Abstract
N-hydroxysuccinimide (NHS) esters are widely used as leaving groups to activate covalent coupling of amine-containing biomolecules onto surfaces in academic and commercial surface immobilizations. Their intrinsic hydrolytic instability is well-known and remains a concern for maintaining stable, reactive surface chemistry, especially for reliable longer-term storage. In this work, we use x-ray photoelectron spectroscopy (XPS) and time-of-flight secondary ion mass spectrometry (ToF-SIMS) to investigate surface hydrolysis in NHS-bearing organic thin films. Principal component analysis (PCA) of both positive and negative ion ToF-SIMS data was used to correlate changes in the well-defined NHS ester oligo(ethylene glycol) (NHS-OEG) self-assembled monolayers to their surface treatment. From PCA results, multi-variate peak intensity ratios were developed for monitoring NHS reactivity, thin film thickness and oxidation of the monolayers during surface hydrolysis. Aging in ambient air for up to seven days resulted in hydrolysis of some fraction of bound NHS groups, oxidation of some resident thiol groups, and deposition of adventitious hydrocarbon contaminants onto the monolayers. Overnight film immersion under water produced complete hydrolysis and removal of the NHS chemistry, as well as removal of some of the thiolated OEG chains. NHS regeneration of the hydrolyzed surfaces was assessed using the same multi-variable peak intensity ratio as well as surface coupling with amine-terminated molecules. Both aqueous and organic NHS regeneration methods produced surfaces with bound NHS concentrations approximately 50% of the bound NHS concentration on freshly prepared NHS-OEG monolayers. Precise methods for quantifying NHS chemistry on surfaces are useful for quality control processes required in surface technologies that rely on reliable and reproducible reactive ester coupling. These applications include microarray, microfluidic, immunoassay, bioreactor, tissue engineering, and biomedical device fabrication.
Introduction
Immobilization of amine-terminated biomolecules onto surfaces is often an essential step for many biotechnologies, including assay technologies and arrays,1–4 biosensors5–7 and biomaterials.8,9 Many amine-reactive conjugation strategies rely on N-hydroxysuccinimide (NHS) active ester chemistry since NHS exhibits high intrinsic reactivity to surface-accessible amines on a wide range of biomolecules.10 In addition to amine reactivity, NHS groups have well-known competitive reactions from numerous nucleophiles that produce the NHS leaving group, including a problematic hydrolysis reaction10 that occurs not only in aqueous solution, but also at surfaces in ambient humidity. Continuous exposure to even trace ambient humidity affects NHS reactivity and shelf-life of commercial diagnostic arrays, resulting in inconsistent (i.e., variable batch-to-batch), or low biomolecule coupling reactivity, subsequent surface coupling yield and assay data reliability problems.11 Methods to analyze and study the aging and regeneration of NHS-bearing films, especially under storage conditions, are important as this conjugation chemistry is widely used. Also, as NHS-based assays proceed toward clinical use, FDA regulations will require the implementation of appropriate quality control processes.
Some traditional characterization techniques, such as fourier transform infrared spectroscopy (FTIR),5,12 contact angle analysis (CA),13 atomic force microscopy (AFM)14 and x-ray photoelectron spectroscopy (XPS)15,16 have been applied to investigate NHS-bearing films, as well as their surface hydrolysis. When these methods are combined with chemical derivatization reactions (fluorescence-tagged,17 fluorine-tagged probe molecules,18 amine-terminated macromolecules,18,19 etc.) the apparent amine reactivity of NHS-bearing surfaces can be addressed. In a series of studies of surface structure – reactivity relationships, Schönherr et al.17,18,20–24 applied quantitative methods, such as XPS and FTIR, to obtain chemical information about surface succinimidyl rings. However, XPS and FTIR do not discriminate chemically bound surface NHS moieties from reacted, surface-resident NHS moieties. As hydrolysis in ambient air is one ubiquitous issue that produces non-reactive, but surface-resident NHS chemistry, alternative methods for conveniently assessing NHS reactivity are required. Our study seeks to (1) develop sensitive surface analytical methods for estimating actual concentrations of surface-bound reactive NHS moieties, (2) assess the loss of NHS reactivity through aging, and (3) restore surface coupling reactivity through in situ regeneration of NHS-bearing films.
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) is a powerful surface analysis technique used to analyze many complex surfaces (self-assembled monolayers (SAMs), polymers, protein films, etc.) due to its high surface sensitivity, high mass resolution and molecular specificity.25 Combined with multi-variate analysis methods, characteristic molecular fragments can be identified that verify the existence, formation and structure of SAM surfaces, as well as detect the presence of trace contaminates.26–28
In this study, ToF-SIMS and XPS methods are combined to investigate the hydrolysis, regeneration and reactivity in organic SAMs containing bound NHS and oligo(ethylene glycol) (OEG7) moieties on gold. Principal component analysis (PCA) applied to the ToF-SIMS data set generates a set of characteristic peaks from positive and negative ion spectra of these NHS-OEG7 monolayers subjected to various aging conditions. Three multi-variate peak ratios are proposed for surface-sensitive measurements that distinguish NHS reactivity, film thickness and monolayer oxidation. Then, two NHS regeneration methods for re-introducing bound NHS moieties to hydrolyzed SAM surfaces are compared using these metrics. Finally, the reactivity of the NHS surfaces to trifluoroethylamine and Protein A are investigated and correlated to the NHS surface analysis data.
Experimental Section
Materials
(2, 2′-Dithiobis-ethyl-hepa (ethylene glycolic) acid)-N-succinimidyl ester (NHS–OEG7 disulfide) from Polypure (Oslo, Norway) was used as received (>95% purity). The chemical structure of NHS-OEG7 disulfide is shown in Figure 1. Ethylenedicarbodiimide (EDC), N-hydroxysuccinimide (NHS), anhydrous dimethylformamide (DMF), dicyclohexylcarbodiimide (DCC), trifluoroethylamine hydrochloride (TFEA · HCl), and Protein A were purchased from Sigma-Aldrich (St. Louis, MO). Ethanol (200-proof) was purchased from the Aaper Alcohol and Chemical Company (Shelbyville, Kentucky). All N2 gas (99.998% minimum purity, O2 < 5ppb and H2O < 3ppb) used in the experiment was purchased from Airgas, Inc. (Seattle, WA). All water used in the experiment was purified by reverse osmosis followed by Millipore polishing (yield: 18 mΩ resistivity). Phosphate buffered saline (PBS, Boston BioProducts) contained 137mM NaCl, 2.7mM KCl and 10mM phosphate salts at pH 7.4.
Figure 1.

The chemical structure of the NHS-OEG7 disulfide used for SAM formation.
Substrate Preparation
Silicon wafers (Silicon Valley Microelectronics, Inc., San Jose), coated with 10 nm Cr and 80 nm Au (99.99%) by electron beam evaporation at pressures below 1 × 10−6 Torr, were used as substrates. Freshly gold-coated substrates were immersed in 0.25mM NHS-OEG7 disulfide ethanolic solutions under N2 for 1 h. After disulfide assembly, samples were removed and rinsed thoroughly with ethanol and finally dried with nitrogen.
Sample Aging
Samples analyzed less than 30 min after preparation are labeled as “fresh.” Surfaces exposed to ambient conditions on the lab bench at room temperature (~21°C) and 30–40% relative humidity for 1 hr to 7 days are labeled as “aged.” Surfaces incubated in a 100% relative humidity chamber overnight are labeled as “100% r.h.” Surfaces soaked in Millipore™ H2O overnight are labeled as “hydrolyzed.”
NHS surface regeneration
Hydrolyzed samples were re-esterified with NHS groups and labeled as “regenerated.” Two NHS regeneration approaches based on common carbodiimide-catalyzed surface NHS re-esterification in different solvents were used as previously reported11: (1) Millipore™-purified H2O (aqueous regeneration method) and (2) DMF (organic regeneration method). After regeneration, samples were blown dry with N2 and analyzed within 30 min.
NHS surface coupling reactivity assays
The surface reactivity of the fresh, aged and regenerated surfaces to amine coupling was tested using TFEA and protein as reported elsewhere.19,29 SAMs after various treatments were immersed into either a 0.1 M solution of TFEA or a 0.01 mg/ml solution of Protein A in PBS buffer for 30 min at room temperature. After removal from solution, samples were washed twice in PBS and Millipore water, then blown dry with N2 and stored under nitrogen.
XPS analysis
XPS is a quantitative, surface analytical tool sensitive to the atomic composition of the outermost 100 Å of a sample surface. XPS SAM surface composition data were acquired on a Surface Science Instruments S-Probe spectrometer equipped with a monochromatized aluminum X-ray source and a hemispherical electron energy analyzer. Compositional survey and detail scans (N 1s, O 1s, S 2p and F 1s) were acquired using a pass energy of 150 eV. All the spectra were taken at 55° takeoff angle unless otherwise noted. The take-off angle is defined as the angle between the sample surface normal and the axis of the XPS analyzer lens. Three spots on two or more replicates of each sample type were analyzed. The compositional data are averages of the values determined at each spot. Data analysis was performed using Service Physics ESCA 2000 A software. XPS high-resolution spectra were acquired on a Kratos AXIS Ultra DLD instrument using a monochromatic Al X-ray source at 0° take-off angles in the hybrid mode. High-resolution spectra (C 1s, S 2p and Au 4f) were acquired using a pass energy of 20 eV. For the high-resolution spectra, peak binding energies (BEs) were referenced to Au 4f 7/2 peak at 84.0 eV. One spot on three replicates of each sample type were analyzed. Data analysis was performed with CasaXPS software (Casa Software Ltd). Reported high-resolution spectral compositions were the average values of the three analysis spots for each sample type.
ToF-SIMS analysis
Positive and negative ion ToF-SIMS data of the SAM surfaces were acquired with a Physical Electronics PHI 7200 reflectron time-of-flight secondary ion mass spectrometer using an 8 keV Cs+ primary ion source in the pulsed mode. The area of analysis for each spectrum was 100 μm × 100 μm, and the total ion dose was maintained below 1012 ions/cm2. Mass resolution (m/Δm) was typically between 6000 and 4500 for the (m/z) 27 and 25 peaks in the positive and negative ion spectra, respectively. Three spots on two replicates of each sample type were examined. Positive ion spectra were mass calibrated using the CH3+, C2H5+, and AuC2H4+ peaks, and negative ion spectra were mass calibrated using the CH−, C2H−, and AuS− peaks. Maximum calibration errors were kept below 10 ppm.
Principal Component Analysis
PCA applications to ToF -SIMS spectral analysis has been described extensively elsewhere.30,31 By applying PCA, a ToF-SIMS data set can be reduced to two cross-product matrices: scores and loadings. Scores plots can be used to visualize the quantitative relationship among samples. Loadings plots can be used to visualize the relationship between original variables (spectral peaks) and new variables (principal components). All of the peaks at least 3 times above background in the 12 – 350 m/z region of the positive and negative ion mass spectra were selected for principal component analysis. Positive and negative spectra were analyzed separately throughout this study. Before PCA, each raw spectrum was normalized to the sum of the intensity of the selected peaks and then mean-centered. PCA was performed using the PLS Toolbox v. 2.0 (Eigenvector Research, Manson, WA) for MATLAB v. 6.5 (MathWorks, Inc., Natick, WA).
Results and Discussion
XPS analysis of NHS-OEG7 SAMs
Elemental composition
XPS data compared the surface elemental compositions of the fresh, aged and hydrolyzed NHS-OEG7 monolayers. XPS detected all elements expected from the NHS-OEG7 disulfide (C, O, N, S), as well as the substrate (Au). No other elemental signals were observed. The resulting elemental composition analysis shown in Table 1 A indicates that for all monolayers, the experimentally measured C/O ratio is close to 2:1, consistent with the 1.9:1 stoichiometric C/O ratio expected for the NHS-OEG7 disulfide.15 The XPS compositions of the fresh and 7-day aged surfaces were the same within experimental error, indicating that 7-day aging did not affect SAM surface composition. Composition trends observed in the table suggest that aging in atmosphere and hydrolysis in water produced different effects on the NHS-OEG7 monolayers. First, compared to the fresh surface, no nitrogen was detected in the hydrolyzed surface, indicating complete removal of NHS groups after hydrolysis. Second, the density of monolayers after hydrolysis, as measured by the degree of attenuation of the substrate Au 4f XPS signal, is consistent with removal of approximately 15% of the SAM thiol molecules from the monolayer.32,33
Table 1.
XPS-determined elemental compositions for NHS-OEG7 SAMs on gold after various treatments.
| Description | Organic Overlayer Atomic Percentage (Std. dev.)
|
Atomic Percentage
|
Atomic ratio
|
||||
|---|---|---|---|---|---|---|---|
| C 1s | O 1s | N 1s | S 2p | F 1s | Au 4f | N/O | |
| Theoretical value | |||||||
| NHS-OEG7 | 62.2 | 32.4 | 2.7 | 2.7 | 0.0 | 0.0 | 0.08 |
| Hydrolyzed | 63.3 | 33.3 | 0.0 | 3.3 | 0.0 | 0.0 | 0.00 |
| TFEA-derivated | 60.0 | 25.7 | 2.8 | 2.8 | 8.6 | 0.0 | 0.11 |
| A. Monolayers under various storage conditions | |||||||
| Fresh NHS | 63.2 (1.9) | 31.3 (1.0) | 3.8 (0.8) | 1.7 (0.2) | ndb | 16.2 (0.1) | 0.12 |
| 7-day aged | 62.3 (0.1) | 32.7 (0.6) | 3.7 (0.5) | 1.3 (0.2) | nd | 15.1 (0.2) | 0.11 |
| Hydrolyzed | 62.2 (0.7) | 35.6 (0.9) | nd | 2.2 (0.2) | nd | 23.4 (0.3) | 0.00 |
| B. Hydrolyzed monolayers after regeneration | |||||||
| Aqueous regeneration | 66.2 (0.8) | 28.8 (0.6) | 3.2 (0.6) | 1.8 (1.0) | nd | 28.1 (1.5) | 0.11 |
| Organic regeneration | 61.5 (0.2) | 33.5 (1.0) | 2.2 (0.7) | 2.8 (0.3) | nd | 24.1 (0.8) | 0.07 |
| NHS adsorptionc | 63.0 (1.1) | 33.9 (0.8) | 1.4 (0.8) | 1.7 (0.6) | nd | 28.5 (0.3) | 0.04 |
| C. TFEA derivatization | |||||||
| Fresh NHS | 57.6 (1.9) | 30.4 (2.1) | 2.4 (0.1) | 2.1 (0.4) | 7.5 (0.5) | 16.9 (1.1) | 0.08 |
| Hydrolyzed | 64.6 (0.7) | 33.5 (0.6) | nd | 1.9 (0.4) | nd | 27.4 (0.6) | 0.00 |
| Aqueous regeneration | 62.4 (0.7) | 30.3 (0.8) | 1.2 (0.1) | 2.6 (0.0) | 3.5 (0.7) | 30.1 (0.6) | 0.04 |
| Organic regeneration | 62.3 (1.1) | 31.5 (0.9) | 0.9 (0.1) | 2.6 (0.2) | 2.7 (0.6) | 26.3 (0.6) | 0.03 |
| D. Protein A immobilization | |||||||
| Fresh NHS | 64.4 (2.5) | 23.7 (1.6) | 10.5 (0.9) | 1.4 (0.5) | nd | 12.2 (0.8) | 0.44 |
| Hydrolyzed | 61.6 (1.6) | 34.0 (1.3) | 2.8 (0.7) | 1.6 (0.3) | nd | 23.4 (0.8) | 0.08 |
| Aqueous regeneration | 65.1 (2.4) | 29.2 (2.1) | 4.2 (0.6) | 1.5 (0.2) | nd | 26.2(1.6) | 0.14 |
| Organic regeneration | 62.8 (1.9) | 31.8 (1.4) | 3.4 (1.1) | 2.0 (0.3) | nd | 22.5(1.1) | 0.11 |
The gold substrate Au4f signal was excluded to better show the elemental composition of the organic overlayers. The remaining signals from the organic SAM were re-normalized to 100%.
nd: not detected.
Hydrolyzed surfaces were incubated in 0.5 M NHS aqueous solution without EDC for 15min.
High resolution XPS analysis
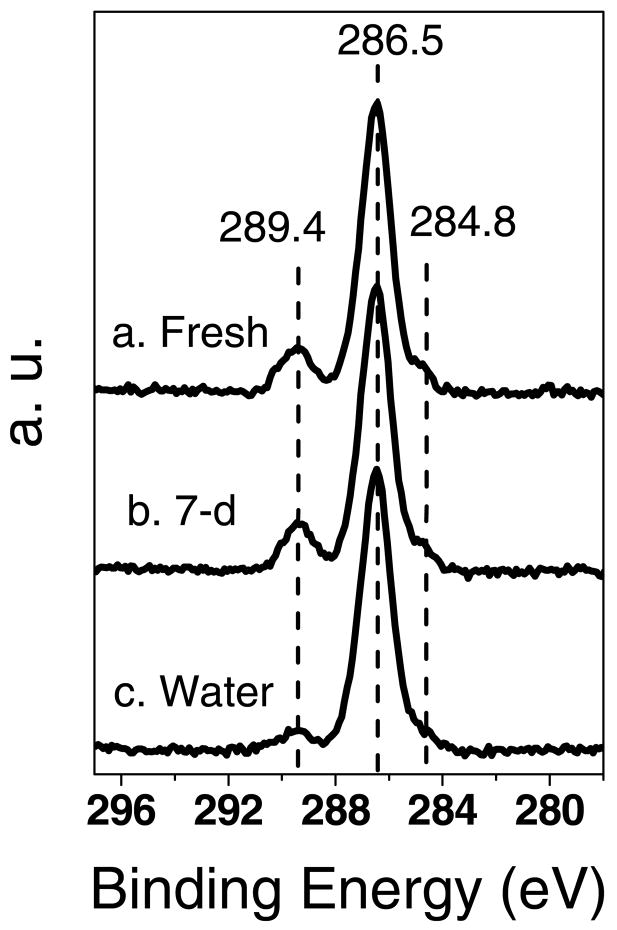
Typical high-resolution XPS C1s spectra for the fresh, 7-day aged and hydrolyzed NHS-OEG7 monolayers are shown in Figure 2. The surface concentrations of the three carbon peaks detected in the C1s spectra are summarized in Table 2. The major carbon peak (286.5 eV) was assigned to the ether carbons in the OEG chains and three β-shifted carbons adjacent to the carbonyl groups.12,19 The low BE small shoulder at 284.8 eV was assigned to hydrocarbon species. The peak at high binding energies (289.4 eV) was assigned to the carbonyl groups in both the esters and NHS moieties, and carboxylate groups of the hydrolyzed samples.12,16,19,34 (Note: Ester and acid carboxyl groups typically have C1s binding energies near 289 ev, but amide groups typically have C1s binding energies near 288 eV. Possible reasons why the amide groups in the NHS moieties have similar C1s binding energies to the ester and acid carboxyl groups include: 1) the strain in the five-membered ring, 2) electron conjugation in the N-succinimidyl esters and 3) trace amounts of carboxylic acid groups in fresh NHS monolayers.) For the fresh and 7-day aged samples, the concentrations of three carbon peaks were the same, confirming that 7-day aging did not affect carbon species composition appreciably. Significantly, the concentration of the carbonyl groups was significantly lower in the hydrolyzed samples compared to the fresh and 7-day aged samples, suggesting the removal of the NHS moiety during the hydrolysis.
Figure 2. High-resolution XPS C1s spectra for (a) fresh NHS (Fresh), (b) 7-day aged (7-d) and (c) hydrolyzed (Water) NHS-OEG7 SAMs on gold.
Peak assignments: (1) 284.8 eV for C-H; (2) 286.5 eV for C-O, C-S and β-shifted carbons adjacent to carbonyl groups; (3) 289.4 eV for carbonyl groups of esters and NHS moieties, and carboxylic groups.12,19
Table 2.
Peak positions and concentrations from high-resolution XPS C 1s spectra for NHS-OEG7 SAMs on gold under various surface treatment conditions, including fresh NHS, 7 -day aged and hydrolyzed surfaces.
| Binding Energy | 284.8 eV | 286.5 eV | 289.4 eV |
|---|---|---|---|
| Species | C-H | C-O, C-S and β-shifted carbons adjacent to carbonyl groups | carbonyl groups in esters and NHS moieties,a and carboxylic groupsb |
| theoretical carbon percentages | |||
| Thin film: | |||
| NHS-OEG7 | 0 | 87.0 | 13.0 |
| Hydrolyzed | 0 | 94.0 | 6.0 |
| experimental carbon percentages (std. dev.) | |||
| Fresh NHS | 7.7 (1.6) | 77.6 (0.7) | 14.7 (1.6) |
| 7-day aged | 6.1 (0.3) | 78.6 (1.8) | 15.3 (1.5) |
| Hydrolyzed | 7.5 (1.8) | 85.3 (1.1) | 7.1 (0.8) |
in NHS-OEG7 SAMs
in hydrolyzed SAMs
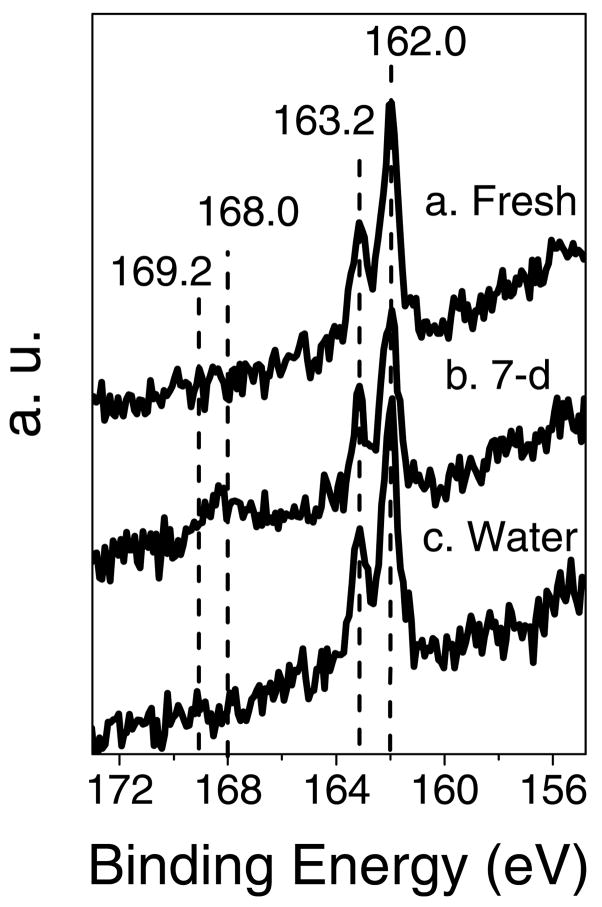
Figure 3 shows high-resolution XPS sulfur 2p spectra for the fresh, 7-day aged and hydrolyzed NHS-OEG7 monolayers. Sulfur species in all 2p spectra exhibited the expected doublet structure containing S 2p3/2 and S 2p1/2 peaks. The S 2p3/2 BE was approximately 162 eV, consistent with sulfur atoms bound to Au substrate as a thiolate species.35 The S 2p1/2 peak BE was 1.2 eV higher with the half the intensity of the S 2p3/2 peaks, as expected. The spectra for 7-day aged samples exhibited a second doublet at high binding energies (ca. 168 eV), indicating the SAM thiol group had oxidized significantly during the 7-day aging process.35,36 Since the aging processes were performed in air on the laboratory bench, the presence of the oxidized sulfur species could result from oxidation by oxygen, water vapor or trace levels of ozone. All three species will be present in ambient laboratory air, though the ozone concentration is expected to be quite small (i.e., no sources of ozone production such as laser printers were located near the samples being aged).
Figure 3. High-resolution XPS S2p spectra for (a) fresh NHS (Fresh), (b) 7-day aged (7-d) and (c) hydrolyzed (Water) NHS-OEG7 SAMs on gold.
Peak assignments: (1) 162.0 eV for bound S 2p3/2; (2) 163.2 eV for for bound S 2p1/2; (3) 168.0 eV for oxidized S 2p3/2; (4) 169.2 eV for oxidized S 2p1/2.35
ToF-SIMS analysis of NHS-OEG7 SAMs
PCA results
Fresh, aged, high humidity and hydrolyzed NHS-OEG7 monolayers were investigated with positive and negative ion ToF-SIMS. By applying PCA to the ToF-SIMS data, the spectral differences resulting from various treatments can be determined. Figure 4 shows the Principal Component (PC) 1 (a) scores and (b) loadings for the positive ion spectra, and the PC 1 (c) scores and (d) loadings for the negative ion spectra.
Figure 4. PCA of ToF-SIMS data. PC 1 (a) scores and (b) loadings from PCA of the positive ion spectra, and PC 1 (c) scores and (d) loadings from PCA of the negative ion spectra of NHS-OEG7 self-assembled monolayers on gold after aging treatments that include fresh NHS (Fresh), 1-hr aged (1-hr), 3-day aged (3-d), 7-day aged (7-d), 100% r.h. (100) and hydrolyzed (Water) surfaces.
The labeled peaks were assigned in Table S1 for positive ion and negative ion spectra. Possible structures of the prominent peaks for the positive and negative ion spectra are proposed in Table S2 and Table S3, respectively.
PC 1 captured 60% and 70% of total variance in the positive ion and negative ion spectra, respectively. Score trends observed in the plots suggest the various surface treatments had significantly affected the NHS-OEG7 spectra. First, the average score of one-hour aged samples and the fresh samples were the same, within experimental error. This means that spectra from SAM surfaces aged for a short time are similar to those from fresh SAM surfaces. Second, SAM surfaces aged for three to seven days had higher positive ion PC 1 scores than fresh SAM surfaces, indicating longer aging times did affect the spectra. The positive ion PC 1 scores continued to increase for the 100% r.h. and hydrolyzed samples, consistent with further hydrolysis and removal of the NHS groups from these monolayers. The negative ion PC 1 scores exhibited similar overall trends to the positive ion PC 1 scores, with the PC 1 score increasing with longer aging times and hydrolysis.
Characteristic peaks analysis
The PCA loadings plots capture important relationships between the original variables (spectral peaks) and the new variables (e.g., PC 1). Peaks with positive loadings on a given PC are relatively more intense in the spectra with positive scores and relatively less intense in spectra with negative score (and vice verse). The peaks with significant PC 1 loadings are listed in Table S1, with some of those peaks labeled in Figure 4 (b) and (d). Possible structures of the prominent peaks for the positive and negative ion spectra are proposed in Table S2 and Table S3, respectively.
For NHS-OEG7 SAMs, many characteristic molecular ion fragments were present in the positive ion spectra. Thus, the loading plots of positive ion spectra provide some unique structure information about the changes that occur upon treatment of these surfaces. Consistent with previous studies of OEG or poly(ethylene glycol)-containing thin films,15,16,31 the following characteristic fragments from the ethylene glycol chains had high loadings: C2H5O+ (m/z = 45), C2H3O+ (m/z = 43), CH3O+ (m/z = 31). Numerous common hydrocarbon fragments were also present in the loading plot (e. g. CH3+, C2H3+, C2H4+ C2H5+, C3H6+, C4H8+, C6H12+, C7H11+, etc.). The fragments C2H5+ (m/z = 29) C2H3+ (m/z = 27) and CH3+ (m/z = 15) were the most prominent in the hydrocarbon series, consistent with the fact the longest hydrocarbon chain in the NHS-OEG7 molecules was two methylene groups.
Since the fresh, aged and hydrolyzed surfaces have very similar chemical structures, it was anticipated that they would exhibit many similar spectral features. However, despite this similarity, PCA was also able to identify several major differences among the samples. First, the positive ion spectra of fresh and aged samples exhibit several nitrogen-containing peaks (C4H6O3N+, m/z = 116; C4H4O2N+, m/z = 98; and C2H2ON+, m/z = 56) originating from NHS groups. The fragment, C4H4O2N+, attributed to (succinimide - H)+, has been previously reported for NHS-containing films.16 Second, a set of peaks from the carboxyl-terminal group (C5H9O3+, m/z = 117; C3H5O2+, m/z = 73; and CHO2+, m/z = 45) were also detected. The large positive loadings of carboxyl-related peaks result in the separation of hydrolyzed surfaces from the remaining SAM surfaces via the positive ion PC 1 scores plot (Figure 4a). This is due to high concentration of carboxyl-terminated OEG groups produced by NHS surface hydrolysis. A difference is also observed in the Au fingerprint peaks. AuSC2H4+ (m/z = 257) and AuC2H4+ (m/z = 225) have the largest loadings in high mass range. Both Au-related peaks and the Cs+ primary ion peak are correlated with spectra from the hydrolyzed surface. As previously reported,31 the increasing peak intensities from substrate and Cs+ primary ion source indicate a decreased overlayer coverage on Au surfaces. Finally, the most important difference among the surfaces is shown in Figure 4b. Three peaks present in the fresh NHS surfaces with high negative PCA loadings (C7H8O4N+, m/z = 170; C6H8O3N+, m/z = 142; and C5H6O3N+, m/z = 128) were not detected in the hydrolyzed surfaces. These three nitrogen-containing peaks are assigned to fragments from covalently bound NHS ester groups. Significantly, these fragments all contain both the succinimidyl ring and a part of the ester-terminated OEG chain: the NHS amine-reactive site of the SAM surfaces.
For NHS-OEG7 monolayers, some characteristic fragments were also observed in the negative ion spectra. Consistent with previous study of poly(L-lysine)-grafted-poly(ethylene glycol) (PLL-PEG) on surfaces,31 the atoms (e.g., C−, O−,) and small clusters (e.g., CH−, OH−, C2H−, CN−) were prominent peaks in the low mass region. The expected characteristic OEG fragments (e.g., C2H3O−, C2H3O2−) were detected from all surfaces. Two series of peaks (C4HyNO2− and C4HyNO3−, where y = 3, 4, 5) with high negative loadings dominated the high-mass range. These fragments are attributed to (succinimide – nH)− and (hydroxysuccinimide – nH)− species. Due to the complicated nature of the ToF-SIMS process, these fragments probably originate from both covalently bound and unbound NHS moieties. Four peaks with medium positive loadings are assigned to SO2−, SO3−, HSO3− and HSO4−. These peaks are commonly observed in negative ion spectra of oxidized SAM monolayers.37
Multivariate peak intensity ratios
Using the positive ion PCA results, the following two multi-variate peak intensity ratios were developed: (1) the NHS reactivity (Bound_NHS ratio) and (2) the thin film thickness (TFT ratio). Bound_NHS ratio represents the relative concentration of chemically bound NHS moieties in the film. TFT ratio represents the thickness of the SAM organic film on the Au substrate.
Bound_NHS and TFT ratios are derived from:
and
where
All intensities (Ix) were obtained from the secondary ion spectra. ICs+ was included in IAu+ because ICs+was primarily due to backscattering of the primary ion beam from the Au surface.
Figure 5(a) shows Bound_NHS from NHS-OEG7 monolayers as a function of surface treatment. As the aging time and humidity increased, Bound_NHS decreased due to surface hydrolysis decreasing the concentration of chemically bound NHS fragments and increasing the concentration of the resulting carboxylic acid fragments. Figure 5(b) shows that TFT also varies with surface treatment. As aging time increases, the thin film thickness increases due to deposition of adventitious hydrocarbon contamination onto the SAM surfaces. The TFT for the 100% r.h. sample was similar to the fresh and 1 hr aged surfaces, but significantly lower than the 3- and 7- day aged SAM surfaces. This is because the 100% r.h. surface was only treated overnight. The TFT ratio for the hydrolyzed surface is significantly lower than all surfaces, including the fresh surface. This indicates that the hydrolysis process thinned the organic overlayer, likely due to both removal of all terminal NHS groups plus removal of some thiolated OEG chains (i.e., oxidized thiols are readily washed off the gold substrate by water), consistent with the higher percentage of gold detected by XPS from the hydrolyzed samples.
Figure 5.
Multi-variate ratios from ToF-SIMS used for determining the (a) NHS reactivity (Bound_NHS ratio) from the positive ion spectra, (b) thin film thickness (TFT ratio) from the positive ion spectra and (c) sulfur oxidation (SO ratio) from the negative ion spectra of NHS-OEG7 SAMs on gold after aging treatments that include fresh NHS (Fresh), 1-hr aged (1-hr), 3-day aged (3-d), 7-day aged (7-d), 100% r.h. (100) and hydrolyzed (Water) surfaces.
The PC 1 loadings from the negative ion spectra are used to develop a sulfur oxidation ratio (SO), useful to estimate the relative concentration of oxidized sulfur species in these SAM organic films.
SO is calculated from:
where
Figure 5(c) shows SO for NHS-OEG7 monolayers on Au. SO for 3- and 7- day aged surfaces is higher than all other SAM surfaces. Since oxygen can penetrate the organic layer to reach the Au-S interface, Au-S thiolate species can be oxidized over time.36,38 Thus, it is not surprising that samples aged for the longest period have the highest SO ratio. Moreover, the oxidized thiolate species formed during the hydrolysis treatment will be removed from the Au surface when exposed to an aqueous environment, resulting in the low SO ratio observed for the hydrolyzed surfaces.
NHS regeneration and reactivity of NHS-OEG7 SAMs
To investigate the NHS regeneration process, hydrolyzed SAM surfaces were activated with both aqueous and organic regeneration methods.11 Once regenerated, resulting NHS reactivity can be assessed by comparing the ToF-SIMS Bound_NHS ratio and direct amine derivatization (e.g. TFEA and Protein A) methods.
Regeneration of NHS moieties
Elemental XPS compositions for both regenerated surfaces are shown in Table 1B. In a control experiment, a hydrolyzed surface was incubated in NHS aqueous solution without EDC. As nitrogen is unique to the succinimidyl ring in NHS moieties and oxygen is predominately in the OEG chains of the monolayers, the nitrogen to oxygen (N/O) ratio represents the amount of NHS moieties in the films. The N/O ratios for two regenerated surfaces are close to 0.1, indicating roughly the same level of NHS introduced by regeneration. From the XPS elemental compositions it is not possible to determine whether or not the NHS is covalently linked to the carboxyl groups on the hydrolyzed SAM surface. Since EDC is a key reagent for chemically binding NHS to carboxyl groups,39 NHS groups are expected to be simply adsorbed onto the hydrolyzed SAM surface in the control experiment. Use of the Bound_NHS ratio developed in the previous section permitted ToF-SIMS data to be used to estimate the relative concentration of chemically attached NHS moieties after the different regeneration treatments.
Both aqueous and organic regeneration methods increase the concentration of bound NHS groups to 0.33±0.01 and 0.31±0.01, respectively, while no bound NHS groups were detected on the control sample. Compared to the Bound_NHS ratio for the fresh surface in Figure 5(a), the amount of chemically attached NHS moieties produced by these regeneration methods is only half of that in the fresh samples. Lower concentrations of NHS observed after aqueous regeneration may reflect the competitive aqueous hydrolysis back-reaction (i.e., in 55M water), but this should not be the case for the anhydrous organic regeneration. One possible explanation for why both regeneration methods yielded lower amounts of bound NHS than the fresh surface is possible steric hindrance of the carboxyl groups in the SAM monolayer, the increased pKa for SAM surface carboxlyates compared to bulk carboxlyates,40 possible inactive hydrogen-bonded carboxylate dimers on the SAM surface or the reduced packing density of SAM molecules in the hydrolyzed samples. The fact that no increase in bound NHS species was detected in the control experiments confirms that EDC (or similar activating agent) is required for reliable regeneration of the covalently bound NHS SAM surfaces.
TFEA derivatization
The amine reactivity of the fresh, hydrolyzed and regenerated surfaces was examined using TFEA derivatization. XPS composition results for the TFEA-derivatized SAM surfaces are summarized in Table 1C. In control experiments, the fresh and hydrolyzed surfaces were treated with TFEA solution. After TEFA treatment of the fresh surface, significant amounts of fluorine were observed. The experimentally measured F/N ratio is equal to the 3:1 stoichiometric F/N ratio in the TFEA molecule, suggesting that nitrogen originates from TFEA derivatization, not from any remaining NHS moieties. Fluorine is not detected on the hydrolyzed surface after TFEA treatment, indicating that the derivatization reaction requires the presence of chemically bound NHS to proceed. For the regenerated surfaces both fluorine and nitrogen signals after TFEA derivatization were significantly lower than TFEA derivatized fresh SAM surfaces, consistent with lower concentrations of NHS produced from both regeneration methods. Compared to TFEA derivatized fresh SAM surfaces, the hydrolyzed and regenerated SAM surfaces showed higher XPS Au 4f signals, consistent with the removal of oxidized thiol chains during the hydrolysis and regeneration treatments.
Protein A immobilization
Immobilization of Protein A was also used to assess the amine reactivity of the fresh, hydrolyzed and regenerated SAM surfaces. The XPS composition results for Protein A immobilization are summarized in Table 1D. After immobilization of Protein A onto the fresh surface a significant increase is seen in the nitrogen concentration, expected from the large nitrogen content of the immobilized protein. Nitrogen concentrations for Protein A immobilized to the regenerated surfaces were significantly lower than that on the fresh SAM surface, consistent with trends observed also with the Bound_NHS ratio and TFEA derivatization. Some nitrogen is also detected after the hydrolyzed surface was exposed to Protein A, indicating this surface exhibited some non-specific protein adsorption. Thus, immobilization of Protein A onto these surfaces involves both covalent immobilization and non-specific adsorption. Exposed side-chain lysine amino groups on Protein A surface will react with SAM-bound NHS moieties to form amide bonds and covalent immobilization of the protein. Although the OEG chain in these surfaces should resist non-specific protein adsorption, both electrostatic interactions between SAM carboxylate groups and Protein A along with lower packing density of SAM molecules in the hydrolyzed and regenerated surfaces probably account for the observed non-specific protein adsorption.
Conclusions
XPS and ToF-SIMS combined with PCA provided new detailed insights into the surface hydrolysis, regeneration and reactivity of NHS-OEG7 SAMs as a model for understanding NHS reactivity on surfaces. The novel combination of these analysis methods allowed systematic and sensitive examination of various aspects of NHS-OEG7 SAMs surface hydrolysis, including cleavage of covalent NHS groups, contamination by adventitious hydrocarbon deposition, thiol oxidation and removal of both NHS and OEG SAM chemistry into aqueous media. XPS data yielded quantitative information for surface elemental composition as well as the carbon and sulfur species in treated NHS-OEG7 surfaces. ToF-SIMS combined with PCA provided more detailed information about surface chemistry and SAM structure, including new, revealing details about hydrolysis, SAM removal and oxidation, as well as the presence of covalent and non-covalent NHS groups. PCA application to ToF-SIMS data allowed reliable identification of characteristic peaks in the positive and negative ion spectra from NHS, covalently attached to the SAM as well as hydrolyzed and released at the surface. Three multivariate peak ratios were developed from the ToF-SIMS/PCA results that correlate with the concentration of covalently bound NHS species, thickness changes for the SAM organic overlayers on Au, and oxidation of anchoring sulfur chemistry. The multi-variate peak ratios from these models can now be used to analyze ToF-SIMS data from other analogous and perhaps more relevant surfaces (i.e., commercial DNA microarray slides containing NHS and OEG groups) without doing PCA. Results from this study provide new characterization methods and associated new metrology useful for assessing the reactive content of surfaces bearing NHS active ester chemistry.
Supplementary Material
Experimental data are available as Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was supported by the National ESCA and Surface Analysis Center for Biomedical Problems (NIH grant EB-002027) and NIH grant EB-001473. Drs. Daniel Graham, Chi-Ying Lee and Roger Michel are thanked for helpful discussions on ToF-SIMS analysis and PCA methods and interpretation.
References
- 1.Gong P, Harbers GM, Grainger DW. Anal Chem. 2006;78:2342–2351. doi: 10.1021/ac051812m. [DOI] [PubMed] [Google Scholar]
- 2.Choi HJ, Kim NH, Chung BH, Seong GH. Anal Biochem. 2005;347:60–66. doi: 10.1016/j.ab.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 3.Liu YJ, Rauch CB. Anal Biochem. 2003;317:76–84. doi: 10.1016/s0003-2697(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 4.Odonnell MJ, Tang K, Koster H, Smith CL, Cantor CR. Anal Chem. 1997;69:2438–2443. doi: 10.1021/ac961007v. [DOI] [PubMed] [Google Scholar]
- 5.Lahiri J, Isaacs L, Tien J, Whitesides GM. Anal Chem. 1999;71:777–790. doi: 10.1021/ac980959t. [DOI] [PubMed] [Google Scholar]
- 6.Nieba L, NiebaAxmann SE, Persson A, Hamalainen M, Edebratt F, Hansson A, Lidholm J, Magnusson K, Karlsson AF, Pluckthun A. Anal Chem. 1997;252:217–228. doi: 10.1006/abio.1997.2326. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi K, Muguruma H, Karube I. Anal Chem. 1996;68:1695–1700. doi: 10.1021/ac950756u. [DOI] [PubMed] [Google Scholar]
- 8.Castner DG, Ratner BD. Surf Sci. 2002;500:28–60. [Google Scholar]
- 9.Ratner BD, Bryant SJ. Annual Review Of Biomedical Engineering. 2004;6:41–75. doi: 10.1146/annurev.bioeng.6.040803.140027. [DOI] [PubMed] [Google Scholar]
- 10.Hermanson GT. Bioconjugate Techniques. Academic Press; 1995. [Google Scholar]
- 11.Gong P, Grainger DW. Surf Sci. 2004;570:67–77. [Google Scholar]
- 12.Voicu R, Boukherroub R, Bartzoka V, Ward T, Wojtyk JTC, Wayner DDM. Langmuir. 2004;20:11713–11720. doi: 10.1021/la047886v. [DOI] [PubMed] [Google Scholar]
- 13.Shovsky A, Schönherr H. Langmuir. 2005;21:4393–4399. doi: 10.1021/la046967o. [DOI] [PubMed] [Google Scholar]
- 14.Dordi B, Pickering JP, Schönherr H, Vancso GJ. Eur Polym J. 2004;40:939–947. [Google Scholar]
- 15.Lu HB, Campbell CT, Castner DG. Langmuir. 2000;16:1711–1718. [Google Scholar]
- 16.Xia N, Hu YH, Grainger DW, Castner DG. Langmuir. 2002;18:3255–3262. [Google Scholar]
- 17.Feng CL, Zhang ZZ, Forch R, Knoll W, Vancso GJ, Schönherr H. Biomacromolecules. 2005;6:3243–3251. doi: 10.1021/bm050247u. [DOI] [PubMed] [Google Scholar]
- 18.Degenhart GH, Dordi B, Schönherr H, Vancso GJ. Langmuir. 2004;20:6216–6224. doi: 10.1021/la049580u. [DOI] [PubMed] [Google Scholar]
- 19.Adden N, Gamble LJ, Castner DG, Hoffmann A, Gross G, Menzel H. Langmuir. 2006;22:8197–8204. doi: 10.1021/la060754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schönherr H, Degenhart GH, Dordi B, Feng CL, Rozkiewicz DI, Shovsky A, Vancso GJ. Ordered Polymeric Nanostructures at Surfaces. Vol. 200. 2006. pp. 169–208. [Google Scholar]
- 21.Feng CL, Vancso GJ, Schönherr H. Langmuir. 2005;21:2356–2363. doi: 10.1021/la047490j. [DOI] [PubMed] [Google Scholar]
- 22.Schönherr H, Feng CL, Shovsky A. Langmuir. 2003;19:10843–10851. [Google Scholar]
- 23.Dordi B, Schönherr H, Vancso GJ. Langmuir. 2003;19:5780–5786. [Google Scholar]
- 24.Feng CL, Vancso GJ, Schönherr H. Adv Funct Mater. 2006;16:1306–1312. [Google Scholar]
- 25.Belu AM, Graham DJ, Castner DG. Biomaterials. 2003;24:3635–3653. doi: 10.1016/s0142-9612(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee CY, Canavan HE, Gamble LJ, Castner DG. Langmuir. 2005;21:5134–5141. doi: 10.1021/la0472302. [DOI] [PubMed] [Google Scholar]
- 27.Graham DJ, Price DD, Ratner BD. Langmuir. 2002;18:1518–1527. [Google Scholar]
- 28.Graham DJ, Ratner BD. Langmuir. 2002;18:5861–5868. [Google Scholar]
- 29.Noiset O, Schneider YJ, MarchandBrynaert J. J Polym Sci, Part A: Polym Chem. 1997;35:3779–3790. [Google Scholar]
- 30.Wagner MS, Castner DG. Langmuir. 2001;17:4649–4660. [Google Scholar]
- 31.Wagner MS, Pasche S, Castner DG, Textor M. Anal Chem. 2004;76:1483–1492. doi: 10.1021/ac034873y. [DOI] [PubMed] [Google Scholar]
- 32.Harder P, Grunze M, Dahint R, Whitesides GM, Laibinis PE. J Phys Chem B. 1998;102:426–436. [Google Scholar]
- 33.Lee JK, Kim YG, Chi YS, Yun WS, Choi IS. J Phys Chem B. 2004;108:7665–7673. [Google Scholar]
- 34.Böcking T, James M, Coster HGL, Chilcott TC, Barrow KD. Langmuir. 2004;20:9227–9235. doi: 10.1021/la048474p. [DOI] [PubMed] [Google Scholar]
- 35.Castner DG, Hinds K, Grainger DW. Langmuir. 1996;12:5083–5086. [Google Scholar]
- 36.Willey TM, Vance AL, van Buuren T, Bostedt C, Terminello LJ, Fadley CS. Surf Sci. 2005;576:188–196. [Google Scholar]
- 37.Hutt DA, Cooper E, Leggett GJ. J Phys Chem B. 1998;102:174–184. [Google Scholar]
- 38.Schöenfisch MH, Pemberton JE. J Am Chem Soc. 1998;120:4502–4513. [Google Scholar]
- 39.Sehgal D, Vijay IK. Anal Biochem. 1994;218:87–91. doi: 10.1006/abio.1994.1144. [DOI] [PubMed] [Google Scholar]
- 40.Creager SE, Clarke J. Langmuir. 1994;10:3675–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental data are available as Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.