processes (Sethi et al., 2008). A key regulatory step in the NF-κB pathway is the activation of a high molecular weight IKK complex in which catalysis is done by kinases, including IKKα and IKKβ, which directly phosphorylate IκB proteins (Sarkar and Li, 2008). Therefore, the precise molecular mechanism by which NF-κB is activated in many disease processes is under intense investigation.
The Notch signaling pathway is another important transduction pathway in cells, and it plays a critical role in controlling cell survival and apoptosis. To date, four vertebrate Notch genes have been identified: Notch-1-4 (Miele, 2006). Notch genes encode proteins which can be activated by interacting with a family of its ligands. Upon activation, Notch is cleaved, releasing ICN (intracellular Notch) which translocates into the nucleus. The ICN associates with transcription factors, regulating the expression of target genes in regulating the development and growth of cells (Miele et al., 2006; Miele, 2006). Recent evidence suggests that the Notch signaling pathway is involved in PC cell survival (Miyamoto et al., 2003; Wang et al., 2006d). Miyamoto et al reported that Notch pathway components and Notch target genes such as Hes-1 are up-regulated in PC (Miyamoto et al., 2003). These reports clearly suggest a possible link between Notch gene over-expression and PC. Recent reports also showed that Notch-1 regulates the NF-κB pathway via activation of molecules in the NF-κB pathway (Jang et al., 2004; Oswald et al., 1998; Wang et al., 2006a).
The fate of cancer chemopreventive strategies relies largely on the ability of chemopreventive agents to maximally exploit the intrinsic anti-tumor potential of in combination without incurring undue toxicity. Targeting multiple signal transduction pathways by a combinatorial approach using natural products would ideally empower the clinician to better delay the progression of cancer especially PC. Emerging evidence suggest that cancer is due to defects in multiple genes, and therefore a multi-targeting agent or the combination of agents targeting multiple pathways without causing any toxicity would be required for the success of prevention and/or treatment of PC. Therefore, we believe that the combination of soy isoflavone and curcumin, or even other agents together would be highly effective in PC cells. In this study, we found that the combination approach is better in killing PC cells, which was partly due to inactivation of Notch-1 and NF-κB signaling and their downstream genes.
Materials and methods
Cell culture and experimental reagent
Human PC cell line BxPC-3 (ATCC, Manassas, VA) was cultured in RPMI-1640 media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin. Colo-357 cells were generously provided by Dr. Paul Chiao at M.D. Anderson Cancer Center (Houston, TX), and grown as a monolayer cell culture in DMEM containing 4.5 mg/mL D-glucose and L-glutamine supplemented with 10% FBS. Isoflavone, curcumin, resveratrol and epigallocatechin-3-gallate (EGCG) were purchased from Amax NutraSource Inc (Eugene, OR).
Cell growth inhibition studies by MTT assay
BxPC-3 and Colo-357 cells (5 × 103) were seeded in a 96-well culture plate and subsequently treated with dietary compounds in different concentrations as described under the figure legend. After treatment, the cells were incubated with 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) reagent (0.5 mg/ml; Sigma) at 37°C for 2 h and then with isopropanol at room temperature for 1 h. The Spectrophotometric absorbance of the samples was measured by using Ultra Multifunctional Microplate Reader (Tecan, Durham, NC, USA). The experiment was repeated three times and the t test was performed to verify the significance of cell growth inhibition after treatment.
Histone/DNA ELISA for detection of apoptosis
The Cell Death Detection ELISA Kit (Roche, Palo Alto, CA, USA) was used for assessing apoptosis in BxPC-3 and Colo-357 cells treated with dietary compounds according to the manufacturer's protocol. Briefly, BxPC-3 and Colo-357 cells were treated the same way as described previously for growth inhibition study. After treatment, the cells were lysed, and the cell lysates were overlaid and incubated in microtiter plate modules coated with anti-histone antibody. Samples were then incubated with anti-DNA peroxidase followed by color development with ABTS® substrate. The optical densities of the samples were determined using the Ultra Multifunctional Microplate Reader (Tecan, Durham, NC, USA) at 405 nm.
Western blot analysis
BxPC-3 and Colo-357 cells were seeded in 100 mm dishes and allowed to attach for 24 h, followed by the addition of isoflavone, curcumin, or a combination of both for 72 h. After incubation, cells were lysed in a buffer containing 62.5 mm Tris-HCl and 2% SDS and were sonicated for 3 × 10 seconds. Protein concentration was measured using the BCA Protein Assay Kit (Pierce, Rockfbrd, IL, USA). Samples were resolved through a 10% SDS polyacrylamide gel and then transferred to a nitrocellulose membrane. After blocking with 5% nonfat dry milk, each blot was incubated with polyclonal anti-Notch-1, anti-Cyclin D1, anti-Bcl-2, anti-Bcl-xL (Santa Cruz Biotechnology, Santa Cruz, CA), or anti-β-actin (Sigma) antibodies. The bound antibodies were detected by secondary antibodies conjugated with peroxidase and Supersignal Chemiluminescent Substrate (Pierce).
Preparation of Nuclear extract
BxPC-3 and Colo-357 cells were plated in 100 mm dishes and cultured for 24 h. Subsequently, the cultures were treated with isoflavone, curcumin, or a combination of both for 72 h. Cells exposed to dietary compounds or kept as control were washed with cold phosphate-buffered saline and suspended in 0.15 ml of lysis buffer [10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, 2 μg/ml leupeptin, 2 μg /ml aprotinin, and 0.5 mg/ml benzamidine). The cells were allowed to swell on ice for 20 min and then 4.8 μl of 10 % Nonidet P-40 was added. The tubes were then vigorously mixed on a vortex mixer for a few seconds and centrifuged for 120 sec in a microfuge. The nuclear pellet was resuspended in 30 μl of ice - cold nuclear extraction buffer [20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.5 mM PMSF, 2 μg /ml leupeptin, 2 μg/ml aprotinin, and 0.5 mg/ml benzamidine], and incubated on ice with intermittent mixing. The tubes were then centrifuged for 5 min in a microfuge at 4°C, and the supernatant (nuclear extract) was collected in a cold eppendorf tube and stored at −70°C for later use. The protein content was measured by BCA method.
Electrophoretic mobility shift assay (EMSA) for measuring NF-κB activity
EMSA was performed by incubating 10μg of nuclear protein extract with IRDye™ 700-labeled NF-κB oligonucleotide (LI-COR, Lincoln, NE). The incubation mixture included 2 μg of poly (dI-dC) in a binding buffer. The DNA-protein complex formed was separated from free oligonucleotide on 8.0% native polyacralyamide gel using buffer containing 50 mM Tris, 200 mM glycine, pH 8.5, and 1 mM EDTA, and then visualized by Odyssey Infrared Imaging System using Odyssey Software Release 1.1 (LI-COR, Lincoln, NE). Super-shift assay using NF-κB p65 antibody was also conducted to confirm the specificity of NF-κB DNA-binding activity. For loading control, 10 μg of nuclear proteins from each sample were subjected to Western blot analysis for retinoblastoma protein, which showed no alternation after genistein treatment.
Densitometric and statistical analysis
The growth inhibition of BxPC-3 and Colo-357 cells by dietary compounds treatment was statistically evaluated using GraphPad StatMate software (GraphPad Software, Inc., San Diego, CA). Comparisons were made between control and dietary compounds treatment. P<0.05 was used to indicate statistical significance.
Results
Cell growth inhibition by isoflavone and curcumin
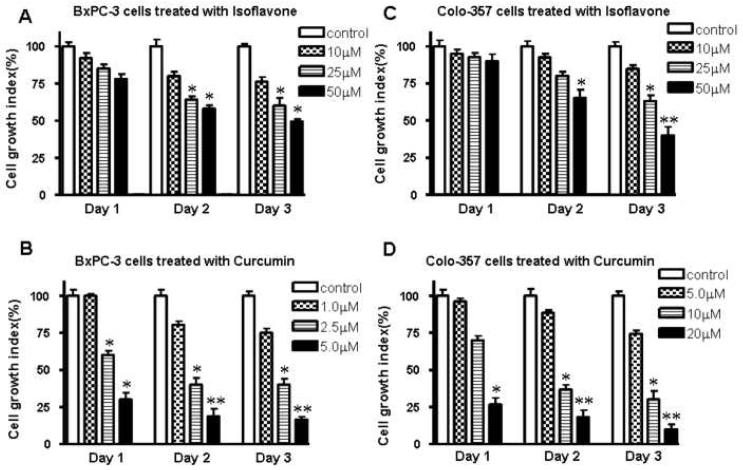
The treatment of BxPC-3 pancreatic cancer cells for 1–3 days with 10, 25, 50 μm of isoflavone and 1.0, 2.5, 5.0 μM curcumin resulted in cell growth inhibition, respectively. The inhibition of cell growth was found to be dose- and time-dependent (Fig.1A, B). Similar results were found in Colo-357 cells treated with isoflavone and curcumin, respectively, although Colo-357 cells were more resistant to curcumin (Fig.1C, D).
Figure 1.
Effect of isoflavone or curcumin on BxPC-3 and Colo-357 cell growth. Dose and time-dependent inhibition of cell growth by isoflavone and curcumin in BxPC-3 and Colo-357 PC cells. Cells were seeded in 96-well plates at 5,000 cells per well and treated with the indicated concentrations of isoflavone or curcumin for various time periods. After treatment, cell viability was determined by MTT assay. Each value represents the mean ± SD (n=6) of three independent experiments. * P<0.05, **P<0.01 compared with the control.
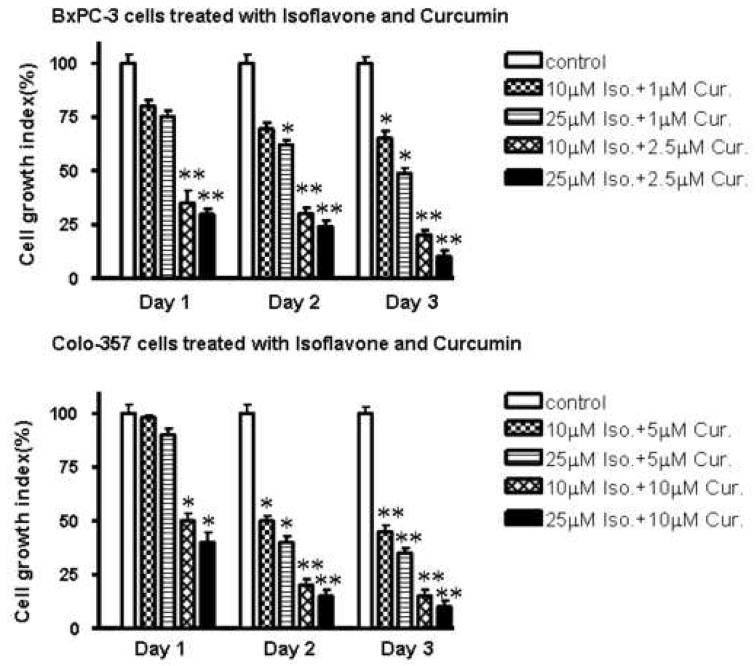
Cell growth inhibition with the combinations of isoflavone and curcumin
In order to detect the effects of the combinations of isoflavone and curcumin on the cell growth inhibition, we used the MTT assay with treatment durations of 24, 48 and 72h. As shown in Fig. 2, the cell growth inhibition increased to 35% and 80%, respectively relative to control in the BxPC-3 cells treated with combinations comprising isoflavone 10 μM + curcumin 1.0 μM and isoflavone 10 μM + curcumin 2.5 μM for 72 hours. Our results showed that 10 μM isoflavone alone did not inhibit BxPC-3 cell growth, and 2.5 μM curcumin alone inhibited about 40% cell growth at 24 h. However, isoflavone 10 μM + curcumin 2.5 μM significantly inhibited (65%) BxPC-3 cell growth at 24 h. The similar changes in cell growth were also observed in Colo-357 cells in response to either isoflavone or curcumin, each alone or in combination. In Colo-357, both 10 μM isoflavone and 5 μM curcumin alone did not significantly inhibit cell growth at 72 h, respectively. However, isoflavone 10 μM + curcumin 5 μM caused cell growth inhibition up to 60%. Taken together, these results suggest that a combinatorial treatment of PC cells with isoflavone and curcumin produced a greater inhibition of cell growth compared to either agent alone. Our results provides scientific basis in support of the role of combination of isoflavone and curcumin at a lower doses for inhibiting cell growth and that the use of low doses are likely to have lower toxicity to normal cells. Inhibition of cell proliferation observed by MTT could also be attributable to the induction of apoptosis in BxPC-3 and Colo-357 cells. Therefore, we explored whether the inhibition of cell growth was also accompanied by the induction of apoptosis induced by isoflavone and curcumin. DNA/histone fragmentation analysis was employed to investigate the degree of apoptosis induced by isoflavone and curcumin.
Figure 2.
Effects of the combination of isoflavone (Iso) and curcumin (Cur) on cell growth of BxPC-3 and Colo-357 cells. Each value represents the mean ± SD (n=6) of three independent experiments. * P<0.05, **P<0.01 compared with the control.
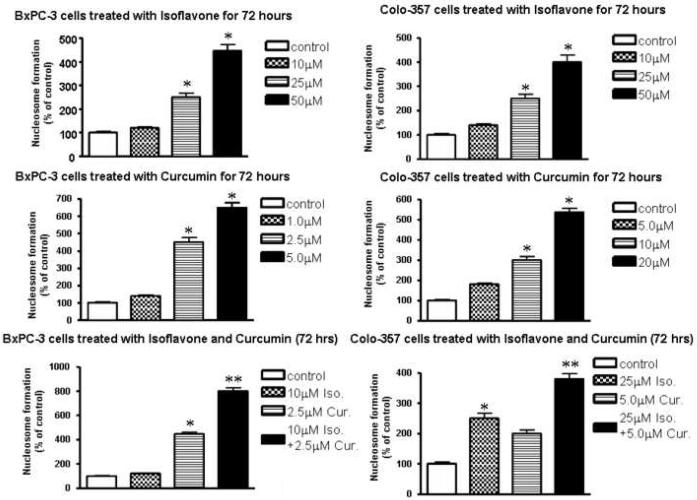
Inhibition of apoptosis with combinations of isoflavone and curcumin
BxPC-3 and Colo-357 cells were treated with different concentrations of isoflavone, curcumin or a combination of both for 72 h, respectively, and apoptosis was assessed. We found that in combinatorial treatment group there was a greater degree of induction of apoptosis than that caused by either agent alone, when compared with the control (Fig. 3). For example, 10 μM isoflavone alone did not induce apoptosis, whereas 2.5 μM curcumin caused approximately 4.5-fold increase in apoptosis of BxPC-3 cells, but the combination group showed 8-fold increase in apoptosis, when compared with the untreated control (Fig. 3). Similar results were also observed in Colo-357 cells in response to either isoflavone or curcumin, each alone or in combination. These results provide convincing data showing induction of apoptosis by the combinatorial treatment of PC cells with isoflavone and curcumin. These results are also consistent with cell growth inhibition observed by MTT assay, suggesting that greater cell growth inhibition by combination treatment is partly due to greater degree of apoptosis in PC cells.
Figure 3.
Effects of isoflavone (Iso) or curcumin (Cur) alone or their combination on apoptosis of BxPC-3 and Colo-357 cells. The cells were incubated for 72 h in the absence (control) or presence of indicated concentrations of isoflavone and/or curcumin. Values represent mean ± SD of 5-6 observations. * P<0.05, **P<0.01 compared with the control.
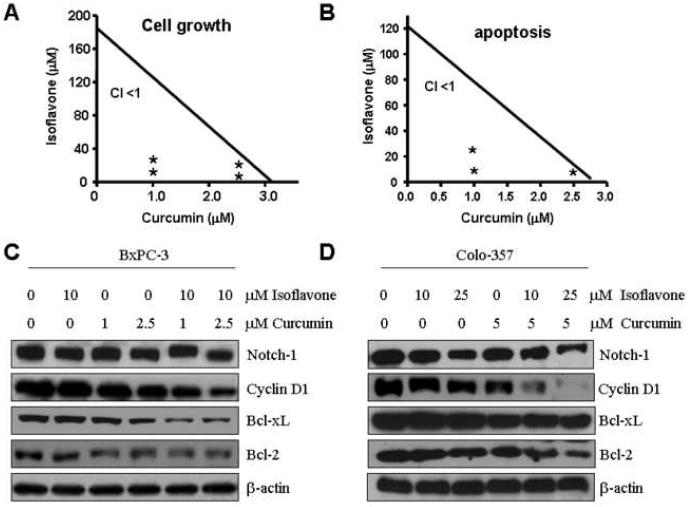
Isobologram analyses and combination indices for the combinations of isoflavone and curcumin
In order to detect whether there was a synergistic interaction observed in cell growth inhibition and apoptosis with the combinations of isoflavone and curcumin, we performed isobologram analyses of our data similar to those reported previously by our laboratory (Li et al., 2005). Isobologram analysis showed that the combination index for every combination treatment examined was <1, suggesting the lower doses of isoflavone with curcumin would be able to elicit synergistic inhibition of cell growth and induction of apoptosis in PC cells (Fig. 4A, B).
Figure 4.
A-B, Isobologram analyses of synergy between combinations of isoflavone and curcumin in BxPC-3 cells. The cells were incubated for 72 h in the absence (control) or presence of indicated concentrations of isoflavone and/or curcumin. Data points (*) are described by concentrations (in μM) of curcumin and isoflavone reflected on x- and y-axes respectively, and are representative of three independent experiments. Combination index (CI): a quantitative measure of the degree of drug interaction. CI < 1 indicates synergism; CI > 1 indicates antagonism; CI = 1 indicates additive effect.
C-D, Western blot analysis showing changes in the levels of Notch-1, Cyclin D1 and Bcl-xL as well as the levels of Bcl-2 in BxPC-3 and Colo-357 cells treated with isoflavone, curcumin, or their combination for 72 h. β-actin represents protein loading control.
Inhibition of the Notch-1 expression by isoflavone and curcumin
The Notch-1 signaling pathway is an important signal transduction pathway that plays a critical role in cell survival and apoptotic process. In our earlier studies, we have shown that isoflavone and curcumin inhibits cell growth by attenuating the signal transduction pathways including Notch-1 pathway (Wang et al., 2006b; Wang et al., 2006c). Because the combinatorial treatment produced a greater effect on cell growth and apoptosis than that elicited by either agent alone, we measured the Notch-1 expression following treatments with isoflavone, curcumin, or the combination of both agents. We found that the protein level of Notch-1 was down-regulated in BxPC-3 and Colo-357 cells treated with isoflavone, curcumin, or the combination of isoflavone and curcumin (Fig. 4C, D). Moreover, the magnitude of reduction of Notch-1 in response to the combinatorial treatment was found to be considerably greater than that observed by either agent alone, when compared with the controls (Fig. 4C, D). To confirm our data, we also found that the expression of Notch-1 target genes including cyclin D1, Bcl-2 and Bcl-xL, was also down-regulated in cells treated with combination of both compounds (Fig. 4C, D).
Inhibition of NF-κB activation by isoflavone and curcumin
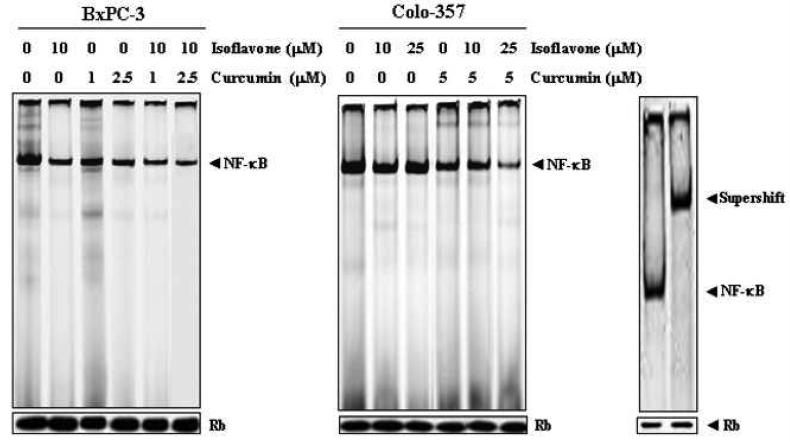
Emerging evidence suggest that Notch-1 regulates the NF-κB via activation of molecules in the NF-κB pathway (Jang et al., 2004; Oswald et al., 1998; Wang et al., 2006a). Therefore, to determine weather attenuation of Notch-1 activation by these agents could lead to reduction in NF-κB activity, we examined the DNA binding activity of NF-κB. The nuclear protein extracts from control and compound-treated BxPC-3 and Colo-357 cells were subjected to analysis for NF-κB DNA-binding activity by EMSA. We found that the combination of isoflavone and curcumin caused greater inhibition of NF-κB DNA-binding activity in BxPC-3 and Colo-357 cells compared to untreated control, although isoflavone or curcumin alone inhibited NF-κB activity to a lesser degree (Fig. 5). The specificity of NF-κB DNA binding to the DNA consensus sequence was confirmed by super-shift assay using anti-p65 antibody.
Figure 5.
Electrophoretic mobility shift assay (EMSA) showing the DNA binding activity of NF-κB in nuclear protein fractions from untreated (control), isoflavone-, curcumin-, or isoflavone + curcumin-treated BxPC-3 and Colo-357 cells. Super-shift assay showed that NF-κB band was shifted because of the formation of bigger complex after addition of anti- NF-κB p65 antibody. This assay confirmed the specificity of NF-κB binding to the DNA consensus sequence.
Cell growth inhibition with the combinations of isoflavone, curcumin, resveratrol and EGCG
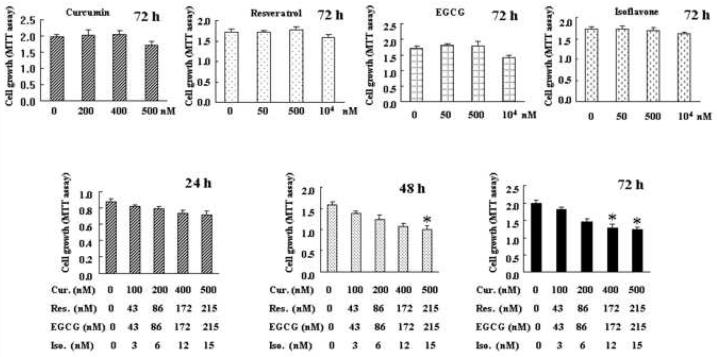
Since consumption of fruits and vegetables provides a complex mixture of different compounds that are associated with reduced cancer risk, we further sought to test weather lower concentrations of a mixture (cocktail) could be effective in the inhibition of PC cell growth. We chose resveratrol and EGCG together with isoflavone and curcumin for our current study. In order to detect the effects of the combinations of these four compounds on cell growth inhibition, we used the MTT assay after treatment of BxPC-3 cells for 24, 48 and 72h. We have tried various ratios of the combinations of four agents and found optimal results with a combination that are shown in Fig. 6. We found that the cell growth was inhibited up to 40% relative to control in BxPC-3 cells treated with combinations comprising isoflavone 10 nM + curcumin 500 nM + resveratrol 125 nM + EGCG 125 nM. However, the same concentrations of curcumin, resveratrol, EGCG, and isoflavone alone for 72 h did not significantly inhibit the cell growth of BxPC-3 cells. Taken together, our results showed that a combinatorial treatment with natural products yields a greater inhibition of cell growth of PC cells, suggesting the importance of a diet or supplement containing a mixture of compounds that apparently have no toxicity could be useful for the prevention and/or treatment of PC and other cancers.
Figure 6.
Effects of isoflavone, curcumin, resveratrol, EGCG alone, or the combination of four compounds on cell growth of pancreatic cancer BxPC-3 cells. Each value represents the mean ± SD (n=6) of three independent experiments. * P<0.05, **P<0.01 compared with the control.
Discussion
Pancreatic Cancer (PC) is a deadly disease, and only 4% of all patients with PC in the U.S. are expected to survive 5 years after diagnosis (Jemal et al., 2008). The low cure rate in PC is in part due to the lack of an effective strategy for the prevention of tumor progression and/or treatment. Emerging evidence suggest that many dietary compounds exhibit beneficial effects toward the prevention of cancer (Sarkar and Li, 2003; Sarkar and Li, 2004; Sarkar and Li, 2006). Although advances have been made in better understanding the biology of human cancers, much of the research focusing on cancer prevention using a variety of chemopreventive agents without any toxicity to normal cells remains to be clinically proven. In recent years combinatorial approach to cancer therapy or prevention is being widely recognized as an effective approach to maximize benefit. Recently, Nair et al. reported combined inhibitory effects of green tea polyphenols and sulforaphane in human colon carcinoma cells (Nair et al., 2008). In addition, combination therapy of gastrointestinal cancer using sulforaphane and dibenzoylmethane was recently reported (Shen et al., 2007). The results from our laboratory have shown that the combination of isoflavone with reduced doses of chemotherapeutic agents could be much more effective compared to higher doses of chemotherapeutic agents in different types of cancers including prostate, breast, lung, and PCs, suggesting that enhanced anti-cancer activity could be achieved without any toxicity to normal cells (Li et al., 2005; Sarkar and Li, 2006). In the present study, we investigated whether the combinations of two dietary natural products (isoflavone and curcumin) could show greater degree of cell growth inhibition and induction of apoptosis in PC cell lines compared to single agents.
Our current data showed, for the first time, that the combined treatment of PC cells with isoflavone and curcumin caused significantly increased growth inhibition and apoptosis, suggesting that this strategy could be an effective approach for the killing of PC cells. In addition, our data provided mechanistic information supporting the concept of combination of two compounds, which exerts their pro-apoptotic effects by inhibiting Notch-1, resulting in the inactivation of NF-κB DNA-binding activity and the inactivation of their target genes.
Notch signaling plays important roles in maintaining the balance between cell proliferation, differentiation and apoptosis (Miele and Osborne, 1999; Miele et al., 2006; Miele, 2006). The Notch gene is abnormally activated in many human malignancies. It has been reported that the Notch signaling is involved in PC cell survival and that Notch pathway components and Notch target genes are up-regulated in PCs (Miyamoto et al., 2003; Wang et al., 2006a; Wang et al., 2006d). It has been known that Notch gene suppresses apoptosis and promotes cell proliferation through a growth factor mediated survival pathway. Nair et al observed that Notch-1 induces cyclin D1 and CDK2 activity and inhibits p53 dependent apoptosis in cervical cancer cells through PI3K/AKT pathway (Nair et al., 2003). Jang et al have shown that Notch-1 expression regulates cell death through both apoptosis and cell cycle pathways in erythroleukemia cells with regulation of Bcl-xL, p21cip1/waf1, p27kip1 and the retinoblastoma protein Rb (Jang et al., 2004). Based on our results, we speculate that one possible mechanism by which isoflavone and curcumin induce apoptosis is in part due to the down-regulation of Notch-1, which leads to the down-regulation of Notch-1 target genes, such as cyclin D1, Bcl-xL, resulting in the inhibition of cell proliferation, and induction of apoptosis. Indeed, we found that isoflavone and curcumin inhibited the expression of Notch-1. Moreover, the combined treatment was more effective, which could be attributed to the inactivation of Notch-1 signaling pathway as supported by greater degree of reduction in the levels of Notch-1 target gene expression (Cyclin D1, Bcl-2 and Bcl-xL) compared to either agent alone.
Notch-1 signaling pathway has been shown to activate NF-κB. Specifically, Notch-1 has been reported to strongly induce NF-κB promoter activity and induce the expression of several NF-κB subunits and NF-κB DNA-binding activity (Jang et al., 2004; Oswald et al., 1998; Wang et al., 2006a). Levels of basal and stimulation-induced NF-κB activity were significantly decreased in mice with reduced Notch levels (Wang et al., 2004). Constitutive levels of Notch activity are essential to maintain NF-κB activity in various cell types. It is generally accepted that Notch and NF-κB pathways are key regulators of numerous cellular processes such as proliferation, differentiation and apoptosis. In the present study, we found that isoflavone and curcumin inhibited NF-κB DNA-binding activity. We also found a greater degree of reduction in the DNA binding activity of NF-κB in PC cells in response to the combinatorial treatment compared to either agent alone, suggesting that these changes are partly responsible for the increased apoptosis or decreased survival of PC cells.
It is becoming increasingly clear that the blocking of multiple signaling pathways is an effective therapeutic approach for the prevention of disease progression and/or treatment of human cancers, including PC. Therefore, identification of inhibitors targeting multiple members of the signaling pathway is likely to provide a better therapeutic outcome in patients diagnosed with cancer, a disease caused by dysregulation of multiple and complex signaling pathways. Reddy et al observed that cell growth inhibition in response to the combination of curcumin and EGFR inhibitor (EGFR Related Protein, ERRP) was significantly greater than that caused by either agent alone and this was associated with decreased activation (tyrosine phosphorylation) of EGFR, ErbB-2, ErbB-3, and/or IGF-1R. (Reddy et al., 2006). Whereas curcumin inhibited constitutive activation of both EGFR and IGF-1R, ERRP decreased activation of EGFR, ErbB-2, and ErbB-3 but had no effect on IGF-1R (Reddy et al., 2006). In addition, we have reported that isoflavone down-regulated the EGFR and Akt pathway (El-Rayes et al., 2006) whereas curcumin inhibited COX-2 and EGFR expression and decreased Erk1/2 activity in PC cells (Lev-Ari et al., 2006). Kotha et al reported that resveratrol inhibits Src and Stat3 signaling and induces apoptosis of malignant PC cells containing activated Stat3 protein (Kotha et al., 2006). Recently, Shankar et al reported that EGCG caused growth arrest at G1 stage of cell cycle through regulation of cyclin D1, cdk4, cdk6, p21/WAF1/CIP1 and p27/KIP1, and induced apoptosis through generation of reactive oxygen species and activation of caspase-3 and caspase-9 (Shankar et al., 2007; Shankar et al., 2008). EGCG also inhibited the expression of Bcl-2 and Bcl-xL and induced the expression of Bax, Bak, Bcl-xS and PUMA in PC cells (Shankar et al., 2007; Shankar et al., 2008). Based on these results, we speculated that one possible mechanism by which four natural products, used in our study, inhibited cell growth to a greater degree is in part due to down-regulation of multiple signaling pathways such as Notch-1, Akt, EGFR, and NF-κB. However, further in-depth studies including clinical trials are needed to fully evaluate the value of isoflavone in combination with curcumin, resveratrol and EGCG for the prevention and/or treatment of human PC.
In Summary, our results demonstrate that exposure of PC cells to isoflavone together with curcumin caused a greater degree of cell growth inhibition than either agent alone. The effect of combinatorial treatment on the inhibition of growth and induction of apoptosis could be attributed to the inhibition of Notch-1 and NF-κB signaling pathways. Because neither isoflavone nor curcumin are known to exert toxic effects, the current combinatorial strategy using more than one natural product could potentially be a superior and nontoxic strategy for the prevention and/or treatment of PC.
Acknowledgements
This work was partly funded by grants from the National Cancer Institute, NIH (5R01CA101870-05) to F.H.S. and also partly supported by a subcontract award (F.H.S.) from the University of Texas MD Anderson Cancer Center through a SPORE grant (1P20-CA010193-01) on pancreatic cancer awarded to James Abbruzzese. We also sincerely thank the Puschelberg Foundation for their generous contribution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Research. 2005;65(19):9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Zhang Y, Wang Z, Che M, Chiao PJ, Abbruzzese JL, Sarkar FH. In vitro and in vivo molecular evidence of genistein action in augmenting the efficacy of cisplatin in pancreatic cancer. Int J Cancer. 2007;120(4):906–917. doi: 10.1002/ijc.22332. [DOI] [PubMed] [Google Scholar]
- El-Rayes BF, Ali S, Ali IF, Philip PA, Abbruzzese J, Sarkar FH. Potentiation of the effect of erlotinib by genistein in pancreatic cancer: the role of Akt and nuclear factor-kappaB. Cancer Research. 2006;66(21):10553–10559. doi: 10.1158/0008-5472.CAN-06-2333. [DOI] [PubMed] [Google Scholar]
- Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R, Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. J.Nutr. 1995;125(3 Suppl):790S–797S. doi: 10.1093/jn/125.suppl_3.790S. [DOI] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem.Pharmacol. 2008;75(4):787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Jang MS, Miao H, Carlesso N, Shelly L, Zlobin A, Darack N, Qin JZ, Nickoloff BJ, Miele L. Notch-1 regulates cell death independently of differentiation in murine erythroleukemia cells through multiple apoptosis and cell cycle pathways. J.Cell Physiol. 2004;199(3):418–433. doi: 10.1002/jcp.10467. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J.Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, Carter B, Turkson J, Jove R. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol.Cancer Ther. 2006;5(3):621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67(8):3853–3861. doi: 10.1158/0008-5472.CAN-06-4257. [DOI] [PubMed] [Google Scholar]
- Lev-Ari S, Starr A, Vexler A, Karaush V, Loew V, Greif J, Fenig E, Aderka D, BenYosef R. Inhibition of pancreatic and lung adenocarcinoma cell survival by curcumin is associated with increased apoptosis, down-regulation of COX-2 and EGFR and inhibition of Erk1/2 activity. Anticancer Res. 2006;26(6B):4423–4430. [PubMed] [Google Scholar]
- Li Y, Ahmed F, Ali S, Philip PA, Kucuk O, Sarkar FH. Inactivation of nuclear factor kappaB by soy isoflavone genistein contributes to increased apoptosis induced by chemotherapeutic agents in human cancer cells. Cancer Res. 2005;65(15):6934–6942. doi: 10.1158/0008-5472.CAN-04-4604. [DOI] [PubMed] [Google Scholar]
- Miele L. Notch signaling. Clin.Cancer Res. 2006;12(4):1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr.Cancer Drug Targets. 2006;6(4):313–323. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J.Cell Physiol. 1999;181(3):393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Maitra A, Ghosh B, Zechner U, Argani P, Iacobuzio-Donahue CA, Sriuranpong V, Iso T, Meszoely IM, Wolfe MS, Hruban RH, Ball DW, Schmid RM, Leach SD. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3(6):565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- Nair P, Somasundaram K, Krishna S. Activated Notch1 inhibits p53-induced apoptosis and sustains transformation by human papillomavirus type 16 E6 and E7 oncogenes through a PI3K-PKB/Akt-dependent pathway. J.Virol. 2003;77(12):7106–7112. doi: 10.1128/JVI.77.12.7106-7112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Hebbar V, Shen G, Gopalakrishnan A, Khor TO, Yu S, Xu C, Kong AN. Synergistic effects of a combination of dietary factors sulforaphane and (−) epigallocatechin-3-gallate in HT-29 AP-1 human colon carcinoma cells. Pharm.Res. 2008;25(2):387–399. doi: 10.1007/s11095-007-9364-7. [DOI] [PubMed] [Google Scholar]
- Oswald F, Liptay S, Adler G, Schmid RM. NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol.Cell Biol. 1998;18(4):2077–2088. doi: 10.1128/mcb.18.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18(44):6013–6020. doi: 10.1038/sj.onc.1202980. [DOI] [PubMed] [Google Scholar]
- Reddy S, Rishi AK, Xu H, Levi E, Sarkar FH, Majumdar AP. Mechanisms of curcumin- and EGF-receptor related protein (ERRP)-dependent growth inhibition of colon cancer cells. Nutr.Cancer. 2006;55(2):185–194. doi: 10.1207/s15327914nc5502_10. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. NF-kappaB: a potential target for cancer chemoprevention and therapy. Front Biosci. 2008;13:2950–2959. doi: 10.2741/2900. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21(5):744–757. doi: 10.1081/cnv-120023773. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. Indole-3-carbinol and prostate cancer. J.Nutr. 2004;134(12 Suppl):3493S–3498S. doi: 10.1093/jn/134.12.3493S. [DOI] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Research. 2006;66(7):3347–3350. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]
- Sethi G, Sung B, Aggarwal BB. Nuclear factor-kappaB activation: from bench to bedside. Exp.Biol.Med.(Maywood.) 2008;233(1):21–31. doi: 10.3181/0707-MR-196. [DOI] [PubMed] [Google Scholar]
- Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front Biosci. 2008;13:440–452. doi: 10.2741/2691. [DOI] [PubMed] [Google Scholar]
- Shankar S, Suthakar G, Srivastava RK. Epigallocatechin-3-gallate inhibits cell cycle and induces apoptosis in pancreatic cancer. Front Biosci. 2007;12:5039–5051. doi: 10.2741/2446. [DOI] [PubMed] [Google Scholar]
- Shen G, Khor TO, Hu R, Yu S, Nair S, Ho CT, Reddy BS, Huang MT, Newmark HL, Kong AN. Chemoprevention of familial adenomatous polyposis by natural dietary compounds sulforaphane and dibenzoylmethane alone and in combination in ApcMin/+ mouse. Cancer Res. 2007;67(20):9937–9944. doi: 10.1158/0008-5472.CAN-07-1112. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, Furukawa K. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc.Natl.Acad.Sci.U.S.A. 2004;101(25):9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of Notch-1 Inhibits Invasion by Inactivation of Nuclear Factor-{kappa}B, Vascular Endothelial Growth Factor, and Matrix Metalloproteinase-9 in Pancreatic Cancer Cells. Cancer Research. 2006a;66(5):2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006c;106(11):2503–2513. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Inhibition of nuclear factor kappab activity by genistein is mediated via Notch-1 signaling pathway in pancreatic cancer cells. Int.J.Cancer. 2006b;118(8):1930–1936. doi: 10.1002/ijc.21589. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang Y, Li Y, Banerjee S, Liao J, Sarkar FH. Down-regulation of Notch-1 contributes to cell growth inhibition and apoptosis in pancreatic cancer cells. Mol.Cancer Ther. 2006d;5(3):483–493. doi: 10.1158/1535-7163.MCT-05-0299. [DOI] [PubMed] [Google Scholar]