Abstract
We had previously observed that treatment utilizing granulocyte–macrophage colony-stimulating factor (GM-CSF) had profound effects on the induction of experimental autoimmune myasthenia gravis (EAMG), a well-characterized antibody-mediated autoimmune disease. In this study, we show that EAMG induced by repeated immunizations with acetylcholine receptor (AChR) protein in C57BL6 mice is effectively suppressed by GM-CSF treatment administered at a stage of chronic, well-established disease. In addition, this amelioration of clinical disease is accompanied by down-modulation of both autoreactive T cell, and pathogenic autoantibody responses, a mobilization of DCs with a tolerogenic phenotype, and an expansion of regulatory T cells (Tregs) that potently suppress AChR-stimulated T cell proliferation in vitro. These observations suggest that the mobilization of antigen-specific Tregs in vivo using pharmacologic agents, like GM-CSF, can modulate ongoing anti-AChR immune responses capable of suppressing antibody-mediated autoimmunity.
Keywords: Myasthenia gravis, Experimental autoimmune myasthenia gravis, Dendritic cells, Regulatory T cells, GM-CSF, Acetylcholine receptor
Introduction
Myasthenia gravis (MG) is an antibody-mediated autoimmune disease in which pathogenic autoantibodies are directed against the skeletal muscle nicotinic acetylcholine receptor (AChR) [1]. Experimental autoimmune myasthenia gravis (EAMG) induced in C57BL/6 mice after repeated immunizations with emulsified Torpedo californica AChR (tAChR) is a useful model for the study of pathogenic mechanisms and therapeutic strategies relevant to MG in humans [2]. Although antibodies to the AChR are directly responsible for the destruction of the muscle endplate resulting in both MG and EAMG, the autoantibody response is T cell dependent, with CD4+ T cells providing help for B cells to produce anti-AChR antibodies [3,4]. Despite the fact that the target antigen is so well defined, there is currently no specific immunosuppressive therapy or cure for MG. Nonspecific immunotherapy utilizing corticosteroids and other immunosuppressive drugs combined with symptomatic therapy with acetylcholinesterase inhibitors results in clinical improvement and substantial control of symptoms in most patients. However, there are significant potential side effects and risks associated with global nonspecific suppression of the immune response, including infections and malignancy.
Ideal specific therapies for MG would have little effect on overall immunity, while targeting the mechanisms that initiate and sustain the autoimmune response to the AChR. While these mechanisms are not completely understood, multiple lines of evidence indicate that the immune system's professional antigen-presenting cells, the dendritic cells (DCs), participate in the onset and progression of autoimmune diseases [5,6]. Animal models show that the transfer of DCs isolated from donors with acute autoimmune disease or propagated in vitro under conditions that induce maturation, generates a strong T helper (Th)-1 response, eventually culminating in autoimmune disease [7]. Conversely, DCs have been shown to have the ability to educate T cells to tolerate self antigens, and to promote the mobilization of regulatory T cell (Treg) subsets [8–10]. It has been shown that the interaction of DCs with antigen-specific Tregs can suppress experimental autoimmunity [11]. Current evidence indicates that the immunogenic or tolerogenic function of DCs is largely determined by differentiation status which may be manipulated using growth factors such as granulocyte–macrophage colony-stimulating factor (GM-CSF) [12], and that DC functional state is important in determining Treg biology and antigen-specific control of experimental autoimmunity [13,14]. Previous work has been published examining the potential of in vivo administration of GM-CSF in experimental autoimmune thyroiditis (EAT) [15,16], and in the experimental model of autoimmune diabetes [14], and mobilization of specific DC subsets and Tregs was reported to critical to the observed effects. But, EAT and autoimmune diabetes are T-cell mediated diseases, and in general, the role of dendritic cells (DCs) in the biology of regulatory T-cells and subsequent control of autoimmunity has been studied primarily in T cell mediated autoimmune diseases. We, however, have previously observed that GM-CSF had profound effects on the induction of experimental autoimmune myasthenia gravis (EAMG), a well-characterized antibody-mediated autoimmune disease [17].
In the current study, we examine the therapeutic potential of GM-CSF in chronic EAMG, and demonstrate that GM-CSF effectively ameliorates clinical disease in mice with ongoing, well-established disease. Furthermore, we show not only an effect of GM-CSF on particular subpopulations of DCs, T cells, and T cell proliferative response to the AChR, but also a significant down-modulation of pathogenic anti-AChR autoantibody production.
Materials and methods
Mice
Eight-week old female C57BL6/J mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were housed in the Biologic Resources Laboratory facilities at the University of Illinois (Chicago, IL) and provided food and water ad libitum. All mice were cared for in accordance with the guidelines set forth by the University of Illinois Animal Care and Use committee.
Purification of tAChR and mouse AChR
Torpedo AChR (tAChR) was purified from the electric organs of T. californica by affinity chromatography using a conjugate of neurotoxin coupled to agarose, as previously described [18]. Similarly, acetylcholine receptor protein was purified from mouse skeletal muscle. Purity of the isolated product was tested by SDS-PAGE. The purified tAChR was used to induce EAMG and as antigen for in vitro testing of immune responses, while the purified mouse AChR was used in the ELISA testing for circulating anti-AChR antibodies.
Induction and clinical scoring of EAMG
Eight-week old female C57BL6/J mice were immunized with 40 µg of tAChR/CFA, 200 µl, s.c, and boosted with 20 µg of tAChR emulsified in IFA in 200 µl volume injected in the flanks and tail base every 30 days. Control mice received an equal volume of PBS in CFA or IFA. Mice were observed and scored every other day. For clinical examination, mice were evaluated for myasthenic weakness and assigned clinical scores as previously described [17]. Briefly, mice were observed on a flat platform for a total of 2 min. They were then exercised by gently dragging them suspended by the base of the tail across a cage top grid repeatedly (20–30 times) as they attempted to grip the grid. They were then placed on a flat platform for 2 min and again observed for signs of EAMG. Clinical muscle weakness was graded as follows: grade 0, mouse with normal posture, muscle strength, and mobility at baseline and after exercise; grade 1, normal at rest but with muscle weakness characteristically shown by a hunchback posture, restricted mobility, and difficulty in raising the head after exercise; grade 2, grade 1 symptoms without exercise during observation period; grade 3, dehydrated and moribund with grade 2 weakness; and grade 4, dead. The evaluator was blinded to treatment status for all clinical evaluations. After initial priming and three booster immunizations, the mice were divided into two groups of 20 mice per group, consisting of equal numbers of mice with various disease severities in each group. An additional booster immunization was given 32 days after initial treatment with GM-CSF or PBS.
GM-CSF treatment
The two groups of mice were treated with either GM-CSF (2 µg daily for 10 days), or PBS administered by IP injection daily for 10 days (first day of treatment designated day 0, and treatment administered on days 0–9). A second course of treatment was administered after the final booster immunization (2 µg daily for 5 days on days 37–41). Separate groups of mice were immunized, treated, and separated into two groups as described above, but were sacrificed 14 days after the second course of treatment for determination of DC phenotype, Th1/Th2 cytokine response, FOXP3 expression and T cell proliferation studies.
Flow cytometry
Three mice from each of the two experimental groups were treated and immunized as described above. Single cell suspensions of splenocytes were prepared from mice sacrificed 48 h after the completion of the second GM-CSF/PBS treatment regimen for DC and Treg analysis. Cells were washed with PBS supplemented with 2% FBS, blocked with antiCD16/CD32 Fc-Block (BD PharMingen, San Jose, CA) on ice for 30 min. FITC-conjugated anti-CD11c and PE-conjugated anti-I-Ab (MHC class II), anti-CD8α, anti-CD80, anti-CD86, and isotype control Abs (BD PharMingen), and FITC-conjugated anti-CD4, PE-conjugated anti-CD25 (Caltag Laboratories) Abs were used in flow cytometry and analyzed using a FACS analyzer (BD Biosciences). Mouse regulatory staining kit (w/PE FOXP3 FJK-16s, FITC CD4, APC CD25) (eBioscience) Abs were used for intracellular staining for FOXP3 expression.
RT-PCR
DCs, CD4+ and CD4+CD25+ cells were isolated from splenocytes using a commercial isolation kit (Miltenyi Biotec, Auburn, CA) and cDNAs were synthesized using a ThermoScript ™ RT-PCR System (Invitrogen, Carlsbad, CA). A multiplex RT-PCR kit (MaximBio, San Francisco, CA) was used to detect Th1/Th2 cytokine transcripts in CD4+CD25+ cells, FOXP3+ and cytokine transcripts in CD4+ cells, and pro-inflammatory cytokines in DCs.
The sequences of primer pairs specific for the FoxP3+ are as follows: FOXP3 (PCR product 735 bp), sense primer [5′ TACACCCAGGAAAGACAGCAACCT 3′]; anti-sense [5′ TGGCTCCTCTTCTTGCGAAACTCA 3′]. Samples were subjected to electrophoresis using a 2% agarose gel to confirm the specificity of the PCR, and relative quantification of the resulting bands was assessed by densitometry as the ratio of the transcript to the housekeeping gene, GAPDH. The above experiments were repeated three times to ensure reproducibility.
ELISA for anti-mouse AChR antibody isotypes
Mice were bled on days 0, 30, and 60 after the first treatment. Affinity-purified mouse AChR (0.5 ug/ml) was used to coat 96-well microtiter plates (Corning Costar 96 wells plate, eBioscience, San Diego, CA) with 0.1 M carbonate bicarbonate buffer (pH 9.6) overnight at 4 °C. Serum samples diluted 1:2000 to 1:10,000 in blocking buffer were added and incubated at 37 °C for 90 min. After four washes, horseradish peroxidase-conjugated (HRPO) goat anti-mouse IgG, IgG1, IgG2b, and IgG2c diluted in 1:2000 (Caltag Laboratories, Burlingame, MA) in blocking buffer were added and incubated at 37 °C for 90 min. Subsequently, TMB substrate solution (eBioscience) was added, and color was allowed to develop at room temperature in the dark for 15 min. The reaction was stopped by adding 2 M H2SO4 and absorbance values were measured at a wavelength of 450 nm using a Bio-Rad microplate-reader (model 550) and the results were expressed as OD values.
CD4+CD25+ T cell coculture and AChR-specific T cell proliferation
Parallel to the flow cytometry experiments described above, three mice/group were sacrificed 14 days after the completion of the second treatment regimen and spleens were collected for the analysis of AChR-specific T cell proliferation. Responder CD4+ cells were isolated from mice in the PBS/TAChR control group (TAChR-immunized mice receiving PBS rather than GM-CSF) using magnetic cell sorting (Miltenyi Biotec) and were stained with CFSE at a concentration of 1 µM for 10 min at 37 °C. Cells were washed three times and plated into 96-well, flat-bottom plates at 0.5 × 106 cells/well. Spleen CD4+CD25+ T cells were isolated from mice in the GM-CSF/tAChR group and the PBS/tAChR group (magnetic cell sorting; Miltenyi Biotec). Isolated CD4+CD25+ T cells from both groups were added to cultures at a 2:1 effector: Treg ratio. T cell-depleted enriched DCs (0.1 × 106 cells/well) from naive mice (also accomplished by magnetic cell sorting; Miltenyi Biotec) were used as feeder cells in these studies. Responder CD4+ cells were cultured either alone, or co-cultured with the isolated Treg cells from GM-CSF-treated mice, or with Treg cells from untreated tAChR-immunized mice. Subsequent experiments were performed with the addition of antibodies to IL-10 (clone JES5-2A5, eBioscience) and TGF-β (clone 1D11, R&D Systems Source). Cells were stimulated with tAChR (5 µg/ml) or ConA (2 µg/ml). Cells were harvested after 7 days in culture and were tested for CFSE dilution using a FACS analyzer (BD Biosciences). These experiments were repeated twice to ensure reproducibility.
Statistical analysis
Mean, SD, and statistical significance were calculated using SPSS software applications. Nonparametric Wilcoxon signed test, Fisher's Exact Test and Student's t-test were utilized as appropriate. A value of p 0.05 was considered significant.
Results
GM-CSF treatment effect in established EAMG
At the end of the disease induction (initial priming immunization and 3 boosters) stage, greater than 90% of the animals had clinically demonstrable fatigable muscle weakness. We then divided these animals into two groups (n=20) with an equal balance of the various disease severities in each group. We treated one group of animals with GM-CSF (2 µg IP daily for 10 days) and the other group of animals with PBS. The day of treatment initiation was designated “day 0” and the mice were clinically graded for disease severity as described in Materials and methods on an every other day basis. At day 32, we administered an additional booster tAChR immunization followed by another course of GM-CSF (2 µg IP daily for 5 days) vs. PBS.
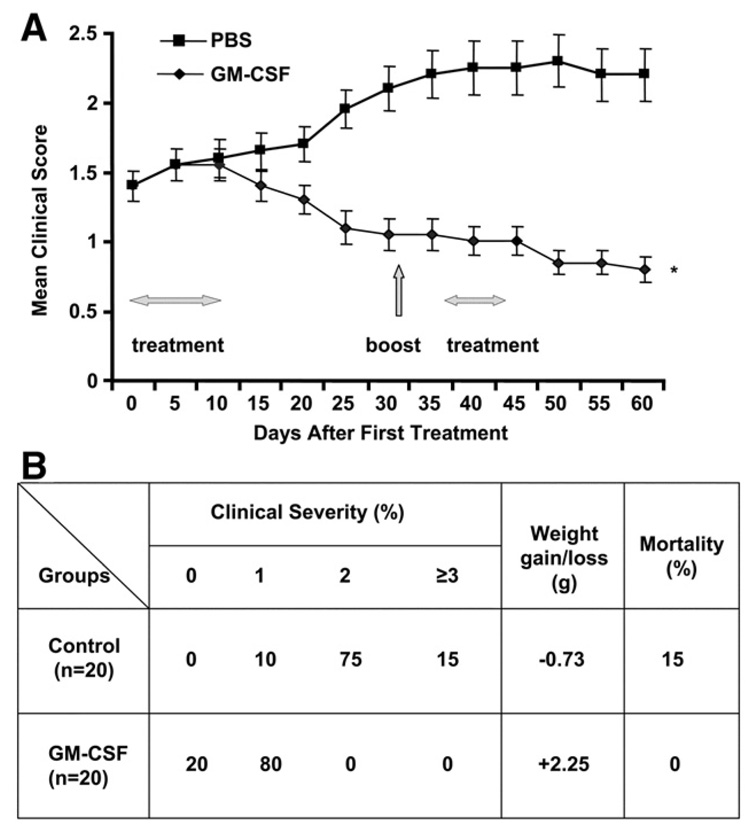
In the PBS-treated group, three mice died due to severe EAMG. As shown in Figure 1, the average disease severity in the GM-CSF-treated animals gradually lessened and continued to do so even after the tAChR boost, while the PBS-treated animals developed significantly more severe disease. At the end of the study 90% of mice in the control group had grade 2 or greater disease compared to 0% of the GM-CSF-treated group. The control group exhibited an average weight loss of 0.73 g compared to an average weight gain of 2.25 g in the GM-CSF-treated group.
Figure 1.
Frequency and severity of EAMG in GM-CSF-treated, compared to PBS-treated, tAChR-immunized mice. Mice were evaluated as described in Materials and methods on an every other day basis beginning at the time of initiation of treatment (Day 0). A) Average clinical score during days 0–60 of the observation period is shown for the two groups (n=20/group). Day 0 corresponds to initiation of treatment (GM-CSF vs. PBS). All mice had received a priming immunization and 3 booster immunizations with tAChR. An additional tAChR booster immunization was given on day 32, after treatment initiation as shown in panel 1. Greater than 90% of animals had clinically demonstrable disease, and the GM-CSF and PBS groups had equal distribution of disease severity at day 0. B) Proportion of animals with each clinical disease severity grade, weight gain/loss, and mortality in each experimental group at the endpoint (Day 60) is shown. Disease severity, percentage weight loss, and mortality were significantly lower in GM-CSF-treated mice compared to the PBS-treated tAChR-immunized controls (*p<0.01). The above experiment was initially performed utilizing smaller numbers in each group (n=8) with similar results.
Anti-AChR antibodies
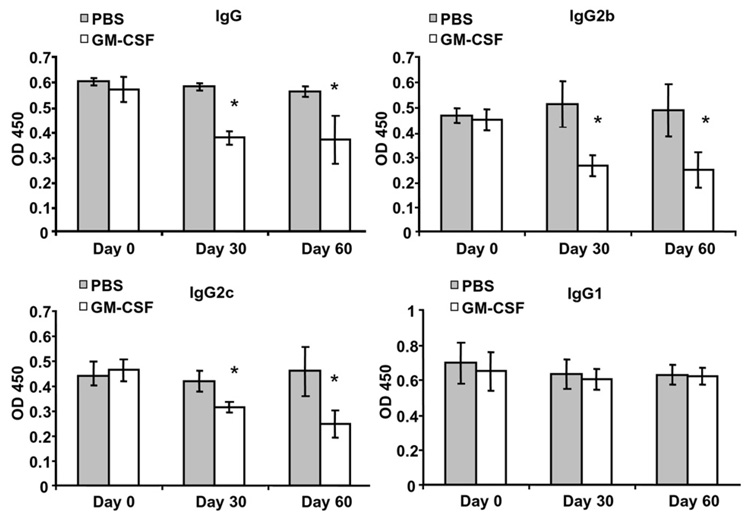
The effects of GM-CSF treatment on the serum anti-AChR Ab responses were monitored on day 0, day 30 and day 60 after initiation of treatment. We measured the serum concentration of anti-mouse AChR Abs by ELISA using affinity-purified mouse AChR as antigen. The titer of anti-AChR Abs did not correlate with disease severity (data not shown). As expected, baseline antibody titers were quite high, approaching saturating values, due to the multiple immunizations and the chronic nature of the induced disease. Despite this, treatment with GM-CSF resulted in a significant decrease in total anti-AChR IgG Ab response compared with the PBS-treated controls (Fig. 2). This decrease was primarily reflected in a prominent decrease in the complement-fixing (and likely pathogenically relevant) IgG2b isotype and the IgG2c isotype, as the IgG1 was relatively unaffected.
Figure 2.
Serum anti-mouse AChR IgG and IgG subclasses for GM-CSF-treated, and PBS-treated mice. Serum was obtained by tail clip and anti-mouse AChR IgG Ab and IgG isotypes were analyzed by ELISA (n=20 per group) on day 0, day 30 and day 60 (n=17 in GM-CSF group due to mortality in 3 mice), with day 0 corresponding to the date of treatment initiation. GM-CSF treated mice showed significantly lower serum levels of anti-tAChR IgG, IgG2b, and IgG2c at day 30 and day 60. Each column represents the mean ± SD of three individual experiments conducted in triplicate (*p<0.05).
Effects of GM-CSF on DC phenotype
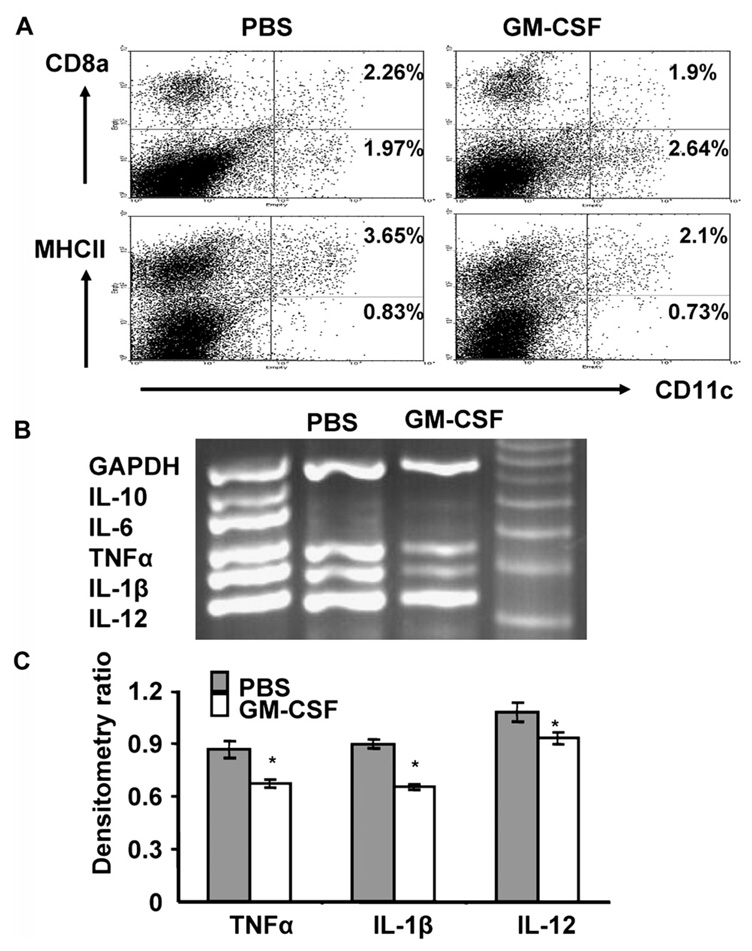
We analyzed the expression of MHC class II, and co-stimulatory molecules, as well as the production of pro-inflammatory cytokines in DCs isolated from GM-CSF-treated animals and untreated controls. There was no significant change in the absolute numbers of DCs as determined by expression of CD11c in splenocytes from the two experimental groups, and there was no significant difference in the expression of co-stimulatory molecules (CD80, CD86) (data not shown). DCs from GM-CSF-treated mice showed a significant reduction in the expression of MHC class II, but only a marginal, (not statistically significant increase in the percentage of CD8α− CD11c+ cells (Fig. 3A). Levels of the pro-inflammatory cytokines TNFα, IL-1β, and IL-12 evaluated by RT-PCR were significantly lower in splenic DCs isolated from GM-CSF-treated animals compared to the untreated controls (Figs. 3B and C).
Figure 3.
Effect of GM-CSF treatment on DC phenotype in established EAMG. B6 mice were immunized and treated with GM-CSF or PBS as described. Mice were sacrificed 48 h after the second treatment (GM-CSF or PBS). (A) Splenocytes were collected and stained with FITC-labeled anti-mouse CD11c and with PE-labeled anti-mouse CD8α and anti-MHC II, and then analyzed by FACS. Mean percentages from three independent experiments are shown in the upper right corner of the scatter plots. Splenic DCs from GM-CSF-treated mice had lower expression of MHC II compared to PBS-treated EAMG controls (2.1% (range 1.78 to 2.74%) vs. 3.65% (range 3.10 to 4.06%); p=0.011), and showed marginally higher (not statistically significant) numbers of CD8α− DCs (1.97% (range 1.18 to 2.51%) compared to 2.64% (range 2.27 to 3.25%); p=0.19), (B) DCs were isolated from spleens using magnetic separation columns, and utilized in a multiplex RT-PCR assay to detect cytokine transcripts. (C) Densitometry ratio of PCR products for TNFα, IL-1β and IL-12 in GM-CSF and PBS-treated groups. DCs from GM-CSF-treated animals had significantly lower levels of transcripts for pro-inflammatory cytokine (TNFα, IL-1β, IL-12) expression (p<0.05). The results shown are representative of three independent experiments using three mice per group.
GM-CSF effects on Treg population and Th1/Th2 balance
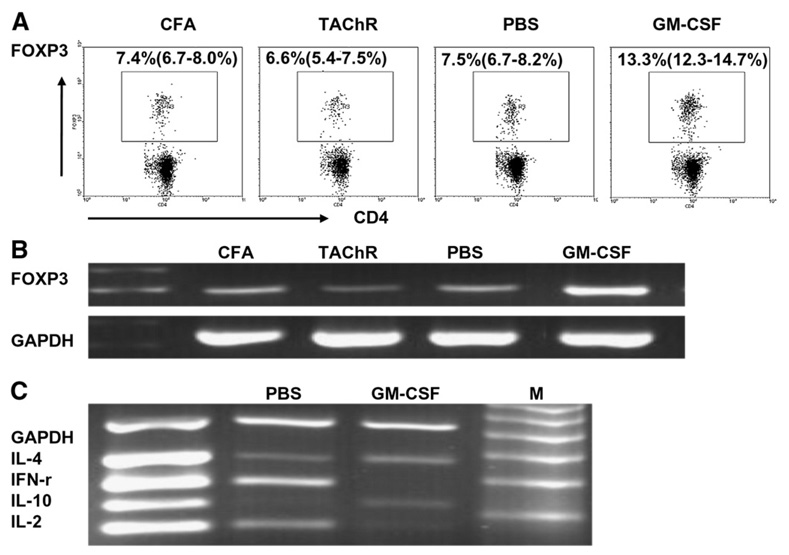
To determine if treatment with GM-CSF affects the relative numbers of regulatory T cells, we tested mouse spleen cells for expression of the known Treg marker, FOXP3, by FACS. As shown in Figure 4A, we found a significant expansion in the percentage of FOXP3-expressing cells in GM-CSF-treated mice (13.3%, range: 12.3–14.7%), compared to PBS-treated mice (7.5%, range: 6.7–8.2%), as well as compared to untreated, unimmunized control animals (CFA) and tAChR-immunized animals not treated with either GM-CSF or PBS (TAChR). We also analyzed the expression of FOXP3 transcripts in CD4+ splenocytes isolated from GM-CSF-treated and untreated mice using a multiplex RT-PCR assay to detect cytokine transcripts (Fig. 4B). As expected, and consistent with the FACS analysis and our previous results [16], a profound increase in the expression of FOXP3 was observed in lymphocytes from GM-CSF-treated animals.
Figure 4.
Effects of GM-CSF on the population of FOXP3+ T cells and Th1/Th2 cytokine response to tAChR stimulation. Mice were treated with GM-CSF or PBS after tAChR immunization as depicted in Figure 1, and sacrificed 14 days after the second treatment. (A) Splenocytes were isolated from animals and stained with FITC-labeled anti-mouse CD4 and PE-labeled anti-FOXP3. Representative plots from three separate experiments (n=3/group) showing the percentage of FOXP3+ regulatory T cells are shown. Gated CD4+ cells are shown and or FOXP3+ cells are marked within each inset. A higher percentage of FOXP3 expressing cells was demonstrated in GM-CSF-treated EAMG mice compared to 1) PBS-treated EAMG mice (PBS) (p=0.0023), 2) B6 mice receiving a priming tAChR immunization plus 3 booster immunizations (TAChR) (p=0.0011) and 3) B6 mice receiving CFA injections alone (CFA) (p=0.0026). (B) CD4+ cells were isolated from splenocytes. RT-PCR was performed to detect FOXP3 transcripts. Higher levels of FOXP3 expression was demonstrated in GM-CSF-treated EAMG mice compared to 1) PBS-treated EAMG mice (PBS), 2) B6 mice receiving a priming tAChR immunization plus 3 booster immunizations (TAChR) and 3) B6 mice receiving CFA injections alone (CFA). (C) CD4+ cells were isolated from splenocytes using a magnetic cell isolation kit. A multiplex RT-PCR kit was used to detect Th1/Th2 cytokine transcripts. GM-CSF treated mice showed a cytokine expression pattern favoring a Th2 polarization (increased IL-4, IL-10, decreased IFN-γ, IL-2) compared with PBS-treated mice which showed an opposite (Th1) pattern.
Similarly, we analyzed cytokine production by assessing expression levels in splenic lymphocytes isolated from these animals (Fig. 4C). GM-CSF-treated mice showed reduced expression of IFN-γ and IL-2, and increased expression of IL-4 and IL-10, consistent with a shift of the immune response to a Th2 polarization.
T cell proliferative responses and the effect of Tregs
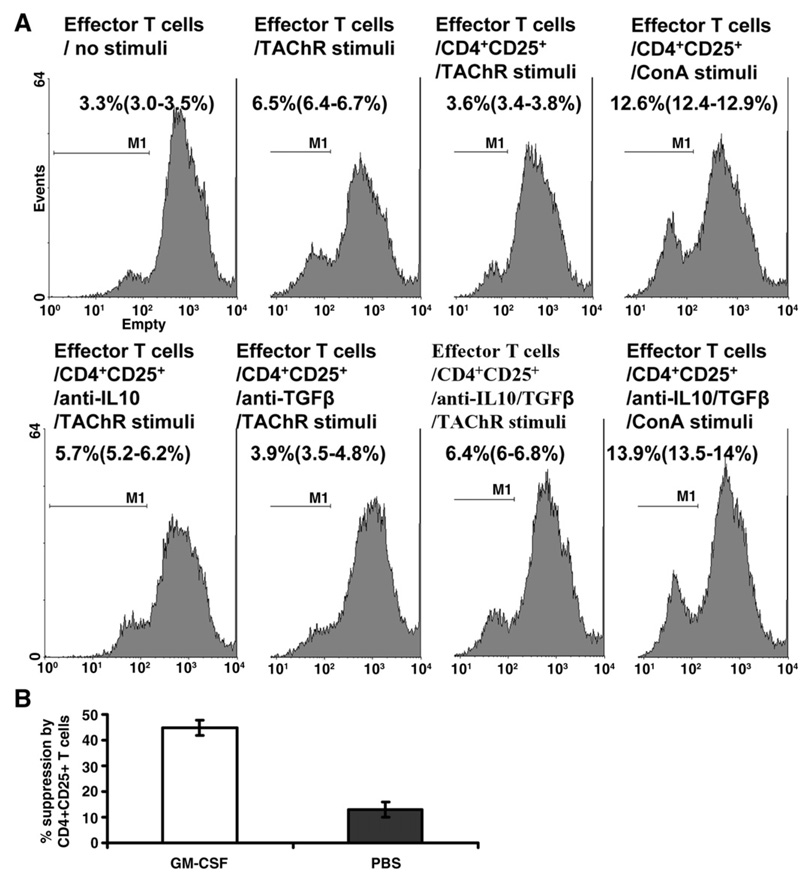
(A) We isolated responder CD4+ cells from untreated EAMG mice, cultured these cells during stimulation with tAChR in the presence of CD4+CD25+ T cells isolated from GM-CSF-treated and control mice and noted the effect on the T cell proliferative response. The addition of CD4+CD25+ cells from mice treated with GM-CSF suppressed the T cell proliferative response to baseline (unstimulated) levels (Fig. 5A). In contrast, the addition of CD4+CD25+ cells from EAMG mice treated with PBS rather than GM-CSF had little suppressive effect (Fig. 5B). Furthermore, CD4+CD25+ cells had no effect on the proliferative response to nonspecific T cell stimulation using the lectin concanavalin A (Con A), suggesting an antigen-specific effect. Baseline T cell proliferation in response to Con A results [without addition of CD4+CD25+ cells] not shown.
Figure 5.
CD4+CD25+ T cells from GM-CSF-treated mice inhibit the tAChR-stimulated proliferation of effector T cells from untreated EAMG mice. Mice were treated with GM-CSF or PBS as described in Materials and methods after immunization with tAChR. (A) Mice were sacrificed to obtain spleen cells, and the proliferative response to tAChR was assayed utilizing CFSE dilution method. Responder CD4+ cells from untreated EAMG mice were stimulated with tAChR (or ConA) as indicated. These cells were co-cultured with CD4+CD25+ cells from GM-CSF-treated mice in vitro, and in subsequent experiments with the addition of antibodies to IL-10 and TGF-β (lower panel). The presence of CD4+CD25+ cells from GM-CSF-treated mice resulted in a significantly lower proliferative response to stimulation with tAChR to baseline unstimulated levels, while anti-IL-10, but not anti-TGF-β, restored the proliferative response. CD4+CD25+ cells from GM-CSF-treated mice did not suppress the proliferative response to nonspecific (ConA) stimulation (baseline ConA results [without addition of CD4+CD25+ cells] not shown). Percentage suppression of AChR-stimulated T cell proliferation when responder CD4+ cells are co-cultured with CD4+CD25+ cells from untreated, tAChR-immunized mice vs. GM-CSF-treated, tAChR-immunized mice. CD4+CD25+ cells from untreated mice have a minimal suppressive effect (13%±2.8) compared to CD4+CD25+ cells obtained from GM-CSF-treated mice (44.9%±2.9).
The addition of antibodies to IL-10 largely restored the T cell proliferative response, while antibodies to TGF-β had no effect (Fig. 5A).
Discussion
In this study, we expanded upon our previous observations that treatment with GM-CSF protected against the induction of EAMG [17], by investigating the therapeutic potential of GM-CSF in the treatment of ongoing, active EAMG, a well characterized antibody-mediated autoimmune disease. We found that treatment with GM-CSF at a stage of chronic, well-established disease, effectively induced improvement in signs of muscle weakness and potently suppressed disease progression. Understanding the molecular mechanism of the effects of GM-CSF in EAMG is critical to the development of enhanced therapeutic strategies that could optimize these effects or lead to the development of GM-CSF or related compounds in MG and other autoimmune diseases. Dendritic cells (DCs) are bone-marrow-derived antigen-presenting cells that play a critical role in the induction and maintenance of an immune response, having the potential to activate or tolerize T cells in an antigen-specific manner [19]. The use of isolated DCs, modified in a number of different ways in vitro, and administered to experimental animals has been reported to be effective in EAMG [20–22].
Growing evidence suggests that DC function is largely determined by differentiation status, which can be manipulated using various growth factors, including GM-CSF [23–26]. Dendritic cells having a “semi-matured” or “tolerogenic” phenotype have been shown to have potential utility in organ transplantation and in the treatment of autoimmune diseases [24,25]. Semi-mature or tolerogenic DCs express relatively high levels of MHC class II and co-stimulatory molecules, but produce low levels of pro-inflammatory cytokines [26]. In secondary lymphoid organs, DCs may also be distinguished by their expression of the marker CD8α [27], with the absence of CD8α expression occurring in an immature or semi-mature stage of DC differentiation [28].
The observed change in splenic DC phenotype in GM-CSF-treated animals in our study, characterized by decreased production of pro-inflammatory cytokines, with no significant change in the expression of co-stimulatory molecules, and reduced expression of MHC class II, is consistent with previously described tolerogenic DC subsets [29]. A direct effect of DCs from GM-CSF-treated mice on AChR-specific lymphocytes was not specifically demonstrated in our study, but is suggested by previously published studies in which GM-CSF was used in other autoimmune animal models [14]. GM-CSF is a multifunctional cytokine which has found widespread current clinical use in the treatment of chemotherapy-induced neutropenia and in mobilizing bone marrow stem cells into the blood [30]. Importantly, GM-CSF is a pivotal mediator of the maturation and function of DCs [31]. It has been shown to not only mobilize “semi-mature” DCs but also to promote a Th2 cytokine response [26,28,32]. Accordingly, in addition to the observed alteration in DC phenotype in our study, the cytokine milieu in CD4+ splenocytes of GM-CSF-treated animals clearly shifted from a Th1 to a Th2 polarization (Fig. 4C), indicating suppression of the anti-AChR Th1 response. The importance of this observed shift to a Th2 cytokine response is emphasized by the IL-10 dependent Treg suppression of AChR-stimulated T cell proliferation in GM-CSF-treated mice (see below).
It is interesting that, as a hemopoietic growth factor, GM-CSF also has purported pro-inflammatory effects, and has even been suggested as a treatment to “boost” the immuneresponse in sepsis [33]. How can the same agent be used to effectively induce immune tolerance in EAMG and other animal models of autoimmunity? While this contrasting range of induced effects is most likely related to a number of factors [34], including the nature of any existing inflammatory response, and the timing of GM-CSF administration, an important determinant may be the dose of GM-CSF used. Previous investigators have observed that low-dose GM-CSF leads to the development of “semi-mature” DCs which are resistant to maturation stimuli in culture, compared to the development of mature, “immunogenic” DCs when high-dose GM-CSF is used [35]. We utilized doses previously found to be effective in other autoimmune models [14,15], but have not yet performed formal dose ranging studies. It is possible that lower doses would be more effective, particularly if administered intravenously, as it is typically administered in cancer patients.
Recent studies have shown that DCs may exert their tolerogenic functions through the generation of regulatory T cells (Tregs) [36,37]. Accordingly, we show a significant expansion in the population of Tregs in GM-CSF-treated mice. Having demonstrated this expansion of Treg cells in response to GM-CSF treatment of EAMG, we set out to determine the importance of these cells for this effect. Treg cells express the transcriptional repressor FOXP3 (forkhead box P3), which is central for their development and function [38]. As a transcription factor specific to Treg cells, FOXP3 is currently the best available marker for these cells. Unfortunately, the availability of specific Treg marker molecules that are expressed on the cell surface is limited. However, Treg cells are enriched within the subset of CD4+ T cells that express CD25 (the α-subunit of the IL-2 receptor), and for this reason, CD25 is often used to identify and isolate Treg cells [39]. We therefore depleted CD4+CD25+ cells from splenocytes of EAMG mice treated with GM-CSF, and showed that co-culturing of these cells with responder CD4+ cells from untreated EAMG mice significantly reduced the AChR-stimulated proliferative response.
It is known that antigen-specific Tregs show a greatly enhanced ability to suppress experimental autoimmunity in comparison to polyclonal Treg populations [40]. In our study, CD4+CD25+ cells isolated from tAChR-immunized mice, treated with GM-CSF had a markedly more potent suppressive effect compared to equal ratios of CD4+CD25+ cells obtained from animals treated with PBS (Fig. 5B). We further demonstrated that the suppressive ability of these CD4+ CD25+ cells was dependent upon IL-10, but not TGF-β. In addition, there was no effect of addition of CD4+ CD25+ cells, or IL-10 antagonism on T cell responses to nonspecific stimulation, also suggesting an antigen-specific immune modulation. These results argue that antigen-specific Tregs are critical to the observed effects of GM-CSF in EAMG. While we hypothesize that these Tregs are mobilized by tolerogenic DCs, it is also quite possible that GM-CSF mobilizes Tregs via a different mechanism, and that expansion of these cells have a direct effect on DC phenotype and function. The dependence of the Tregs on IL-10 argues that a Th2 cytokine shift also plays an important role. In fact, the function of Tregs, Th2 cells and DCs and their effects on AChR-specific T cells are very likely interrelated. The precise mechanisms and relative importance of DCs and Th1/Th2 cells in the therapeutic effect of GM-CSF in EAMG, and how these cells affect/interact with Tregs will be the focus of further study.
In summary, we have shown that GM-CSF treatment of chronic EAMG suppresses both clinical disease and down-modulates T cell and antibody-specific responses to the AChR. We further show that this effect is accompanied by the mobilization of DCs with a tolerogenic phenotype, and a shift in the cytokine response to a Th2 pattern. Most importantly, we demonstrate an expansion of Tregs in treated animals which suppress anti-AChR T cell proliferation in an antigen-specific manner. These findings demonstrate that by modulating early events in an autoimmune response, one can impact upon subsequent steps that eventually lead to clinical disease development in a well-defined, antibody-mediated autoimmune disease. Therapeutic administration of GM-CSF or other DC differentiation modulators could represent a novel and antigen-specific approach to the therapy of MG in humans.
Acknowledgments
This work was supported by the NIH (National Institute of Neurologic Disorders and Stroke, K08NS058800-01, MNM; and National Institute of Allergy and Infectious Diseases, RO1 AI 058190-01, BSP); and by the Myasthenia Gravis Foundation of America — Postdoctoral Fellowship Award to JRS.
Abbreviations
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- MG
myasthenia gravis
- EAMG
experimental autoimmune myasthenia gravis
- DC
dendritic cell
- AChR
acetylcholine receptor
- Treg
regulatory T cell
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Vincent A. Unraveling the pathogenesis of myasthenia gravis. Nat. Rev. Immunol. 2002;2:797–804. doi: 10.1038/nri916. [DOI] [PubMed] [Google Scholar]
- 2.Berman PW, Patrick J. Experimental myasthenia gravis: a murine system. J. Exp. Med. 1980;151:204–223. doi: 10.1084/jem.151.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes BW, Moro De Casillas ML, Kaminski HJ. Pathophysiology of myasthenia gravis. Semin. Neurol. 2004;24:21–30. doi: 10.1055/s-2004-829585. [DOI] [PubMed] [Google Scholar]
- 4.Elson CJ, Barker RN. Helper T cells in antibody-mediated, organ-specific autoimmunity. Curr. Opin. Immunol. 2000;12:664–669. doi: 10.1016/s0952-7915(00)00160-6. [DOI] [PubMed] [Google Scholar]
- 5.Hardin JA. Dendritic cells: potential triggers of autoimmunity and targets for therapy. Ann. Rheum. Dis. 2005;64:iv86–iv90. doi: 10.1136/ard.2005.044560. Suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludewig B, Junt T, Hengartner H, Zinkernagel RM. Dendritic cells in autoimmune diseases. Curr. Opin. Immunol. 2001;13:657–662. doi: 10.1016/s0952-7915(01)00275-8. [DOI] [PubMed] [Google Scholar]
- 7.Dittel BN, Visintin I, Merchant RM, Janeway CA., Jr Presentation of the self antigen myelin basic protein by dendritic cells leads to experimental autoimmune encephalomyelitis. J. Immunol. 1999;163:32–39. [PubMed] [Google Scholar]
- 8.Rossi M, Young HW. Human dendritic cells: Potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 10.Jonuleit H, Schmitt E, Steinbrink K, Enk AH. Dendritic cells as tools to induce anergic and regulatory T cells. Trends Immunol. 2001;22:394–400. doi: 10.1016/s1471-4906(01)01952-4. [DOI] [PubMed] [Google Scholar]
- 11.Tarbell KV, Yamazaki S, Steinman RM. The interactions of dendritic cells with antigen-specific regulatory T cells that suppress autoimmunity. Sem. Immunol. 2006;18:93–102. doi: 10.1016/j.smim.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Pulendran BJ, Banchereau S, Burkeholder E, Kraus E, Guinet E, Chalouni C, Caron D, Maliszewski C, Davoust J, Fay J, Palucka K. Flt3-Ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 13.Cools N, Ponsaerts P, Van Tendeloo VFI, Berneman ZN. Balancing between immunity and tolerance: an interplay between dendritic cells, regulatory T cells, and effector T cells. J. Leukoc. Biol. 2007;82:1365–1374. doi: 10.1189/jlb.0307166. [DOI] [PubMed] [Google Scholar]
- 14.Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A. Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J. Immunol. 2007;179:3638–3647. doi: 10.4049/jimmunol.179.6.3638. [DOI] [PubMed] [Google Scholar]
- 15.Vasu C, Dogan RE, Holterman MJ, Prabhakar BS. Selective induction of dendritic cells using granulocyte macrophage-colony stimulating factor, but not fms-like tyrosine kinase receptor-3 ligand, activates thyroglobulin-specific CD4+/CD25+ T cells and suppresses experimental autoimmune thyroiditis. J. Immunol. 2003;170:5511–5522. doi: 10.4049/jimmunol.170.11.5511. [DOI] [PubMed] [Google Scholar]
- 16.Gangi E, Vasu C, Cheatem D, Prabhakar BS. IL-10-producing CD4+CD25+ regulatory T cells play a critical role in granulocyte-macrophage colony-stimulating factor-induced suppression of experimental autoimmune thyroiditis. J. Immunol. 2005;174:7006–7013. doi: 10.4049/jimmunol.174.11.7006. [DOI] [PubMed] [Google Scholar]
- 17.Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis (EAMG) by Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) is associated with an expansion of FoxP3+ regulatory T cells. J. Immunol. 2006;177:5296–5306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 18.Wu B, Goluszko E, Christadoss P. Experimental autoimmune myasthenia gravis in the mouse. In: Coligan JE, Shevach EM, Strober W, editors. Current Protocols of Immunology. vol. 3. New York, NY: John Wiley & Sons; 1997. [Google Scholar]
- 19.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J. Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 20.Xiao BG, Duan RS, Link H, Huang YM. Induction of peripheral tolerance to experimental autoimmune myasthenia gravis by acetylcholine receptor-pulsed dendritic cells. Cell. Immunol. 2003;223:63–69. doi: 10.1016/s0008-8749(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 21.Duan RS, Adikari SB, Huang YM, Link H, Xiao BG. Protective potential of experimental autoimmune myasthenia gravis in Lewis rats by IL-10 modified dendritic cells. Neurobiol. Dis. 2004;16:461–467. doi: 10.1016/j.nbd.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Duan RS, Link H, Xiao BG. Long-term effects of IFN-γ, IL-10, and TGF-β-modulated dendritic cells on immune responses in Lewis rats. J. Clin. Immunol. 2005;25:50–56. doi: 10.1007/s10875-005-0357-4. [DOI] [PubMed] [Google Scholar]
- 23.O'Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt3 ligand, granulocyte colony-stimulating, and pegylated granulocyte colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 24.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 25.Lu L, Thomson AW. Manipulation of dendritic cells for tolerance induction in transplantation and autoimmune disease. Transplantation. 2002;73:S19–S22. doi: 10.1097/00007890-200201151-00008. [DOI] [PubMed] [Google Scholar]
- 26.Daro E, Butz E, Smith J, Teepe M, Maliszewski CR, McKenna HJ. Comparison of the functional properties of murine dendritic cells generated in vivo with flt3 ligand, GM-CSF and flt3 ligand plus GM-CSF. Cytokine. 2002;17:119–130. doi: 10.1006/cyto.2001.0995. [DOI] [PubMed] [Google Scholar]
- 27.Maldonado-Lopez R, De Smedt T, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Maliszewski CR, Moser M. Role of CD8+ and CD8− dendritic cells in the induction of primary immune responses in vivo. J. Leukoc. Biol. 1999;66:242–246. doi: 10.1002/jlb.66.2.242. [DOI] [PubMed] [Google Scholar]
- 28.Martinez del Hoyo G, Martin P, Arias CF, Marin AR, Ardavin C. CD8alpha+ dendritic cells originate from the CD8alpha− dendritic cell subset by a maturation process involving CD8alpha, DEC-205 and CD24 up-regulation. Blood. 2002;99:999–1004. doi: 10.1182/blood.v99.3.999. [DOI] [PubMed] [Google Scholar]
- 29.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 30.Hamilton JA, Anderson GP. GM-CSF Biology. Growth Factors. 2004;22:225–231. doi: 10.1080/08977190412331279881. [DOI] [PubMed] [Google Scholar]
- 31.Tarr PE. Granulocyte-macrophage colony-stimulating factor and the immune system. Med. Oncol. 1996;13:133–140. doi: 10.1007/BF02990841. [DOI] [PubMed] [Google Scholar]
- 32.Arpinati M, Green CL, Heimfeld S, Heuser JE, Anasetti C. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood. 2000;95:2484–2490. [PubMed] [Google Scholar]
- 33.Pugin J. Immunostimulation is a rational therapeutic strategy in sepsis. Novartis Found. Symp. 2007;280:21–27. [PubMed] [Google Scholar]
- 34.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 35.Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rossner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur. J. Immunol. 2000;30:1813–1822. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Mahnke K, Enk AH. Dendritic cells: key cells for the induction of regulatory T cells? Curr. Top. Microbiol. Immunol. 2005;293:133–150. doi: 10.1007/3-540-27702-1_7. [DOI] [PubMed] [Google Scholar]
- 37.Groux H, Fournier N, Cottrez F. Role of dendritic cells in the generation of regulatory T cells. Semin. Immunol. 2004;16:99–106. doi: 10.1016/j.smim.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat. Rev. Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 39.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 2001;193:285–294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinmen RM. CD25+ CD4+ T cells expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J. Exp. Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]