1. Introduction
The developing axons of many neuronal cell types exhibit saltatory growth, alternating between distinct periods of rapid, sustained elongation and periods of relative quiescence or retraction. This pattern of axonal growth can be observed in vitro (Katz et al., 1984; Argiro et al., 1984; Aletta and Greene, 1988; Ruthel and Banker, 1999), in semi-intact living preparations (Godement et al., 1994) and in intact brain (Kaethner and Struermer, 1992). Alternation between these two states of elongation activity can be associated with important features of axon development, including axon establishment (Dotti et al., 1988), branch formation (Szebenyi et al., 1998) and branch competition (Futerman and Banker, 1996). More recently, molecular and pharmacological treatments have been shown to selectively alter specific aspects of the typical growth pattern, such as the relative duration of periods of sustained growth (Walker et al., 2001) or extent of retraction during pausing periods (Lindsley et al., 2003). However, quantitative tools designed specifically for the analysis of saltatory axon elongation have not been available.
Studying axon growth dynamics typically requires the collection of length data from a series of time-lapse images of axon growth. In order to analyze the features of this time-series data it must first be divided into distinct periods of time. For example, the data can be divided into an arbitrary number of periods of equal duration (Gonzalez-Billault et al., 2002), or alternatively, divided into two periods that represent the time before and after a treatment (Mendez-Otero and Friedman, 1996) depending on what feature of elongation dynamics is being investigated. Since alternation between periods of sustained elongation and periods of quiescence or retraction is the defining feature of saltatory axonal growth, directly analyzing saltatory elongation requires first parsing the data with respect to the transitions between these states.
Ruthel and Hollenbeck (2000) presented simple criteria for analyzing saltatory growth of axon branches in order to study growth competition between ‘sibling’ branches. Periods of sustained growth were identified as periods with an average elongation rate >7 μm/h for at least 50 min until a break in trend (average elongation at a rate below that) lasting 30 min. or more. Generalized, these criteria can provide the framework for parsing any elongation time-series data by identifying points of transition in growth behavior.
We developed quantitative algorithms that parse time-series data into periods of ‘growth’ and ‘nongrowth’ by applying a modified version of the criteria presented by Ruthel and Hollenbeck (2000) and other optional criteria, such as minimum net elongation. These algorithms were implemented in a novel software tool, NeuroRhythmics©, which assists specifically with the analysis of time-lapse length measurements from neuronal processes that exhibit saltatory elongation. To ensure that it could be used to analyze process elongation data from a variety of paradigms, algorithms in NeuroRhythmics© depend on a number of flexible parameters that are easily defined and stored in the user interface. NeuroRhythmics© also includes convenient statistical, graphing, and export options.
2. Materials and Methods
2.1. Programming
NeuroRhythmics© was developed using Microsoft Visual Basic and has been run under Microsoft Windows 98, NT, 2000, and XP and NT. It requires Microsoft Excel 97 or later for data import and export. Technical information, the executable program and instructions for its use are available for download as Supplementary Material. Also available as Supplementary Material is a macro, Process Tracker, that assists with the manual collection of axon-length measurements and exports them to Microsoft Excel (Redmond, WA).
2.2. Neuron cultures
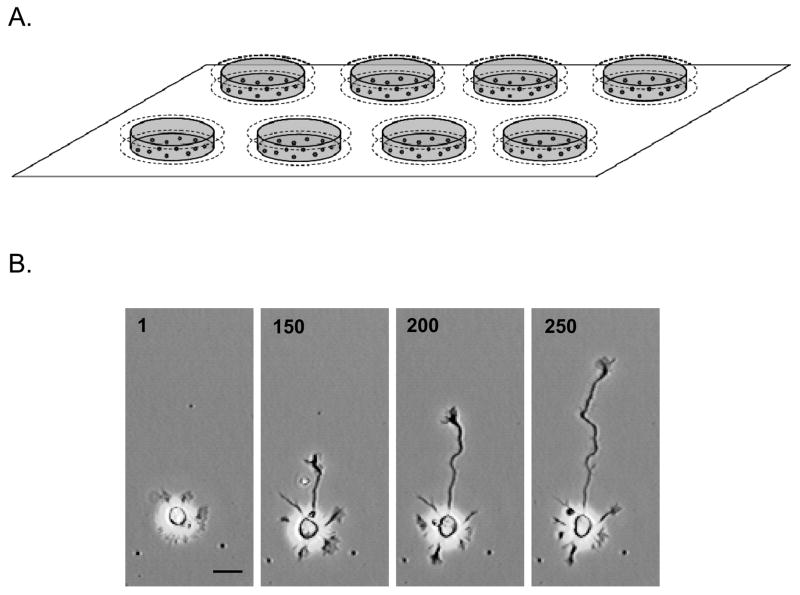
The examples of axon growth data shown in this manuscript are from low-density hippocampal pyramidal neuron cultures, prepared from hippocampi of embryonic Sprague-Dawley rats (Taconic Farms) at gestational day 19, essentially as described by Kaech and Banker (2006). Briefly, hippocampi were dissected from the cerebral hemispheres, cleaned of meninges, then dissociated by treatment with trypsin (0.25% for 15 min at 37°C), and triturated with a fire-polished Pasteur pipette. The cells were plated in Minimal Essential Medium (MEM) with 10% heat-inactivated horse serum at a density of 5650 cells/cm2 into custom wells precoated with poly-D-lysine. Wells were constructed by attaching a 5 mm × 18 mm glass ring (Micro slide rings, Thomas Scientific #6705R24, internal diameter of 15 mm) to a 22 mm glass coverslip using sterile silicone grease. After approximately 3 h the medium was replaced with astrocyte-conditioned MEM with N2 supplements, 0.1 % ovalbumin, 0.1 mM pyruvate, and 10 mM HEPES. Just prior to imaging, the wells were sealed with a sterile 22 mm coverslip and affixed with silicone grease over 16 mm holes in a metal stage insert machined to the specifications of the microscope stage (Fig. 1A). Neurons in these cultures develop a lamella surrounding the cell body shortly after plating, then 3–5 undifferentiated neurites, one of which becomes the axon (Fig. 1B).
Figure 1. Custom wells for automated time-lapse imaging of neuronal development in vitro.
(A) A rectangular stage insert for an inverted microscope holds up to 8 custom wells that are affixed with silicone grease over 16-mm holes in the insert. Each well consists of a bottom, polylysine-coated, glass coverslip plated with the neurons, a glass ring forming a well filled with culture medium, and a top glass coverslip that seals the well. (B) Phase contrast images of a neuron before and during axon outgrowth, selected from a time series of 500 images (image number is indicated). Scale bar = 20 μm.
2.3. Time-lapse Digital Microscopy
Time-lapse imaging of developing neurons was performed with an Olympus IX70 inverted phase contrast microscope (Tokyo, Japan) equipped with a Dage CCD-300 camera (Michigan City, IN), Ludl Motorized Stage System (Hawthorne, NY), and Uniblitz shutter (Vincent Associates). The microscope was contained in an enclosure at 37°C throughout the recording period. The imaging system was controlled by ImagePro Plus Software, including StagePro and ScopePro modules that automate the shutter and stage movements respectively (Media Cybernetics, Inc., Silver Spring, MD). The imaging software maintains images of each field in temporal order for merging into movie clips (avi file format). The user programs the stage positions (corresponding to the fields to be imaged), the number of images to be captured per field and the time interval between image capture. For examples presented here, images were collected every 2.5 min over a period of 24 h, so the time interval between each image was 2.5 min. and the resulting 500 images were merged into the movie. An example of a time-lapse movie showing salutatory axonal growth in low-density embryonic rat hippocampal neuron culture is viewable online at http://www.amc.edu/academic/research/Tlindsley/NewerBrowser.htm.
3. Program Description
3.1 Criteria for detecting growth periodicity
NeuroRhythmics© analyzes a set of process length measurements (non-negative values in microns) collected at a constant time interval and entered into a Microsoft Excel spreadsheet. These data may be obtained by manually tracing the axon tip in a stack of images (see Supplementary material for a macro that assists manual tracing) or more efficiently using software that automates tracking of axon growth, such as Automated Analysis of Axon Growth (A3G) (Keenan et al., 2006). NeuroRhythmics© initially identifies growth periodicity by searching process growth data for periods of time that satisfy a set of criteria specified by the user for growth periods. A fundamental criterion for identifying periods of sustained growth is average growth rate. An ‘average growth rate’ (in μm/hr) requirement must be specified in NeuroRhythmics© and directs it to search for time periods during which the process extends at a rate greater than or equal to that requirement. It should be noted that NeuroRhythmics© calculates the average rate of growth during a period as a running average, and it is the running average that is tested against any rate criterion specified by the user (see section 3.3 below for details).
While ‘average growth rate’ is the only criterion that is absolutely required by NeuroRhythmics© to conduct an analysis, specifying a ‘break in trend’ criterion is normally necessary to obtain sensible results (Fig. 2A,B). ‘Break in trend’ criterion applies the rule that a time period will not be identified as a period of growth if it contains a period during which the rate of growth drops below a specified value (in μm/hr) for a specified minimum amount of time (in min). In order to be logical, the limiting rate specified under ‘break in trend’ must be less than that specified for the ‘average growth rate’ criterion.
Figure 2. The importance of the ‘break in trend’ criterion to the analysis of time-lapse axon length measurements.
Data was collected from a fragment of time-lapse video of a rat hippocampal neuron in culture, analyzed using 3 different criteria settings and shown in plots A-C. Upward arrows represent the start of a growth period (boldface lines) and downward arrows represent the end of a growth period. (A) Results of analysis with a ‘break in trend’ criterion specified. The axon exhibits a period of rapid sustained growth between 18 and 19.5 HIV (31 μm/h) then a period of slight retraction (−6 μm/h for ~3 h), before another period of sustained growth (14 μm/h for ~1.5 h). The following settings were used: ‘average growth rate’ > 7 μm/h; ‘break in trend’ < 3 μm/h for 30 min; ‘duration’ > 50 min; ‘net elongation’ > 10 μm; ‘running average sensitivity’ = 12 min. (B) Results of analysis with the same settings as A, except no ‘break in trend’ criterion was specified. Bursts of growth at the beginning of potential growth periods drive the running average very high. Only after the inclusion of periods of quiescence or retraction does the running average approach the specified limit (7 μm/h) and terminate a growth period. (C) Results of analysis with the same settings as B, but with the special ‘exclude terminal retraction’ setting enabled. Periods of growth are not allowed to contain a final period of retraction. Note that after this "trimming" a period of growth must still meet all other criterion, such as ‘duration’ and ‘net elongation’. This option is normally not necessary if an appropriate ‘break in trend’ setting is specified as in graph A.
‘Duration’ and ‘net elongation’ criteria are both optional. The ‘duration’ criterion requires that the duration of a growth period be greater than or equal to the specified value (in min). The ‘net elongation’ criterion requires that the net change in length over the growth period be greater than or equal to the specified value (in μm).
3.2 Refinement of data analysis
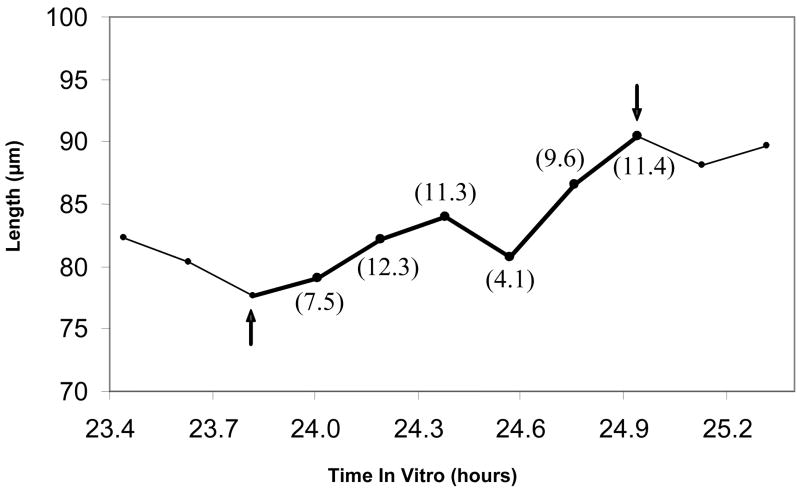
As mentioned above, any rate requirements specified in NeuroRhythmics© are compared against a running average growth rate at each measurement point of a time period. For example, suppose a 60 min period contains 7 points, endpoints included. First, the rate of growth between the initial 2 points (10 min) would be compared against the rate requirement. Then, the rate of growth between points 1 and 3 (20 min) would be compared against the rate requirement, and so on until the final comparison of the rate of growth between points 1 and 7 against the requirement. By default, if at any point during this procedure the average is less than the rate requirement, the time period under analysis is disqualified as a period of growth. The ‘running average sensitivity’ settings in NeuroRhythmics© can modify how strictly this is applied. It allows the running average rate of growth to temporarily violate the rate requirement, as long as it subsequently conforms to the rate requirement within a specified period of time (Fig. 3). Increasing this setting decreases the sensitivity of NeuroRhythmics© to brief fluctuations in the growth rate or “noise” in the data due to slight errors of measurement. The sensitivity of the ‘average growth rate’ and ‘break in trend’ rate requirements can be specified individually.
Figure 3. The ‘running average sensitivity’ setting modifies how strictly rate requirements are applied.
Values in parentheses are the running average rate of growth (in μm/h) from the starting point of 23.8 h. An axon exhibits a period of growth between 23.8 and 24.4 h in vitro that is above 7 μm/h. The axon length then briefly fluctuates, rapidly retracting and then rapidly elongating. The running average rate of growth drops below 7 μm/h for at least 10 min, then immediately returns to a rate above 7 μm/h. Using the following settings, NeuroRhythmics would identify this as a period of growth only if the ‘running average sensitivity’ was set to allow the average growth rate to drop below the rate requirement for at least 10 min : ‘average growth rate’ > 7 μm/h; ‘break in trend’ < 3 μm/h for 30 min; ‘duration’ > 50 min; ‘net elongation’ > 10 μm.
NeuroRhythmics© contains another option to deal with “noise” in growth data that may be used as an alternative to manipulating ‘running average sensitivity’ or in conjunction with it. The ‘smooth data’ option executes a routine that transforms each raw measurement into the average of itself and the two measurements adjacent to it. ‘Smooth data’ by order n, specifies that this transformation should be repeated n times before the data is analyzed. While this is a simple and powerful method to decease noise in the analysis, we generally find that adjusting the ‘running average sensitivity’ to be the more precise method. Analysis utilizing the ‘smooth data’ option can displace the exact time of a dramatic change in growth dynamics. This could be especially problematic if the results of the analysis are being compared to the timing of experimental manipulations.
If the ‘break in trend’ criterion is not specified, or permits especially long periods of slow growth, it is possible that a period of growth identified by NeuroRhythmics© could include a terminal period of retraction, during which the running average gradually dropped below the ‘average growth rate’ requirement. The ‘exclude terminal retraction” option specifies that no period of growth will contain a terminal period of retraction (Fig. 2C).
3.3 Display and output functions
Once an analysis has been completed, NeuroRhythmics© displays a window containing information on each “growth period” and “nongrowth period” (periods of time bounded by periods of growth or the bounds of the recording period), including the start point, end point, duration, and growth rate of the period. The window also contains an array of summary information (Table I).
Table I. NeuroRhythmics analysis of data from time-lapse imaging of the axon of a hippocampal neuron for ~23 hours.
‘Start Point’ and ‘End Point’ values reference the positions of measurements in the ordered time-series. Growth periods and nongrowth periods are numbered in chronological order of their occurrence. Information about the time-series irrespective of growth state is provided under the heading ‘Overall’. Information about the time-series after separation into all growth and all nongrowth periods is provided under the heading ‘Summary’. ‘Average Duration’ (Σdurations/total periods) and ‘Average Rate’ (Σrates/total periods) provide information about the typical growth or nongrowth period in this time series. ‘Combined Duration’ (Σdurations) and ‘Combined Rate’ (Σ(rates * durations)/Σdurations) provide information about the total amount of time that was spent in each state and the duration-weighted average rate of growth during each state, respectively. For this example axon, elongation rate during growth periods was about 30 μm/h, regardless of growth period duration. While the axon spent only ~25% of the time-lapse in this growth state, elongation during these periods can account for the entire increase in length that occurred over the 23 hour imaging session.
| Growth: | Period | Start Point | End Point | Duration (min) | Avg Rate (μm/h) |
|---|---|---|---|---|---|
| 1 | 10 | 16 | 67.5 | 30.08 | |
| 2 | 47 | 55 | 90 | 31.01 | |
| 3 | 109 | 125 | 180 | 28.18 | |
| NonGrowth: | Period | Start Point | End Point | Duration (min) | Avg Rate (μm/h) |
| 1 | 1 | 10 | 101.25 | 3.51 | |
| 2 | 16 | 47 | 348.75 | −3.37 | |
| 3 | 55 | 109 | 607.5 | 0.10 | |
| Summary: | Growth | NonGrowth | Overall: | ||
| # of Periods | 3 | 3 | Duration (min) | 1395 | |
| Average Duration (min) | 112.50 | 352.50 | Change in Length (μm) | 152.3 | |
| Average Rate (μm/h) | 29.76 | 0.08 | Rate (μm/h) | 6.55 | |
| Combined Duration (min) | 337.5 | 1057.5 | |||
| Combined Rate (μm/h) | 29.31 | −0.72 | |||
| % Total Time | 24.2 | 75.8 | |||
| Change in Length (μm) | 164.9 | −12.6 | |||
| % Total Length Change | 108.3 | −8.3 | |||
The “ignore first nongrowth period” option excludes data from the first “nongrowth period” from the displayed results and calculations that derive the summary information. This option is included as a convenience for experiments where process length was recorded for substantial period of time before the onset of growth alternation. The option does not affect the fundamental identification process, only the final displayed information.
NeuroRhythmics© also contains functions to export the analysis results to Excel, highlight the measurement data in Excel that correspond to points within a period of growth, and to highlight the corresponding segments on an Excel graph of process length versus time.
4. Discussion
Time-lapse video recordings of axons, in vitro and in vivo, have demonstrated that they exhibit saltatory growth throughout many different cell types and developmental stages (Katz et al., 1984). The dynamics of neuronal process elongation, or alternating nature of growth states, is thought to be an intrinsic property that can be modulated by various extrinsic conditions. The absence of analytic tools that can quantify these different growth states has led many investigators to measure net growth, or to limit their analyses to processes undergoing active extension, which likely obscures important mechanistic insights regarding intracellular events regulating process growth and guidance. NeuroRhythmics© is designed to investigate the details of this growth pattern in relation to the normal events of axon development or in response to extracellular, molecular, or pharmacological manipulations.
A number of approaches have previously been employed to analyze axon growth dynamics. A fundamental issue is how to parse the data for analysis. One common approach is to simply divide a long recording into arbitrary periods of equal duration and then assess saltatory elongation qualitatively or by comparing the periods that exhibit the minimum and maximum rate of growth. The time periods selected for such analysis have ranged from 10 min (Katz et al., 1984) to 1 hour (Dotti et al., 1988). Saltatory axonal elongation has also been detected in time-lapse images by binning each change in length measurement into categories based on whether the growth cone advanced, retracted, or remained in the same position compared to the previous image (Halloran and Kalil, 1994; Walker et al., 2001). Yet another approach parses elongation data with respect to a secondary phenomenon that appears to be associated with changes in growth state, such as stereotyped changes in growth cone morphology (Kaethner and Stuermer, 1992).
While the above methods of analysis may provide sufficient information regarding a particular experiment, the results of the analysis will be difficult to compare to other studies. Technical differences, such as sampling rate or spatial resolution of time-lapse imaging, change how saltatory elongation is identified by the above methods. For example, when categorized by instantaneous change in position, the same axon imaged with a higher temporal resolution but lower spatial resolution would appear to spend more time “remaining in the same position” than with a lower temporal resolution and higher spatial resolution. Methods of analysis based on secondary phenomenon also fail to be applicable to all cell types and experimental conditions. For example, while growth cone morphology correlates well with states of elongation or quiescence in developing zebrafish retinotectal axons (Kaethner and Stuermer, 1992), hippocampal axons exhibit clear saltatory elongation even when growth cones are abolished by cytochalasin E treatment (Ruthel and Hollenbeck, 2000).
NeuroRhythmics© utilizes only time-series length data in its analysis, and thus its analysis is independent of particular experimental conditions. While time-lapse resolution may affect the precision of results generated by NeuroRhythmics, the criteria it applies to identify saltatory elongation are not directly altered. By using standardized criteria with easily modifiable parameters, NeuroRhythmics© is amenable to the study of any process that exhibits saltatory elongation, regardless of cell type or developmental stage, and analyses from processes that exhibit dramatically different dynamics can still be compared quantitatively.
NeuroRhythmics’ approach to analysis also helps distinguish biologically significant changes in saltatory elongation from “noise” that is the result of the experimental methods or is intrinsic to the growth process. Developing axons frequently undergo rapid fluctuations that result in no net change in length, a phenomenon that appears to be stochastic (Katz et al., 1984). Therefore, results from the analyses mentioned above that simply compare short term rates in isolation may reflect these stochastic fluctuations rather than significant features of saltatory elongation. NeuroRhythmics©, on the other hand, has criteria to specifically distinguish sustained periods of growth that result in sustained net elongation. It also contains convenient options to modify the stringency with which it applies these criteria so it can be easily adapted to the “noisiness” of the data.
By parsing axon elongation data with respect to transitions between the two states of saltatory elongation, NeuroRhythmics© can enable the detection of specific changes of growth pattern in response to various experimental manipulations. Recent experiments demonstrate that molecular and pharmacological treatments can selectively alter aspects of axon growth dynamics, such as the relative durations of growth and nongrowth periods (Walker et al., 2001; Walz 2002) or the degree of retraction (Ruthel and Banker, 1999). Such effects are difficult to detect from measurements of the overall axon growth rate, due to large variability or simultaneous changes in multiple aspects of the pattern. For example, some studies have reported that ethanol treatment enhances neurite growth on polylysine substrates (Zou et al., 1993) whereas others describe inhibitory effects when neurons grow on L1, but not on N-cadherin or laminin (Bearer et al., 1999), raising the possibility that the substrate may influence ethanol effects on neurite growth. NeuroRhythmics© analysis of ethanol effects on axons elongating on polylysine revealed that ethanol delays axon specification and the onset of salutatory elongation (an inhibitory action), but retractions during nongrowth periods were shorter, which resulted in longer axons at 24 h (Lindsley et al, 2003). These data illustrate how the timing of analysis may influence conclusions regarding overall rate of growth, and how NeuroRhythmics© could be used to directly test the hypothesis that substrate influences effects of ethanol on neurite outgrowth.
NeuroRhythmics© is currently the only tool available that simultaneously provides detailed information on the characteristics of each period that was delimited by transitions in elongation state (i.e. initiation time, average rate, duration) and information regarding the overall pattern of saltatory growth (i.e. time of pattern onset, frequency of transitions, relative time spent in a state of growth vs. nongrowth). Quantitative methods to analyze saltatory growth have been designed for use in other areas of biology, from tomato leaf elongation (Price et al., 2001) to the daily growth of human infants (Lampl et al., 2001), but these analyses depend on complex model fitting or transformations of the data. NeuroRhythmics© analyzes saltatory growth by directly comparing series of length measurements against simple criteria, such that the results of its analysis can be easily related back to an original plot of the axon length vs. time.
There are a number of potential applications for NeuroRhythmics© outside studying axon growth. Early dendrite elongation is saltatory (Ruthel and Banker, 1999), but little attention has been given to cellular mechanisms related to this pattern of growth or its potential disruption in disease. NeuroRhythmics© could also be used to analyze saltatory movement, by collecting measurements of the net translocation of a moving object. For example, migrating neuronal precursors in developing cortex (Leavitt et al., 1999) and granule cells in cerebellum (Komuro and Rakic, 1998) exhibit periods of rapid cell body translocation, interspersed with stationary periods. Theoretically, NeuroRhythmics© could be used to analyze any one-dimensional time-series that exhibits saltatory dynamics.
Supplementary Material
Acknowledgments
The authors wish to thank Ms. Lisa Rising for excellent technical assistance and Gary Banker, Ph.D. for helpful discussions related to quantification of axon behavior. This research was supported by NIAAA R01AA11416 (to TAL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aletta JM, Greene LA. Growth cone configuration and advance: a time-lapse study using video-enhanced differential interference contrast microscopy. J Neurosci. 1988;8:1425–35. doi: 10.1523/JNEUROSCI.08-04-01425.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiro V, Bunge MB, Johnson MI. Correlation between growth form and their dependence on neuronal age. J Neurosci. 1984;4:3051–62. doi: 10.1523/JNEUROSCI.04-12-03051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer CF, Swick AR, O’Riordan M, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–70. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker GA. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–68. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Banker GA. The economics of neurite outgrowth – the addition of new membrane to growing axons. Trends Neurosci. 1996;19:144–9. doi: 10.1016/s0166-2236(96)80025-7. [DOI] [PubMed] [Google Scholar]
- Godement P, Wang LC, Mason CA. Retinal axon divergence in the optic chiasm: dynamics of growth cone behavior at the midline. J Neurosci. 1994;14:7024–39. doi: 10.1523/JNEUROSCI.14-11-07024.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Billault C, Owen R, Gordon-Weeks PR, Avila J. Microtubule-associated protein 1B is involved in the intial stages of axonogenesis in peripheral nervous system cultured neurons. Brain Res. 2002;943:56–67. doi: 10.1016/s0006-8993(02)02534-9. [DOI] [PubMed] [Google Scholar]
- Halloran MC, Kalil K. Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci. 1994;14:2161–77. doi: 10.1523/JNEUROSCI.14-04-02161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nature Protocols. 2006;1:2406–15. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kaethner RJ, Stuermer CA. Dynamics of terminal arbor formation and target approach of retinotectal axons in living zebrafish embryos: a time-lapse study of single axons. J Neurosci. 1992;12:3257–71. doi: 10.1523/JNEUROSCI.12-08-03257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ, George EB, Gilbert LJ. Axonal elongation as a stochastic walk. Cell Motil. 1984;4:351–70. doi: 10.1002/cm.970040505. [DOI] [PubMed] [Google Scholar]
- Keenan TM, Hooker A, Spilker ME, Li N, Boggy GJ, Vicini P, Folch A. Automated identification of axonal growth cones in time-lapse image sequences. J Neurosci Meth. 2006;151:232–38. doi: 10.1016/j.jneumeth.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J. Neurobiol. 1998;37:110–30. [PubMed] [Google Scholar]
- Lampl M, Johnson ML, Frongillo EA., Jr Mixed distribution analysis identifies saltation and stasis growth. Ann Hum Biol. 2001;28:403–11. doi: 10.1080/03014460010016662. [DOI] [PubMed] [Google Scholar]
- Leavitt BR, Hernit-Grant CS, Macklis JD. Mature astrocytes transform into transitional radial glia within adult mouse neocortex that supports directed migration of transplanted immature neurons. Exp Neurol. 1999;157:43–57. doi: 10.1006/exnr.1999.6982. [DOI] [PubMed] [Google Scholar]
- Lindsley TA, Kerlin AM, Rising LJ. Time-lapse analysis of ethanol’s effects on axon growth in vitro. Dev Brain Res. 2003;147:191–9. doi: 10.1016/j.devbrainres.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Mendez-Otero R, Friedman JE. Role of acetylated gangliosides on neurite extension. Eur J Cell Biol. 1996;71:192–8. [PubMed] [Google Scholar]
- Price LE, Bacon MA, Young PC, Davies WJ. High-resolution analysis of tomato leaf elongation: the application of novel time-series analysis techniques. J Exp Bot. 2001;52:1925–32. doi: 10.1093/jexbot/52.362.1925. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Banker G. Role of moving growth cone-like “wave” structures in the outgrowth of cultured hippocampal axons and dendrites. J Neurobiol. 1999;39:97–106. doi: 10.1002/(sici)1097-4695(199904)39:1<97::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Ruthel G, Hollenbeck PJ. Growth cones are not required for initial establishment of polarity or differential axon branch growth in cultured hippocampal neurons. J Neurosci. 2000;20:2266–74. doi: 10.1523/JNEUROSCI.20-06-02266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebenyi G, Callaway JL, Dent EW, Kalil K. Interstitial branches develop from active regions of the axon demarcated by the primary growth cone during pausing behaviors. J Neurosci. 1998;18:7930–40. doi: 10.1523/JNEUROSCI.18-19-07930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KL, Yoo HK, Undamatla J, Szaro BG. Loss of neurofilaments alters axonal growth dynamics. J Neurosci. 2001;21:9655–66. doi: 10.1523/JNEUROSCI.21-24-09655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A, Anderson RB, Irie A, Chien C, Holt CE. Chondroitin sulfate disrupts axon pathfinding in the optic tract and alters growth cone dynamics. J Neurobiol. 2002;53:330–42. doi: 10.1002/neu.10113. [DOI] [PubMed] [Google Scholar]
- Zou J, Rabin RA, Pentney RJ. Ethanol enhances neurite outgrowth in primary cultures of rat cerebellar macroneurons. Brain Res Dev Brain Res. 1993;72:75–84. doi: 10.1016/0165-3806(93)90161-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.