Abstract
The acyl amidine represented by the 4,5-dihydro-2(3H)-pyrazinone ring system 2 is isosteric to the vinylogous amide of the 1,2-dihydro-3(6H)-pyridinone 1, but its assembly from separate amine and amide components enables ready incorporation of an amino acid side chain with correct regio- and stereochemistry. β-Strand peptidomimetics incorporating amino acid analogues based on 2 have recently been shown to be potent, protease-resistant ligands to a PDZ protein-interaction domain. Two routes to the protected dipeptide analogue 3 are described.
Keywords: peptidomimetics, β-strand, amino acid analogues, heterocycle synthesis
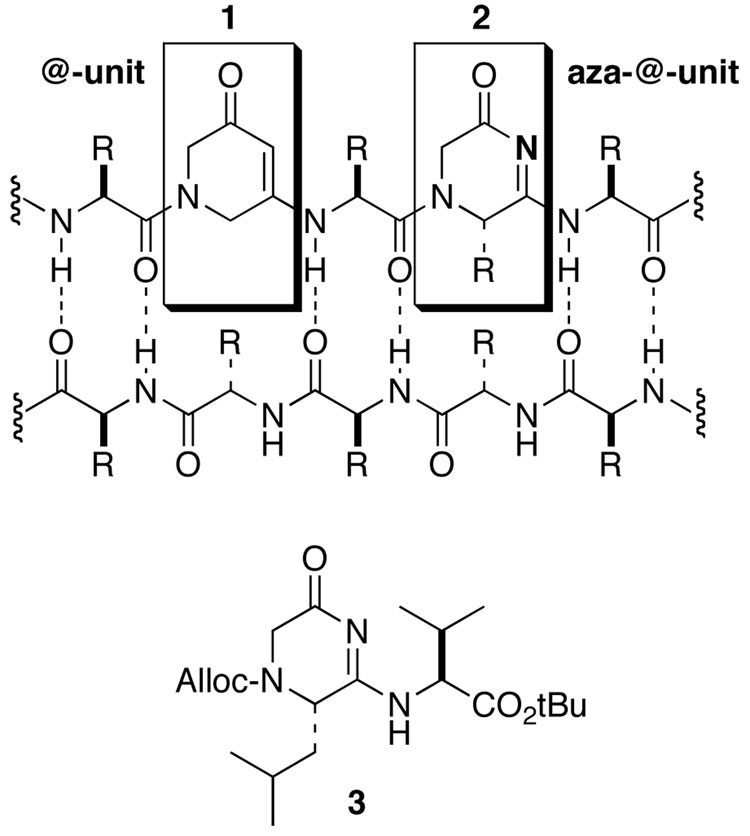
The class of protein-protein interactions mediated by β-strand association offers many opportunities for therapeutic intervention, including an entire panel of neurodegenerative diseases that so far have no preventative treatment or cure.1,2 With the goal of developing a general strategy for disrupting or modeling β-strand interactions, we have designed modular peptidomimetics, nicknamed "@-tides", based on the dihydropyridinone 1.3,4 These peptidomimetics utilize cyclic amino acid analogues that stabilize the extended conformation and maintain the canonical hydrogen bonding pattern of a β-strand along one edge (Fig. 1). Recently, we demonstrated that substituted @-tides, peptidomimetics that incorporate side chain-substituted modules 2, serve as potent ligands to a PDZ protein-interaction domain.5 Here we describe the synthesis of protected dipeptide analogue 3, abbreviated Alloc-@Leu-Val-OtBu, which addresses two crucial points early in the construction of these peptidomimetics: assembly of the 4,5-dihydro-2(3H)-pyrazinone ring system 2 and condensation with the preceding C-terminal amino acid.
FIGURE 1.
Cyclic β-strand peptidomimetic modules based on the dihydropyridinone ("@-unit", 1) and dihydropyrazinone ("aza-@-unit", 2) ring systems. Target dipeptide analogue 3.
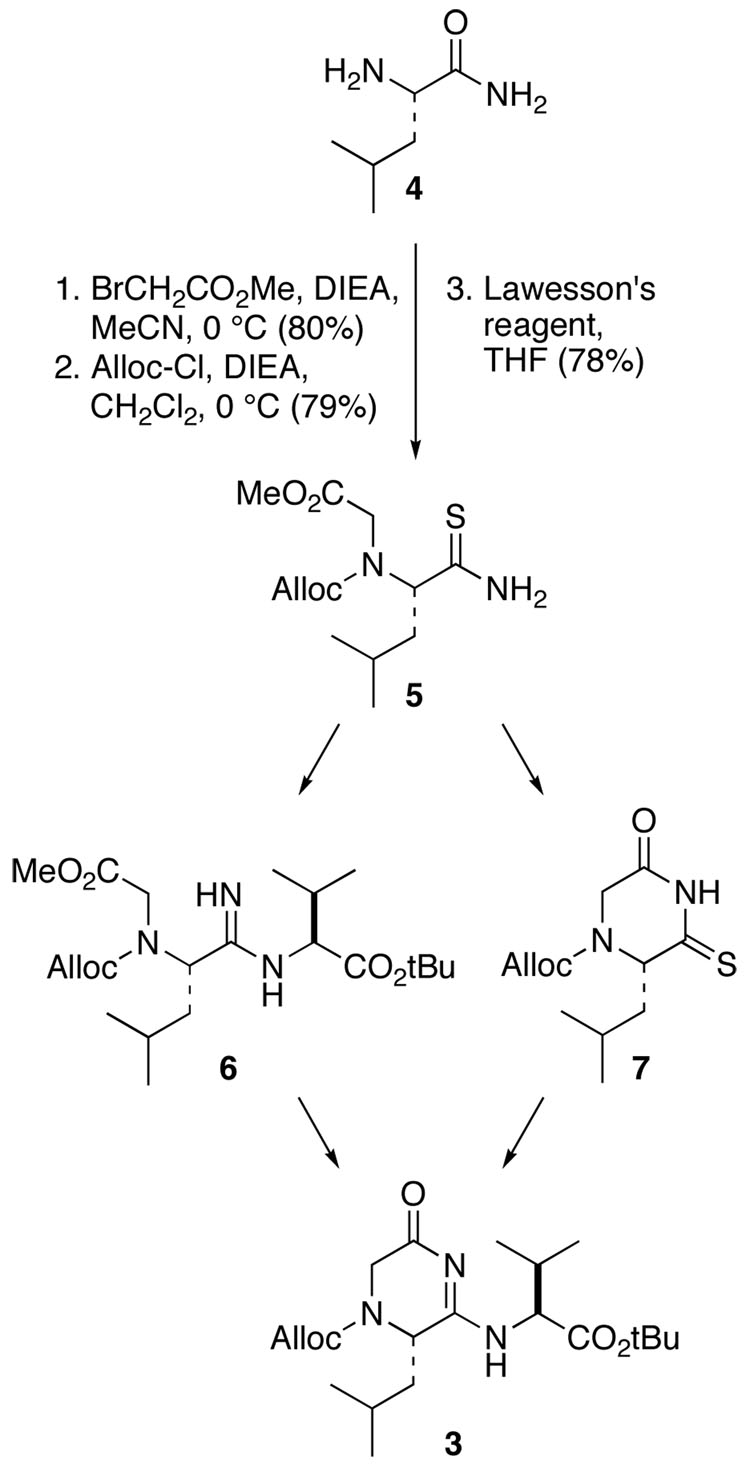
We evaluated two possible routes to 3, each starting from the commercially available L-leucine amide but differing in the order in which the amidine functionality is generated and the ring is formed (Scheme 1). In both sequences, the amidine is constructed via condensation of valine t-butyl ester with thiocarbonyl precursors that are activated by S-methylation; thioamide 5 is a common intermediate in both routes. The greater reactivity of primary amides toward thionation by Lawesson’s reagent (2,4-bis(4-methoxyphenyl)-1,3-dithia-2,4-diphosphetane-2,4-disulfide) suggested that thioamide 5 could be formed without interference from ester or carbamate protecting groups.6 Accordingly, leucine amide 4 was alkylated with methyl bromoacetate and DIEA and then protected as the alloc derivative (Scheme 1). Thionation with 0.7 equiv of Lawesson’s reagent in THF at room temperature affords intermediate 5 in 78% yield. Dithionated material was only observed with extended reaction times or elevated temperatures.
SCHEME 1.
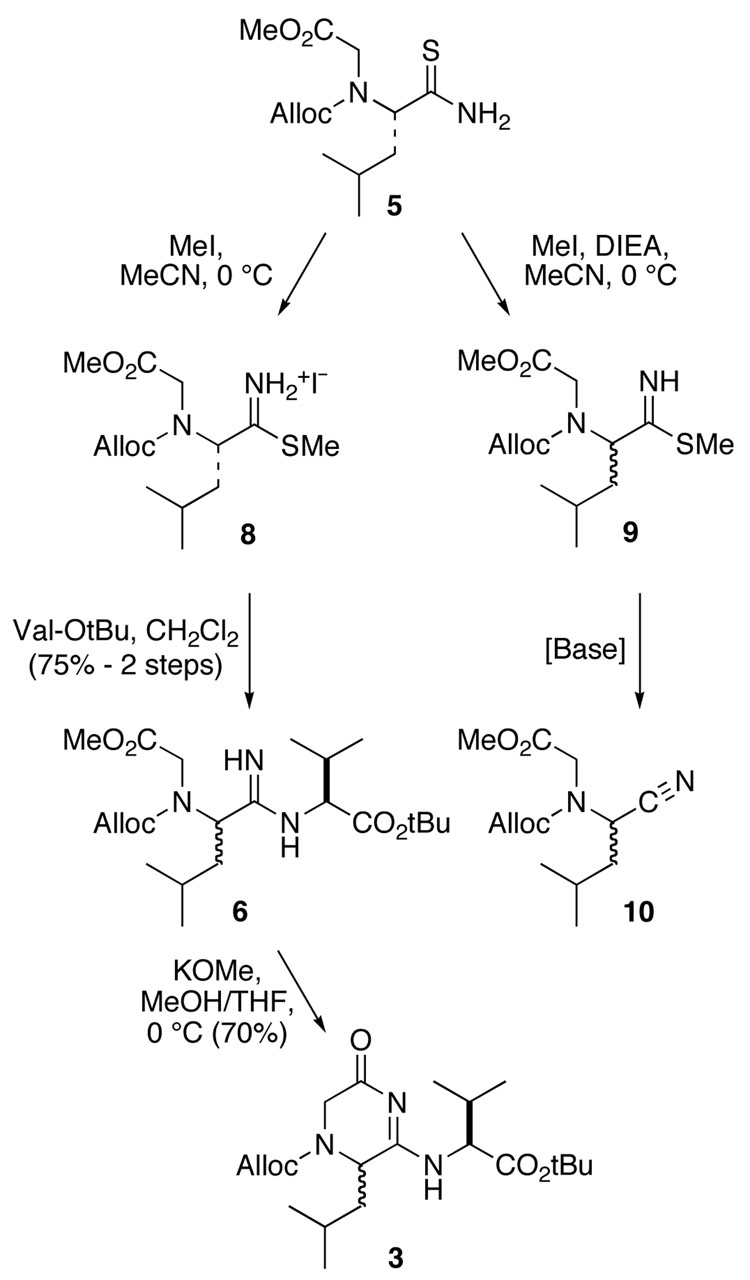
We first explored the “acyclic” route via amidine 6 (Scheme 2). Alkylation of thioamide 5 with excess methyl iodide in MeCN in the absence of base affords the thioimidate salt 8; this material could be coupled with one equiv of valine t-butyl ester, also without added base, to give the acyclic amidine 6 in 75% overall yield. Use of base at either step results in formation of nitrile 10 as the major side product. During the course of the amine coupling, some (~10%) of the product 6 cyclized spontaneously to dihydropyrazinone 3; this conversion also could be accomplished in 70% yield on treatment of the crude amidine 6 with potassium methoxide in a mixture of THF and methanol at 0 °C.
SCHEME 2.
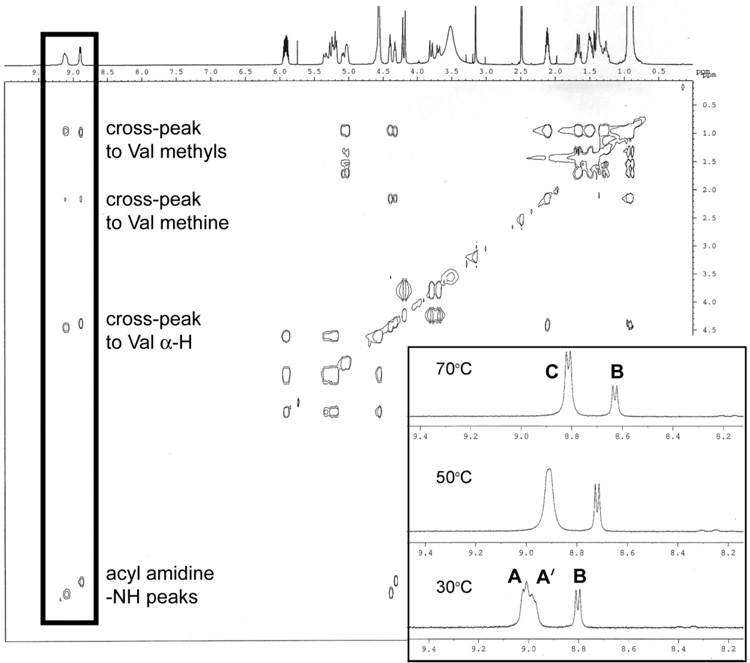
Analysis by 1H-NMR and TOCSY experiments confirmed that cyclization occurs on the less substituted amidine nitrogen to give the desired regioisomer 3, since strong cross-peaks were observed between the NH peaks and other signals in the valine spin system (Fig. 2). However, several acyl amidine NH doublets (signals at 8.7–9.2 ppm) as well as other doubled signals in the 1H-NMR in d-DMSO revealed the presence of multiple isomeric species in the sample which are inseparable by normal or reverse phase chromatography at this stage and have a single product mass (MH+ 410). VT-NMR experiments demonstrated that the two downfield NH signals (A and A') arise from conformational rotamers, since they coalesce to one doublet (C) at 70 °C. However, the upfield NH signal (B) remains unchanged and must therefore represent a diastereomer of 3 (Fig. 2).7 Epimerization of the leucyl α-proton appears to occur during the amine coupling step, since chiral HPLC analysis indicated that unreacted starting material, isolated as thioimidate 9, is almost completely racemized. The polarity of amidine 6 made it difficult to analyze this material directly, but the dihydropyrazinone 3 was obtained with diastereomeric ratios ranging from 2:1 to 1:1 as established by 1H-NMR. A report on the synthesis of cyclic amidine sugars using thioimidates describes a similar problem with epimerization.8 Although the acyclic route provides a method to obtain cyclic acyl amidines in six synthetic steps starting from a commercially available amino amide, the sensitivity of the thioimidate intermediate to epimerization would be problematic for the assembly of longer or multiply substituted β-strand peptidomimetics. Therefore, we turned to the alternative “cyclic” route in our search for a serviceable synthesis of substituted @-tides.
FIGURE 2.
TOCSY and VT-1H-NMR (inset) spectra of cyclic amidine 3 from the "acyclic" route.
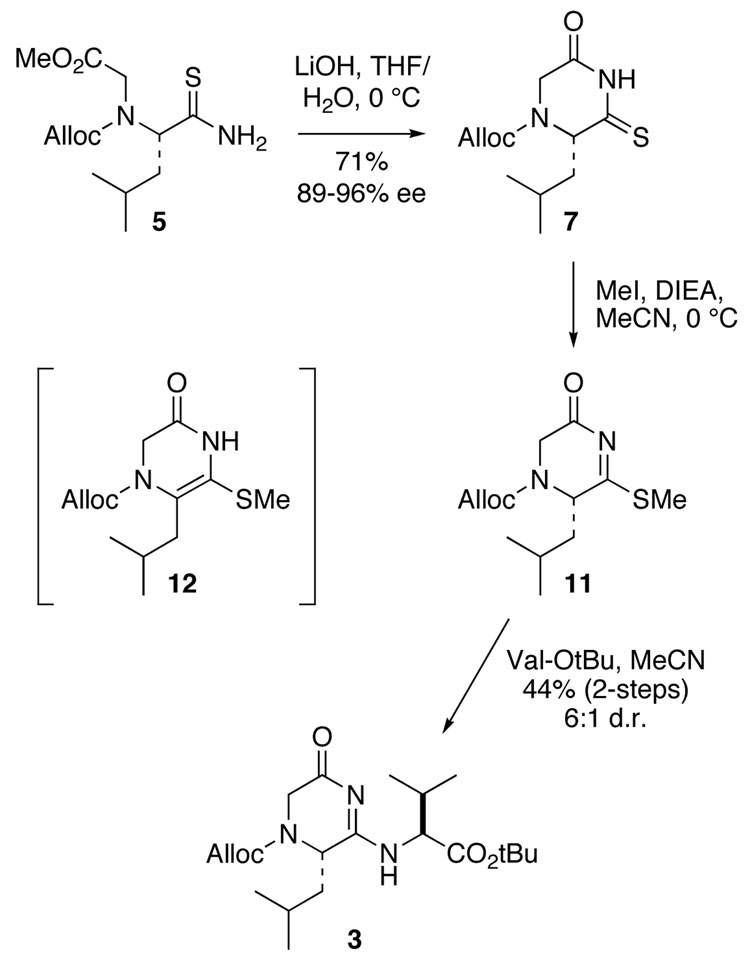
In exploring the alternative sequence, we found that epimerization can be minimized by cyclizing thioamide 5 to monothioimide 7 with LiOH in aqueous THF (Scheme 3). Under these conditions, deprotonation of the thioamide hydrogen is favored kinetically, cyclization occurs rapidly, and ionization of the resulting imide NH prevents product racemization. The thioimide 7 was obtained in 71% yield and greater than 89% enantiomeric excess, as determined by chiral HPLC analysis. In contrast, largely racemized product is obtained if cyclization is induced with a base such as DBU, which allows thermodynamic equilibration. Activation of the thioimide by S-methylation presented an additional complication, since the desired 4,5-dihydro-2(3H)-pyrazinone 11 isomerizes to the achiral 3,4-dihydro-2(1H) tautomer 12 in the presence of base. This side reaction was minimized by carrying out the alkylation with an excess of methyl iodide in MeCN at 0 °C and slow addition of only 1 equiv of DIEA. The greater thermodynamic stability of 12 actually proved to be advantageous, since ionization of the α-proton from 11 leads to isomerization to an unreactive species, rather than epimerization. Initial experiments involving reaction of enantio-enriched 11 (92% e.e.) with valine t-butyl ester in MeCN afforded the coupled product 3 as a 6:1 ratio of stereoisomers, to which we assigned the major component (exemplified by NH signal B) as the desired S,S-diastereomer. The anticipated preference for 1,4-over 1,2-addition to the acyl thioimidate, resulting in the desired product 3, was verified by comparison of 1H-NMR spectra with the product from the acyclic route. The slight degradation in stereochemical enrichment during the coupling reaction, from 96:4 for 7 to 82:14 for 3, appears to arise primarily from kinetic resolution rather than epimerization, since synthesis of the coupled product starting with racemic thioimide 7 affords a 2:1 ratio favoring the undesired R,S-diastereomer.
SCHEME 3.
In addition to converting some of the starting material 11 to unreactive 12, reaction with the amine nucleophile leads initially to buildup of an intermediate adduct (M+SCH3), as monitored by LCMS. However, this adduct is broken down upon addition of excess base to the reaction mixture, resulting in the formation of the desired product 3 (44% final yield), along with a small amount of material from addition of two valine moieties (M+Val-OtBu). We have since found that use of catalytic amounts (0.05 equiv) of a lanthanide Lewis acid such as ytterbium triflate prevents buildup of the methanethiol adduct and consequently improves both the coupling efficiency and stereoretention for the 1,4-addition reaction dramatically.9 For example, in an optimized procedure using the analogous Cbz-protected thioimide with >99% e.e., the coupling to valine t-butyl ester was performed with almost complete stereoretention (98:2 d.r.) and 77% overall yield over two steps.5
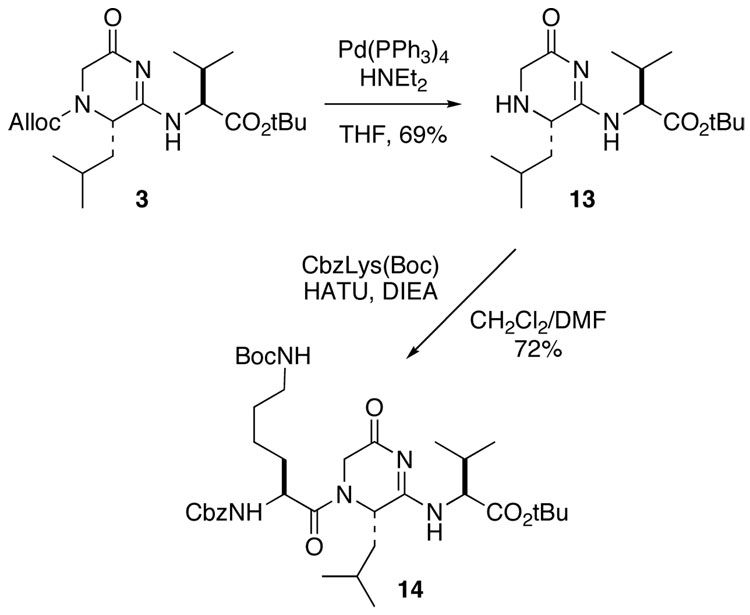
Importantly, no epimerization of the acyl amidine α-stereocenter was observed in subsequent reactions for deprotection and elongation of the substituted @-tide peptidomimetics, including allyl deprotection and amino acid coupling in the presence of excess base (Scheme 4). The diastereomers of the di-@-tide were separated by normal phase chromatography after alloc deprotection. The S,S-diastereomer 13 then was coupled with an N-protected amino acid following the normal protocol to generate 14.
SCHEME 4.
In conclusion, we have developed two concise, six-step routes to the dipeptide analogue 3, one of which preserves the chirality of the L-leucine amide starting material. Compound 3 served as the model system to develop the chemistries for construction of the cyclic acyl amidine moiety of our substituted @-tide β-strand peptidomimetic. The generality of the cyclic route detailed here has been further demonstrated by the successful synthesis of a pentapeptide analogue containing 4,5-dihydro-2(3H)-pyrazinone units incorporating both leucine and glutamate side chains.5 Key to the facile adaptation of the synthesis to oligomers of varying sequence and length is the assembly of the cyclic acyl amidine from an amino acid precursor that is commercially available with diverse and appropriately protected side chains.
Experimental Section10
N-Allyloxycarbonyl-N-(2-methoxy-2-oxoethyl)-L-leucyl Methyl Thioimidate Hydroiodide (8)
To 0.1 g (0.33 mmol) of thioamide 5 in 1 mL of dry MeCN under argon atmosphere was added 0.1 mL (1.6 mmol) of methyl iodide. The reaction flask was sealed and the solution was stirred for 7 h, during which time it progressively turned dark yellow. After concentration of the reaction mixture, the crude product was used without further purification. 1H NMR (500 MHz, CDCl3, crude) δ 0.86–0.93 (m, 6), 1.48–1.56 (m, 1), 1.73 (m, 1, J = 4.6, 9.4, 14.0), 2.05 (m, 0.3, J = 3.6, 10.6, 14.3), 2.13 (m, 0.7, J = 3.9, 10.4, 14.4), 2.92 (s, 2), 2.94 (s, 1), 3.74 (s, 3), 4.15–4.29 (m, 2), 4.50 (d, 1.3, J = 5.5), 4.56 (d, 0.7, J = 6.5), 5.14–5.23 (m, 3), 5.75 (m, 0.7, J = 5.4, 10.7, 16.1), 5.8–5.85 (m, 0.3); MS (ES) m/z 317.3 (M+H+).
N-(2RS-(N-Allyloxycarbonyl-N-(2-methoxy-2-oxoethyl)amino)-1-imino-4-methyl)-Lvaline t-Butyl Ester (6)
To a crude product mixture containing thioimidate 8 in dry CH2Cl2 was added roughly 1.1 equiv of valine t-butyl ester in CH2Cl2, and the reaction solution (which turned colorless after a few minutes) was stirred under argon for 24 h. After concentration of the reaction mixture, the product was isolated by flash chromatography (EtOAc/hexanes 1:1 → EtOAc → MeOH/EtOAc 1:9) as a yellow oil (75% over two steps, mixture of diastereomers). During the course of the reaction, some of the amidine product had spontaneously cyclized to 3 (12% yield over two steps). Rf = 0.13 (UV, EtOAc/hexanes 1:1); 1H NMR (500 MHz, (CD3)2SO) δ 0.85–1.0 (m, 12), 1.44 (s, 9), 1.50–1.60 (m, 1), 1.55–1.75 (m, 1), 1.85–1.95 (m, 1), 2.20 (m, 1), 3.70 (s, 3), 4.15–4.40 (m, 3), 4.55 (s, 2), 4.75–4.90 (m, 1), 5.17–5.25 (m, 2), 5.80–5.95 (m, 1), 9.17 (s, 1), 9.48 (s, 1); resonance assignments and correlation to amino acid side chains by TOCSY confirmed Leu, Val; MS (ES) m/z 442.4 (M+H+).
1-Allyloxycarbonyl-2-(S)-isobutyl-5-oxo-3-thionopiperazine (7)
To 0.24 g (0.78 mmol) of thioamide 5 in 16 mL of THF/H2O 3:1 at 0 °C was added 1 M LiOH (0.78 mL, 0.78 mmol) dropwise. Upon addition of base, the reaction mixture immediately turned yellow. The reaction solution was stirred under nitrogen and neutralized after 6 min by addition of solid KHSO4. After concentration of the reaction mixture, the aqueous solution was extracted with EtOAc (2×10 mL). The organic layer was further washed with 1 M KHSO4 (3× 10 mL), saturated NaHCO3 (3× 10 mL), and brine (10 mL), dried over Na2SO4, filtered, and concentrated. No further purification was necessary to afford the cyclic thioimide 7 as a yellow oil (0.15 g, 71% yield, 89% e.e. as determined by chiral HPLC). Rf = 0.6 (UV, EtOAc/hexanes 1:1); retention time (analytical HPLC): 15.8 min; retention time (chiral HPLC, AS column, 90/10 hexanes/isopropanol): 10.9 min (S-isomer), 13.2 (R-isomer); [α]23 D = −102 (c = 1.02, 89% e.e., in CHCl3); 1H NMR (500 MHz, CDCl3) δ 0.92 (d, 3, J = 6.5), 0.94 (d, 3, J = 5.9), 1.58–1.74 (m, 3), 3.6–3.8 (m, 1), 4.58 (m, 2, J = 4.8), 4.8–5.0 (m, 1), 5.18 (dd, 1, J = 1.1, 10.4), 5.26 (dd, 1, J = 1.3, 17.2), 5.2–5.4 (m, 1), 5.8 (m, 1, J = 5.6, 10.8), 10.4 (bs, 1); 13C NMR (125 MHz, CDCl3) δ 21.0, 22.8, 24.6, 41.2, 42.2, 61.1, 67.0, 118.5, 131.6, 154.0, 165.1, 206.5; MS (ES) m/z 271.1 (M+H+); HRMS (FAB) m/z 271.1123 (M+H+ C12H19N2O3S requires 271.1116).
1-Allyloxycarbonyl-6-(S)-isobutyl-5-methylthio-3-oxo-3,6-dihydro-2H-pyrazine (11)
To 0.11 g (0.4 mmol) of cyclic thioimide 7 (92% e.e.) in 3 mL of dry MeCN under argon atmosphere at 0 °C was added 0.14 mL (2.2 mmol) of methyl iodide followed by addition of 65 µL (0.4 mmol) of DIEA. The flask then was sealed and the solution was stirred for 30 min, during which it progressively turned dark yellow. The crude product mixture was concentrated and used without further purification. Rf = 0.52 (UV, EtOAc/hexanes 1:1); 1H NMR (400 MHz, CDCl3) δ 0.98 (m, 6, J = 4.4), 1.4–1.5 (m, 1), 1.6–1.7 (m, 2), 2.51 (s, 3), 3.9–4.0 (m, 1), 4.58–4.63 (m, 1), 4.62 (d, 2, J = 4.8), 4.73–4.74 (m, 0.5), 4.85–4.95 (m, 0.5), 5.26 (d, 1, J = 10.4), 5.32 (d, 1, J = 17.2), 5.92 (m, 1, J = 5.6, 10.8); MS (ES) m/z 285.1 (M+H+).
Alloc-@L,DLeu-Val t-Butyl Ester (3)
Via acyclic route
To 1.3 g (2.9 mmol) of amidine 6 in 120 mL of THF/MeOH 3:1 at 0 °C was added 0.22 g (3.2 mmol) of potassium methoxide. The reaction solution was allowed to stir under nitrogen for 20 min, after which time the solvent was removed and the product was redissolved in 50 mL EtOAc. The organic layer was washed with NaH2PO4 pH 5.0 (1× 50 mL), the acidic aqueous layer was extracted with EtOAc (2× 25 mL), and the combined organic layers were washed with brine (1× 30 mL), dried over Na2SO4, and concentrated. The crude product was purified by flash chromatography (EtOAc/ hexanes 1:2 → 1:1) to afford acyl amidine 3 (0.84 g, 70% yield, 2:1 d.r. of S/R stereoisomers of @Leu).
Via cyclic route
To a crude product mixture containing acyl thioimidate 11 was added roughly 1.1 equiv of valine t-butyl ester in 3 mL of MeCN and the reaction solution was stirred under argon for 48 h, after which time 1 equiv of DIEA was added and the mixture was stirred for an additional 3 d. After concentration, the crude product was purified by flash chromatography (EtOAc/ hexanes 1:3 → 1:2 → 1:1) to afford 3 (44% over two steps, 6:1 d.r.). Rf = 0.28 (UV, EtOAc/hexanes 1:1); IR (film) 1390.6, 1709.8, 2872.8, 2961.1 cm−1; 1H NMR (500 MHz, (CD3)2SO, 1:1.5 d.r. of S/R stereoisomers of @Leu) δ 0.89–0.97 (m, 12), 1.40 (s, 5.5), 1.40 (s, 4.5), 1.23–1.31 (m, 1), 1.46–1.55 (m, 1), 1.67 (dq, 1, J = 4.0, 12.0), 2.12 (m, 1, J = 6.5), 3.70 (d, 0.4, J = 18.5), 3.81 (d, 0.6, J = 18.5), 4.20 (d, 1, J = 18.5), 4.34 (t, 0.4, J = 6.0), 4.41 (t, 0.6, J = 6.5), 5.00–5.11 (m, 1), 5.19–5.37 (m, 2), 5.93 (m, 1, J = 5.0, 10.5), 8.91 (d, 0.4, J = 7.5), 9.11 (d, 0.3, J = 7.0), 9.14 (d, 0.3, J = 8.0); resonance assignments and correlation to amino acid side chains by TOCSY confirmed @Leu, Val; MS (ES) m/z 410.2 (M+H+), 354.3 (M+H+-tBu).
Supplementary Material
General experimental procedures, synthesis of thioamide 5, synthetic procedures and characterization data for compounds 9, 10, 12–14, and NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
This work was supported by a grant from the National Institutes of Health (grant no. GM28965 to P.A.B.) and a predoctoral fellowship to M.C.H. from the Howard Hughes Medical Institute. The Center for New Directions in Organic Synthesis is supported by Bristol-Myer Squibb as a sponsoring member and Novartis Pharma as a supporting member.
References
- 1.Loughlin WA, Tyndall JDA, Glenn MP, Fairlie DP. Chem. Rev. 2004;104:6085–6117. doi: 10.1021/cr040648k. [DOI] [PubMed] [Google Scholar]
- 2.Maitra S, N JS. In: The Amide Linkage: Selected Structural Aspects in Chemistry, Biochemistry, and Materials Science. Greenberg A, Breneman CM, Liebman JF, editors. New York: John Wiley & Sons, Inc; 2000. pp. 495–518. [Google Scholar]
- 3.Phillips ST, Blasdel LK, Bartlett PA. J. Am. Chem. Soc. 2005;127:4193–4198. doi: 10.1021/ja045122w. [DOI] [PubMed] [Google Scholar]
- 4.Phillips ST, Piersanti G, Bartlett PA. Proc. Natl. Acad. Sci. USA. 2005;102:13737–13742. doi: 10.1073/pnas.0506646102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond MC, Harris BZ, Lim WA, Bartlett PA. Chem. Biol. 2006;13:1247–1251. doi: 10.1016/j.chembiol.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Cava MP, Levinson MI. Tetrahedron. 1985;41:5061–5087. [Google Scholar]
- 7.The ease with which the diastereomers could be purified at the dimer stage depends strongly on the particular sequence; for example, the diastereomers of alloc-@Leu-Phe-OMe are readily separable chromatographically.
- 8.Heck MP, Vincent SP, Murray BW, Bellamy F, Wong CH, Mioskowski C. J. Am. Chem. Soc. 2004;126:1971–1979. doi: 10.1021/ja037822r. [DOI] [PubMed] [Google Scholar]
- 9.In coupling reactions with longer substrates, we have observed 1,2-addition to the acyl amidine with concommitant ring opening and formation of the nitrile; this side reaction was also suppressed by Yb(OTf)3
- 10.General experimental methods are described in the Supporting Information
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General experimental procedures, synthesis of thioamide 5, synthetic procedures and characterization data for compounds 9, 10, 12–14, and NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.