Abstract
A high throughput screening protocol is proposed for chiral selector discovery. It is modeled after the protocol for biological screening of candidate drugs from chemical libraries. The procecure works based on target distribution between an aqueous phase and an organic phase. The target may be a racemate or separate enantiomers. Screening for noncovalent intermolecular association between target and candidate selectors is carried out by partitioning experiments in the presence and absence of the candidate chiral selectors in the organic phase (plasticized poly(vinylchloride)).The partition ratio measurement uses 96-well plates for high throughput. The feasibility of this approach is validated by working with a known target/chiral selector pair, N-(3,5-dinitrobenzoyl)-α-phenylglycine and 2,2,2-trifluoro-1-(9-anthryl)ethanol. The validated protocol is applied to a small library of 12 cyclopropyl dipeptide isosteres. Eight bind the racemic target, econazole. Among them, one has measurable chiral selectivity. The advantage of the method is that it does not require the covalent attachment of either the analyte or the selector, and the required amount of the potential chiral selector is about 100 micrograms.
The most widely used technique for chiral separations at the analytical and semipreparative scale is liquid chromatography (LC) on chiral stationary phases (CSPs)1. Numerous CSPs exist1,2. The desire for more generality, better selectivity, more robustness and predictability drives the search for new CSPs3. While understanding the mechanism of chiral selectivity will certainly advance the search4 for better selectors, screening of libraries is showing promise for CSP discovery5. We note that all the current screening methods require the immobilization of either the target or the selector to a stationary phase; some of them also require packing and using the CSP in a column. These steps require time, labor and material. It would be extremely useful to have a library screening protocol for chiral selector discovery that would function within the standard regimen for biological screening of combinatorial libraries. That means using sub-mg quantities of candidate selectors in DMSO solution in 96- or 384-well microtiter plates.
Here we introduce such a method based on target distribution between an aqueous phase and an organic (film) phase in a microtiter plate6, 7. Partitioning experiments are performed in the presence and the absence of a candidate selector in the organic phase. The difference in the observed distribution of the target reports on the binding of the target to the selector. Since ordinary organic solvents are difficult to work with, especially at low volume, we prefer thin polymer films as the organic phase. Plasticized polyvinyl chloride (PVC) films have been used to study molecular recognition8, so it is naturally a good choice for chiral recognition. Figure 1 gives the sequence of operations for the screening procedure. The films are 50:50 (w/w) dioctyl sebacate (DOS) and PVC and occupy about 5.0 μL. The aqueous phase contains target initially. Alternatively, target and selector may be combined in the film phase initially.
Figure 1.
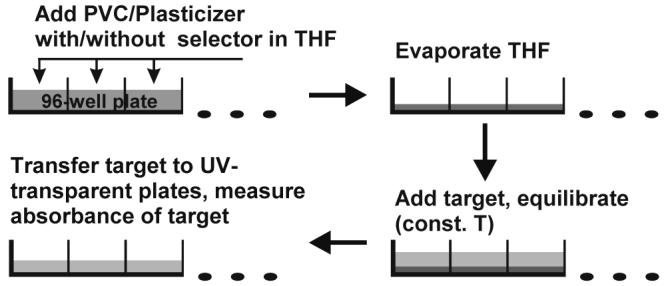
Screening protocol
As a validation of our method prior to application (for detailed protocol, see SI), we consider a known chiral selector and target. N-(3,5-dinitrobenzoyl)-phenylglycine (DNBPG, 1), when attached to a stationary support, has a selectivity (α) of 1.3 ∼ 1.69-10 to 2,2,2-trifluoro-1-(9-anthryl)ethanol (TFAE, 2). We define  TFAE as the selector, and observe the release of DNBPG from the PVC/DOS film to the aqueous buffer solution, with or without the selector in the film. Kinetic studies show that the target distribution needs 5 hours to reach equilibrium with the microplate in a shaker (500 rpm, 25 °C). Figure 2 shows the dependence of the concentration of target in the aqueous phase (10 mM HCl) at equilibrium on the selector concentration in the PVC/DOS film.
TFAE as the selector, and observe the release of DNBPG from the PVC/DOS film to the aqueous buffer solution, with or without the selector in the film. Kinetic studies show that the target distribution needs 5 hours to reach equilibrium with the microplate in a shaker (500 rpm, 25 °C). Figure 2 shows the dependence of the concentration of target in the aqueous phase (10 mM HCl) at equilibrium on the selector concentration in the PVC/DOS film.
Figure 2.

Effect of TFAE concentration on DNBPG (7.2 mM) release from the PVC/DOS film (5.0 μL) to the 10 mM HCl solution (200 μL). Error bars are the standard error of the mean (16 repeats).
Both selectors hindered the release of target in a concentration-dependent manner. At all selector concentrations, compared with (R)-TFAE, the (S)-TFAE kept more (R)-DNBPG in the film phase; when (S)-DNBPG was the target, the opposite held true. The higher the selector concentration, the greater was the difference. Since a relatively low selector concentration minimizes the effect of TFAE self-association in the film (if it occurs), we determined Kf, (the formation constant for the (+)- and (−)-targets with the selector) and the selectivity, α, at the selector concentration of 36 mM, and Kp (partition ratio) with no selector present. Table 1 shows the results.
Table 1.
Values of Kp for DNBPG going from PVC/DOS (50:50 w/w) film phase to 10 mM HCl phase and its Kf with TFAE in the film
| Kp |
Kf (M−1) |
α | |
|---|---|---|---|
| R-R or S-S | R-S or S-R | ||
| 39.2 ± 0.2 | 5.8 ± 0.6 | 10.5 ± 0.6 | 1.8 ± 0.2 |
The formation constants are extremely small, yet complex formation influences partitioning. In a case like this, where material supply is not limited, we were able to perform a number of repeat measurements, adding significance to the measured absorbance differences. The value of selectivity is significant, and similar to the value found in chromatography cited above. It is likely that in normal organic solvents, the formation constants would be larger8a, 11. In order for the solute distribution process to reveal binding, the solute partition coefficient (distribution without selector) must be in a certain range (which depends on phase ratio). For accurate calculation of Kf, the selector should not be soluble in water and should have no self-association in film. The effect of partitioning of selector into the aqueous phase is to reduce the sensitivity of the measurement to binding.
We applied this method to screen a small library of potential chiral selectors for econazole (3), an antifungal agent. This small library contains 12 cyclopropyl dipeptide isosteres12. Racemic econazole solutions (120 μM) were prepared in phosphate buffer (25 mM, pH 3.0) and equilibrated with PVC/DOS films (phase ratio, Φ = 40), without selector and with selectors I-XII (structures in Supporting Information). The equilibrium optical absorbances in the aqueous phase for candidate selectors I-XII were compared to the control using the z distribution (Table 2). Eight of the 12 compounds show significant binding to econazole at the 99% confidence level.
Table 2.
Binding of econazole to the potential selectors
| Selector | UV abs.(218nm) | Na | zb | Kf (M−1) |
|---|---|---|---|---|
| No | 0.737 | 24 | ||
| I | 0.724 | 2 | 1.36 | -- |
| II | 0.685 | 2 | 5.43 | 48 ± 9 |
| III | 0.680 | 2 | 5.96 | 53 ± 9 |
| IV | 0.661 | 2 | 7.94 | 73 ± 10 |
| V | 0.741 | 2 | 0.42 | -- |
| VI | 0.724 | 2 | 1.36 | -- |
| VII | 0.677 | 2 | 6.27 | 57 ± 9 |
| VIII | 0.641 | 2 | 10.0 | 96 ± 11 |
| IX | 0.696 | 2 | 4.29 | 38 ± 9 |
| X | 0.502 | 2 | 24.6 | 298 ± 17 |
| XI | 0.676 | 2 | 6.38 | 58 ± 9 |
| XII | 0.714 | 2 | 2.40 | -- |
Number of repeats
Difference in absorbances divided by the error of the difference in absorbances.
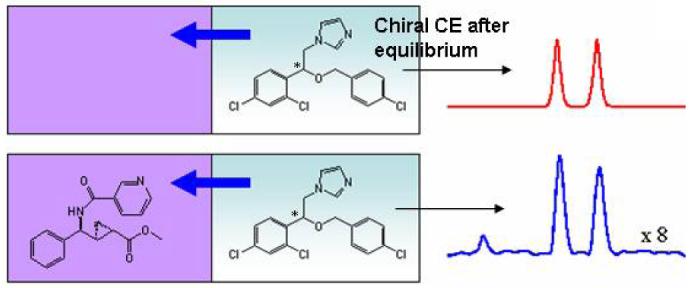
As the racemate was used, chiral capillary electrophoresis (CE) was needed to determine the selectivity of econazole distribution. Among the 8 compounds, only selector X (4) showed measurable enantioselective binding (Table 3).
Table 3.
Peak area ratios from CE of econazole
| Econazole | Area Ratio (Peak 1 / Peak 2) |
SEM | n | |
|---|---|---|---|---|
| Before extraction | 0.980 | 0.002 | 6 | |
| After | No selector | 0.973 | 0.003 | 6 |
| extraction | Selector X | 1.051 | 0.009 | 16 |
As indicated by the Peak 1/Peak 2 area ratio in the chiral CE trace (Supporting Information), selector X binds the two enantiomers of econazole differently. Assuming no selector X was back-extracted to the aqueous phase, the selectivity is calculated to be 1.2. Though the selectivity is too low to use this compound as a chiral selector, it is remarkable that in this small sample we identified a selector.
There is a great deal of flexibility in this system. The sensitivity of the technique can be adjusted. While here we have demonstrated applicability to small values of α, it is also possible to set up a system in which a particular combination of Kf and α is discovered. In this high throughput screening application, only 200 nmol (<100 μg) of each library member (in the second example) was used. Because of the small mass requirements, it opens up huge numbers of compounds as candidate selectors. The same procedures can be used to test one selector vs. many solutes (i.e. generality).
Supplementary Material
Acknowledgment
This project was supported by grants from NIH (P50 GM067082) and the NSF (CHE 0315188)
Footnotes
Supporting Information Available: Protocol for assays, capillary electrophoresis separation of econazole enantiomers, chemical structure of the 12 library members, and a detailed comparison of this screening method to others.
References
- 1.Francotte ER. J. Chromatogr. A. 2001;906:379. doi: 10.1016/s0021-9673(00)00951-1. [DOI] [PubMed] [Google Scholar]
- 2.Pirkle WH, Pochapsky TC. Chem. Rev. 1989;89:347. [Google Scholar]
- 3.Huang J, Zhang P, Chen H, Li T. Anal. Chem. 2005;77:3301. doi: 10.1021/ac050050s. [DOI] [PubMed] [Google Scholar]
- 4.(a) Pirkle WH, Sikkenga DL. J. Org. Chem. 1977;42:1370. [Google Scholar]; (b) Lipkowitz KB, Stoehr CM. chirality. 1996;8:341. [Google Scholar]; (c) Aboul-Enein HY, Ali I. Chiral Separations by Liquid Chromatography and Related Technologies. Marcel Dekker, Inc.; New York: 2003. [Google Scholar]
- 5.(a) Welch CJ, Pollard SD, Mathre DJ, Reider PJ. Org. Lett. 2001;3:95. doi: 10.1021/ol0003329. [DOI] [PubMed] [Google Scholar]; (b) Lewandowski K, Murer P, Svec F, Frechet JMJ. Chem. Commun. 1998;20:2237. [Google Scholar]; (c) Murer P, Lewandowski K, Svec F, Frechet JMJ. Chem. Commun. 1998;20:2559. [Google Scholar]; (d) Chiari M, Desperati V, Manera E, Longhi R. Anal. Chem. 1998;70:4967. doi: 10.1021/ac9806557. [DOI] [PubMed] [Google Scholar]; (e) Weingarten MD, Sekanina K, Still WC. J. Am. Chem. Soc. 1998;120:9112. [Google Scholar]; (f) Wu Y, Wang Y, Yang A, Li T. Anal. Chem. 1999;71:1688. doi: 10.1021/ac9900073. [DOI] [PubMed] [Google Scholar]; (g) Wang Y, Li T. Anal. Chem. 1999;71:4178. doi: 10.1021/ac9905017. [DOI] [PubMed] [Google Scholar]; (h) Bluhm LH, Wang Y, Li T. Anal. Chem. 2000;72:5201. doi: 10.1021/ac000568q. [DOI] [PubMed] [Google Scholar]; (i) Wang Y, Bluhm LH, Li T. Anal. Chem. 2000;72:5459. doi: 10.1021/ac000529e. [DOI] [PubMed] [Google Scholar]
- 6.(a) Kim SB, Cho HC, Cha GS, Nam H. Anal. Chem. 1998;70:4860. doi: 10.1021/ac980322+. [DOI] [PubMed] [Google Scholar]; (b) Dai S, Ye Q, Wang E, Meyerhoff ME. Anal. Chem. 2000;72:3142. doi: 10.1021/ac000060n. [DOI] [PubMed] [Google Scholar]
- 7.Welch CJ, Shaimi M, Biba M, Chilenski JR, Szumigala RH, Jr., Dolling U, Mathre DJ, Reider PJ. J. Sep. Sci. 2002;25:847. [Google Scholar]
- 8.(a) Li S, Sun L, Chung Y, Weber SG. Anal. Chem. 1999;71:2146. doi: 10.1021/ac980587o. [DOI] [PubMed] [Google Scholar]; (b) Zhang X, Zhao H, Weber SG. Anal. Chem. 2002;74:2184. doi: 10.1021/ac0255227. [DOI] [PubMed] [Google Scholar]; (c) Zhang X, Zhao H, Chen Z, Nims R, Weber SG. Anal. Chem. 2003;75:4257. doi: 10.1021/ac0342267. [DOI] [PubMed] [Google Scholar]
- 9.Pirkle WH, Finn JM, Schreiner JL, Hamper BC. J. Am. Chem. Soc. 1981;103:3964. [Google Scholar]
- 10.Malyshev OR, Vinogradov MG. J. Chromatogr. A. 1999;859:143. doi: 10.1016/s0021-9673(99)00871-7. [DOI] [PubMed] [Google Scholar]
- 11.(a) Li S, Weber SG. Anal. Chem. 1997;69:1217. [Google Scholar]; (b) Valenta JN, Sun L, Ren Y, Weber SG. Anal. Chem. 1997;69:3490. doi: 10.1021/ac961279y. [DOI] [PubMed] [Google Scholar]; (c) Sun L, Weber SG. J. Mol. Recognit. 1998;11:28. doi: 10.1002/(SICI)1099-1352(199812)11:1/6<28::AID-JMR385>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Wipf P, Werner S, Woo GHC, Stephenson CRJ, Walczak MAA, Coleman CM, Twining LA. Tetrahedron. 2005;61:11488. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


