Abstract
CD39 can exist in at least two distinct functional states depending on the presence and intact membrane integration of its two transmembrane helices. In native membranes, the transmembrane helices undergo dynamic rotational motions that are required for enzymatic activity and are regulated by substrate binding. In the present study we show that bilayer mechanical properties regulate conversion between the two enzymatic functional states by modulating transmembrane helix dynamics. Alteration of membrane properties by insertion of cone shaped or inverse cone shaped amphiphiles or by cholesterol removal switches CD39 to the same enzymatic state as does removing or solubilizing the transmembrane domains. The same membrane alterations increase the propensity of both transmembrane helices to rotate within the packed structure, resulting in a structure with greater mobility but not an altered primary conformation. Membrane alteration also abolishes the ability of substrate to stabilize the helices in their primary conformation, indicating a loss of coupling between substrate binding and transmembrane helix dynamics. Removal of either transmembrane helix mimics the effect of membrane alteration on the mobility and substrate sensitivity of the remaining helix, suggesting that the ends of the extracellular domain have intrinsic flexibility. We suggest that a mechanical bilayer property, potentially elasticity, regulates CD39 by altering the balance between stability and flexibility of its transmembrane helices and, in turn, of its active site.
Keywords: CD39, NTPDase, transmembrane helices, bilayer, mechanical
Of the various enzymes that process nucleotides at the cell surface and in the lumen of intracellular organelles, the ectonucleoside triphosphate diphosphohydrolases (eNTPDases) have emerged as the major family responsible and specific for breaking the terminal phosphoanhydride bonds of tri- and dinucleotides (1, 2). As such, they have been shown or hypothesized to modulate many of the signaling and biosynthetic processes in which extracytoplasmic nucleotides play a role, including vascular homeostasis, cell size maintenance, neuronal signaling, immune function, and protein and lipid modification (3–8). Consistent with the variety of tasks they perform on and in the cell, different family members exhibit different localizations and specificities: some reside on the plasma membrane and others in the Golgi, lysosomes, or endoplasmic reticulum, while each has a characteristic hierarchy of preferences for di- vs trinucleotides as well as for different bases (1, 9–11). The defining structural feature shared by all family members is a set of five short sequences called apyrase conserved regions (ACRs) (12, 13), two of which are thought to constitute phosphate binding loops based on homology to the nucleotide binding domain of the actin/hsp70/hexokinase ATPase superfamily (12, 14), and all of which are required for enzymatic activity (15–18). Like the enzymatic activity, all five ACRs are located in the extracytoplasmic domain, consistent with their putative identity as the active site.
Like most other ectoenzymes (19), some eNTPDases are secreted into the extracytoplasmic space or anchored in the membrane by a single transmembrane helix (20). However, several family members exhibit a topology commonly observed among channels, transporters, and receptors but unusual for ectoenzymes: a large extracellular domain flanked by two transmembrane helices (9–11, 20, 21). Why the active site would be held down by transmembrane domains on both sides rather than by a single protein or lipid link was originally a mystery, but in recent years it has become clear that the two transmembrane helices are intricately linked to active site function rather than simply serving as anchors (22). In particular, CD39 (eNTPDase1), a plasma membrane bound apyrase, loses ninety percent of its activity upon removal of either or both transmembrane helices as well as upon disruption of their native state by detergent solubilization (23). Furthermore, kinetic and mutational analysis of the native and truncated forms has revealed that they differ not only in total activity but also in hydrolysis mechanisms, role of a putative phosphate binding loop ACR1, substrate specificity, and presence or absence of intermediate ADP release during ATP hydrolysis (24–26). All of these enzymatic features line up one way when CD39 is in its native state and a different but consistent way regardless of whether the native state is disrupted by removing the N-terminal, the C-terminal, or both transmembrane helices or by detergent solubilization. Thus, native CD39 function requires both transmembrane helices to be present and in the membrane, and the state of the transmembrane helices governs which of at least two distinct functional states the active site resides in (24).
These studies established that several physiologically important features of the enzyme depend on the transmembrane domains, but the fact that these insights were won by wholesale removal of the transmembrane domains or complete extraction from the bilayer left open two major questions: 1) what do the transmembrane domains do that makes them so important? and 2) do they ever change organization and regulate switching between enzymatic states in vivo? Recently, using disulfide crosslinking of cysteines substituted for TM1 and TM2 residues, we found that the transmembrane helices interact strongly both within and between subunits, in particular near the extracellular side of the membrane, suggesting a potential role for specific intra- and intermolecular helical interactions (28). However, each helix exhibits a high degree of rotational mobility within the packed structure; significant crosslinking takes place between all faces of TM1 and TM2 within a molecule as well as between all faces of TM1 and TM1′ and of TM2 and TM2′ of different subunits. Specific primary interaction surfaces were identified by differences in temperature dependence, supporting a distinct packing arrangement rather than complete randomness. Nevertheless, locking the helices in the primary or any other orientation results in activity loss comparable to that induced by solubilization or by complete removal of the transmembrane domains. These results indicate that specific helix interactions and orientation do matter but suggest that the ability of the helices to move relative to each other is at least if not more important than their ability to stabilize one optimal arrangement. Substrate binding in turn regulates mobility, further suggesting that a balance between stability and flexibility of specific interactions underlies the transmembrane domains’ functional relationship with the active site.
Are any of these features subject to regulation by the environment? Several lines of reasoning suggest that the transmembrane helices might be primed to respond to changes in the membrane and to translate them into changes in enzymatic functional state. Energetically, altering membrane physical properties may alter the bilayer deformation energy for a given protein conformational change (29); if the energy difference between protein conformations is relatively low compared to the associated bilayer deformation energy, appropriate alteration of membrane properties can be sufficient to allow the conformational change. For CD39, the extracellular domain appears not to pose a barrier to whatever conformational change alters its function, since it achieves the altered state by default in the absence of transmembrane domains (24). The mobility discussed above suggests that the transmembrane helices themselves are also not optimized for strong association (28), potentially leaving the membrane as a significant factor keeping the protein in its native state. Physiologically, extracellular ATP is a first line response to and normalizing agent for a wide range of alterations in membrane physical properties, such as those due to swelling, sheer stress, or inflammatory responses (3–5); as a primary modulator of cell surface ATP concentrations CD39 activity might be expected to be tailored to the state of the membrane as well. A recent report provided direct evidence that CD39 activity does indeed vary based on the cholesterol content of the membrane (30), although whether this reflects a specific requirement for cholesterol or a response to mechanical bilayer properties is unknown.
In the present study we ask whether mechanical properties of the membrane regulate CD39 function and, if so, what changes in the transmembrane helices translate membrane properties to the active site. We find that a range of amphiphiles with unrelated structures that alter membrane curvature in opposite ways all convert CD39 to the same functional state as removing the transmembrane helices. In parallel they, as well as cholesterol removal, increase the rotational mobility of both transmembrane helices. We suggest that a general mechanical bilayer property, potentially elasticity, regulates CD39 by altering the balance between stability and mobility of its transmembrane helices, thereby modulating the coupling between transmembrane domains and active site.
Experimental procedures
DNA Construction
Full length CD39 containing single cysteine substitutions in TM1 or TM2 or paired cysteine substitutions in TM1 and TM2 were constructed previously as described in ref 28. Figure 1 shows the positions of the cysteine substituted residues in the context of the entire CD39 molecule as well as their organization in helical wheel format. CD39 lacking TM1 (NT) and containing single cysteine substitutions in TM2 was constructed by replacement of the SacII-NotI fragment from pcDNA3-NTmyc (described in ref 23) with the SacII-NotI fragment from the TM2 cysteine substituted versions of pcIneo-CD39HA (described in ref). This procedure also replaced the C-terminal myc tag with an HA tag. CD39 lacking TM1 (NT) and containing single cysteine substitutions in TM2 was constructed by PCR amplification of the TM1 and extracellular domain coding regions from pcIneo-CD39HA containing TM1 cysteine substitutions. T7EEV (Promega) was used as the forward primer, and the reverse primer annealed to the CD39 sequence coding for MIPAEQP prior to TM2 (as in ref 23) and contained coding sequence for an HA tag followed by a NotI site. The resulting fragment was digested with NheI and NotI and inserted into pcIneo.
Figure 1.
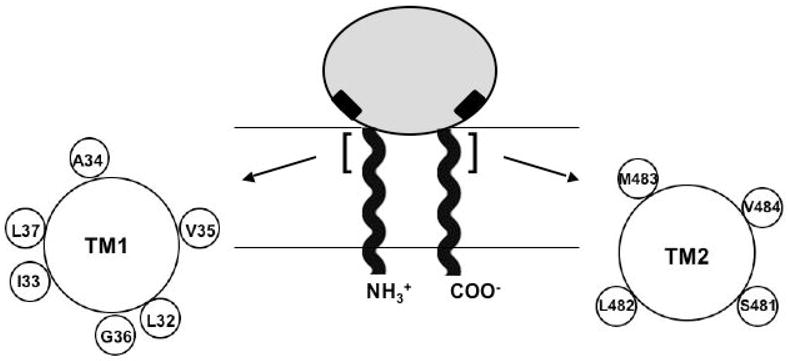
Organization of CD39 extracellular and transmembrane domains. Shaded oval represents the extracellular domain. N- and C-terminal helical strands represent the transmembrane domains TM1 and TM2. Black rectangles adjacent to TM1 and TM2 represent active site regions ACR1 and ACR5. The TM1 and TM2 residues examined in this study are highlighted with brackets, and their relative positions around the helix are shown in helical wheel format.
Preparation of COS7 Cell Crude Membranes
COS7 cells were transiently transfected with 6 μg plasmid per 100 mm dish using Lipofectamine (Invitrogen). Cells were harvested 72 h after transfection, and crude membranes were prepared as described in ref 31. Membranes were resuspended in 50 μL of Tris-HCl (pH 7.8) per 100 mm plate, and aliquots were flash frozen in liquid nitrogen and stored at −80°C.
Nucleotidase Assays
Nucleotidase assays were carried out in a 200 μL solution containing 50 mM Tris-HCl pH 7.5, 1 mM EDTA, I mM HEDTA, 2 mM ATP or ADP, and MgCl2 or CaCl2 to give the indicated concentrations of free Mg2+ or Ca2+ as calculated and described in ref 24. In most cases MgCl2 was used in order to be consistent with the fact that the cytoplasmic side of the membrane is not usually exposed to high Ca2+ concentrations and thus to avoid any potential nonphysiological interference of Ca2+ with amphiphile insertion or distribution. Nevertheless, similar results were obtained with Ca2+. Amphiphiles were diluted in assay buffer from stock solutions as follows. AA (Sigma) stock solution was 300 mM in EtOH; OA (Sigma) was 200 mM in EtOH; DOHA (Sigma) was 300 mM in EtOH; stearic acid (Sigma) was 80 mM in EtOH; arachidic acid (Sigma) was 150 mM in chloroform; all LPLs were 10 mM in H2O (LPC and LPI from Sigma; LPG and LPE from Avanti); Triton X-100 was 10% in H2O (solution from Pierce); cyclodextrin was 6% in H2O. Membrane concentrations were approximately 5 μg/mL. Reaction mixtures containing all components except nucleotide were preincubated 10 minutes at 37°C, and reactions were started by addition of nucleotide, incubated 20 minutes at 37°C, and stopped by addition of 300 μL 10% SDS. Phosphate concentration was determined by the colorimetric method (32). For the reversibility experiments, membranes were preincubated 10 minutes at 37°C in reaction solutions containing the indicated amphiphiles, BSA at 10 mg/mL in assay buffer was added to a final concentration of 0.5 mg/mL, solutions were preincubated for an additional 10 minutes at 37°C, and reactions were carried out as described above.
Oxidative Crosslinking
Cysteine substituted CD39 constructs were crosslinked in crude membranes using copper phenanthroline (CuP) as the oxidizing agent. Copper phenanthroline was prepared by combining cupric sulfate and 1,10-phenanthroline (Sigma) at a 1:3 molar ratio in water and used at a final concentration of 0.3 mM (expressed as Cu2+ concentration) except where otherwise indicated. For all constructs time courses were initially carried out at 0.03, 0.3, and 3 mM CuP to confirm that CuP concentration was not the rate limiting factor. Reactions were carried out in 12 mM Tris-HCl at 37°C for 30″, 1′, 2′, or 5′, except for intramolecular crosslinking of A34C-V484C, which was crosslinked at 22°C in order to obtain an observable rate. Reactions were performed at the same membrane concentrations as those used for activity measurement; in order to facilitate using the dilutions of membranes and amphiphiles as described above, a 500 μL reaction volume was used. Membranes were added to buffer containing amphiphiles, and reactions were started by addition of a 100X solution of CuP to avoid significant changes in amphiphile concentrations. Reactions were stopped by adding 20 μL of 0.5 M EDTA for a final concentration of 20 mM EDTA, and membranes were collected by centrifugation in a Ti70.1 rotor at 40,000 rpm for 30′ at 4°C. The centrifugation step also served the purpose of confirming that protein was not extracted from the membrane during any of the amphiphile treatments. Membranes were resuspended in 30 μL nonreducing SDS loading buffer containing 20 mM N-ethylmaleimide (NEM) and 20 mM EDTA and used immediately for SDS-polyacrylamide gel analysis.
Western Blot Analysis
Samples resuspended as described above were resolved on a 5.5% SDS-polyacrylamide gel as described in ref 33 and transferred to nitrocellulose at 250 mA for 2.5 h. Nitrocellulose membranes were probed with anti-HA11 monoclonal antibody (Covance) in 2% milk in Tris-buffered saline/0.1% Tween, followed by a secondary anti-mouse horseradish peroxidase conjugated antibody (Sigma) in 3% milk in Tris-buffered saline/0.1% Tween. The protein was visualized by chemiluminescence (substrate from Pierce) and exposure to film, and the percent crosslinked was determined by quantitation of dimer band as a percent of the sum of monomer and dimer bands in a given sample.
Results
Mechanical bilayer properties can be altered by direct pressure, by reversible insertion of amphiphilic compounds, or by variations in lipid composition. CD39 most likely encounters each of these situations in vivo; here we have employed a series of amphiphilic compounds that have been shown to mimic the effects of physical pressure and to alter bilayer deformation energy for several types of mechanosensitive channels (29, 34–37). Compounds were added to isolated native membranes to allow direct comparison of the native and altered states and to avoid potential indirect effects mediated by cytoplasmic components.
Unsaturated fatty acids reversibly alter enzymatic functional state
Cis-unsaturated fatty acids are thought to alter bilayer properties by virtue of their inverse cone shape; the combination of a wide hydrocarbon tail and a narrow headgroup alters the packing of the native lipids, introducing negative spontaneous curvature as well as changes in membrane tension and elasticity. As shown in Figure 2A, adding arachidonic acid (AA) to membranes from COS7 cells transfected with CD39 reduces ATPase activity by approximately 80%, similar to the activity loss that occurs when the transmembrane domains are removed. Although the molar fraction of AA in the membrane is unknown, the Ki of approximately 3 μM is comparable to that observed for other proteins known to be sensitive to membrane mechanical properties (34, 35). The same effect is observed for other unsaturated fatty acids with varying degrees of unsaturation; oleic acid (OA), which has 18 carbons and one degree of unsaturation, and docosahexaenoic acid (DOHA), which has 22 carbons and six degrees of unsaturation, inhibit ATPase activity to the same extent as does AA, which has 20 carbons and four degrees of unsaturation. OA, the smallest and least unsaturated molecule in the series, has the highest Ki of approximately 6μM.
Figure 2.
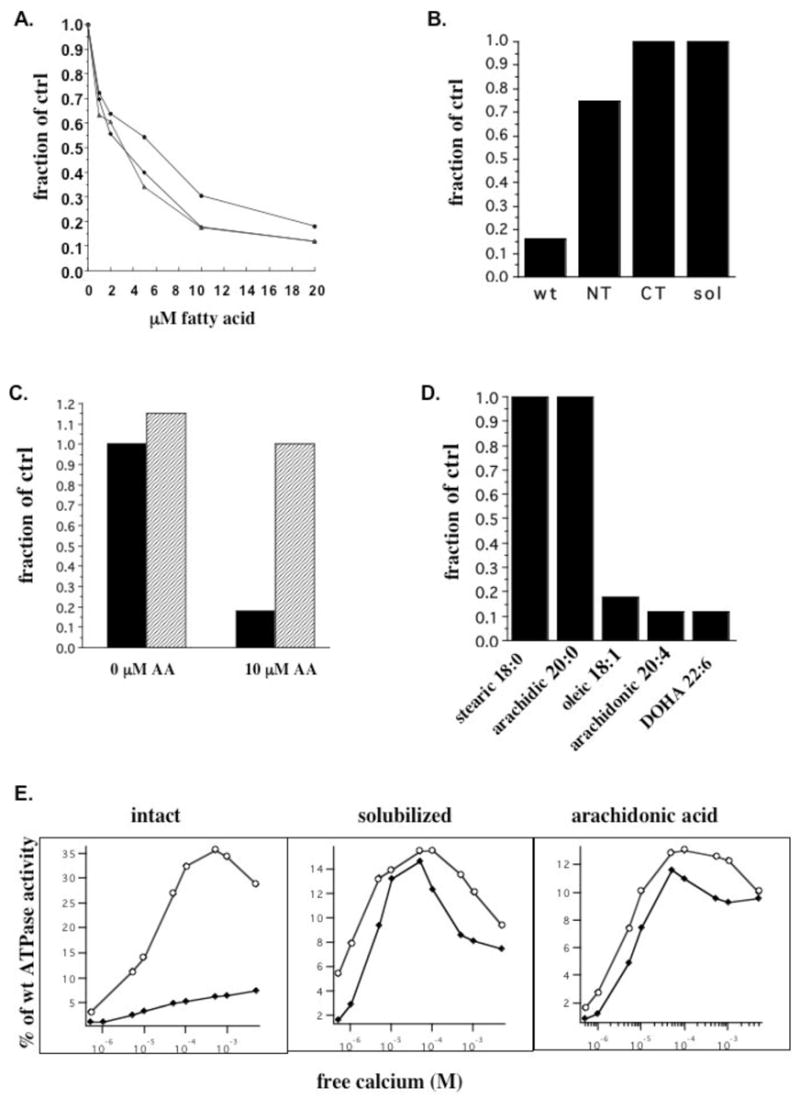
ATPase activity in the presence of unsaturated fatty acids. (A) ATPase activity of full length CD39 was measured in isolated membranes in the presence of the indicated concentrations of arachidonic acid (AA) (◆), oleic acid (OA) (●), or docosahexaenoic acid (DOHA) (▲). (B) ATPase activity in the presence of 10 μM AA was measured for full length CD39, single truncated NT lacking TM1, single truncated CT lacking TM2, and soluble CD39 lacking TM1 and TM2. Activities are expressed as a fraction of native full length CD39 ATPase activity. (C) Full length CD39 was exposed to 0 or 10 μM AA followed by direct measurement of ATPase activity (black bars) or followed by treatment with BSA prior to activity measurement (hatched bars). (D) Full length CD39 activity was compared in the presence of saturated fatty acids stearic and arachidic acid and their unsaturated counterparts OA and AA as well as DOHA, each at 20 μM. (E) ATPase (◆) and ADPase (O) activities in the presence of the indicated free Ca2+ concentrations were measured for full length CD39-H59G in intact membranes (panel 1), solubilized in 1% Triton X-100 (panel 2), and in the presence of 10 μM AA (panel 3) and expressed as a percent of maximal native wild type CD39 ATPase activity.
As shown in Figure 2B, the transmembrane domains are required for sensitivity to AA. The membrane-bound construct lacking TM1 (NT) is only slightly sensitive to AA up to concentrations as high as 100 μM, similar to the minor degree of inhibition observed upon detergent solubilization. Both CT, the membrane-bound counterpart lacking TM2, and soluble CD39 lacking both TM1 and TM2, are completely insensitive to AA up to the same concentrations. The same results were obtained with DOHA and OA. In contrast, inhibitors that are expected to inhibit via the extracellular domain, such as FSBA, trinitrophenol, and low concentrations of SDS, have comparable effects on both native and truncated constructs (data not shown). These results suggest that low concentrations of unsaturated fatty acids mimic removal of the transmembrane helices or detergent solubilization, consistent with the idea that they inhibit via their effects on the membrane.
In order to verify that unsaturated fatty acids act via a reversible process rather than by destroying the membrane, we pretreated membranes with AA and either measured activity directly or used bovine serum albumin (BSA) to extract AA from membranes before measuring activity. Figure 2C shows that pretreatment and activity measurement in the presence of AA produces the same inhibitory effect as observed previously; however, pretreating with AA, adding BSA, and measuring activity in the presence of both produces the same result as not adding AA. The same result was obtained with OA and DOHA. Unsaturated fatty acids therefore appear to inhibit activity via reversible insertion in the membrane, consistent with the conditions under which they alter the membrane mechanical properties discussed above.
As summarized in Figure 2D, unsaturation is required for inhibition of activity. The saturated counterparts of OA and AA, stearic and arachidic acid respectively, have no effect despite their expected ability to partition in the membrane. The wide hydrocarbon chain and resulting inverse cone shape therefore appear to underlie the effects of unsaturated fatty acids on the membrane and on CD39.
Do unsaturated fatty acids convert CD39 to the same altered functional state as does solubilization or removing the transmembrane domains? A key distinction between the two states is their difference in ATP and ADP hydrolysis mechanisms; in the native state residue H59 in ACR1 is critical to ATPase activity but less critical to ADPase activity, while in the solubilized state both activities are independent of this residue. As a result, native CD39 with H59G substitution is an ADPase but solubilized CD39-H59G has equal ATPase and ADPase activities each comparable to solubilized wild type CD39 (24). We therefore compared the kinetic profiles of CD39-H59G in intact membranes, when solubilized in 1% Triton X-100, and in native membranes treated with 10 μM AA. As illustrated in Figure 2E, AA mimics solubilization in its effect on ATPase:ADPase ratio as well on total ATPase and ADPase activities. Activities are expressed as the percent of wild type CD39 ATPase activity in intact membranes, as determined previously for native and solubilized CD39-H59G; like solubilization, AA decreases ADPase activity, increases ATPase activity, and changes the ATPase:ADPase ratio from 1:6 to approximately 1:1. AA, like 1% Triton X-100, also reduces the apparent Km for Ca2+ by about one order of magnitude. The identity of the kinetic profile to the solubilized state and its marked difference from the native state suggest that, rather than denaturing or otherwise incapacitating the enzyme, addition of AA to native membranes converts CD39 to the same mechanistic functional state as does solubilization or truncation.
Cone shaped amphiphiles mimic unsaturated fatty acids
In contrast to unsaturated fatty acids, lysophospholipids (LPLs) and Triton X-100 have narrow hydrocarbon tails and wide headgroups. As a result their insertion in the membrane at concentrations below their critical micelle concentrations (CMC) also alters packing of the native lipids; like inverse cone shaped molecules they change various mechanical features of the membrane including tension and elasticity, but they induce positive rather than negative spontaneous curvature. In Figure 3A we show that addition of lysophosphatidycholine (LPC) to native membranes reduces CD39 ATPase activity by 80%, similar to unsaturated fatty acids, solubilization, and truncation. The Ki of approximately 2 μM is below the CMC and is comparable to that observed for mechanosensitive channels (34, 35).
Figure 3.
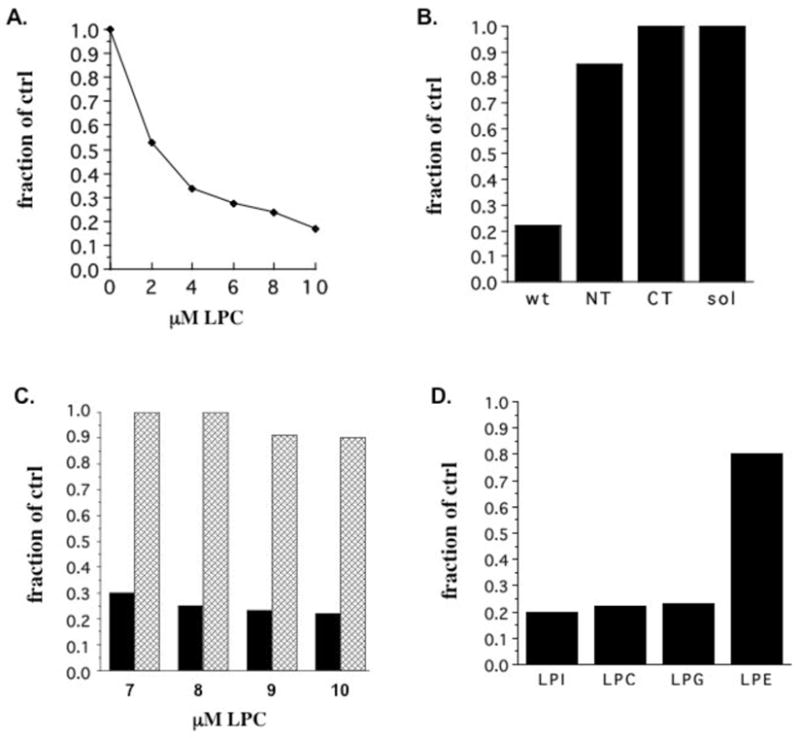
ATPase activity in the presence of lysophospholipids. (A) ATPase activity of full length CD39 was measured in membranes in the presence of the indicated concentrations of lysophosphatidylcholine (LPC). (B) ATPase activity in the presence of 8 μM LPC was measured for full length CD39, NT, CT, and soluble CD39 and expressed as a fraction of native full length CD39 activity. (C) Full length CD39 was exposed to the indicated concentrations of LPC followed by direct measurement of ATPase activity (black bars) or followed by treatment with BSA prior to activity measurement (hatched bars). (D) Full length CD39 ATPase activity was compared in the presence of 8 μM lysophospholipids with large (lysophosphatidylinositol, LPC, and lysophosphatidylglycerol) and small (lysophosphatidylethanolamine) headgroups and expressed as a fraction of native CD39 activity.
As shown in Figure 3B, both transmembrane domains are required for sensitivity to LPC; as with unsaturated fatty acids and solubilization, NT is only slightly sensitive to LPC and CT and soluble CD39 are completely insensitive, suggesting that LPC acts via the membrane. LPC inhibits activity in a reversible manner, as illustrated in Figure 3C; treating membranes with LPC followed by extraction by BSA and measurement of activity in the presence of both reveals that the inhibitory effect is completely reversible up to 8 μM LPC and 90% reversible at 9 and 10 μM LPC, most likely due to the fact that LPC approaches its CMC at the latter concentrations. As with fatty acids, the shape of the molecule rather than its specific identity correlates with its inhibitory potential; as summarized in Figure 3D, LPLs with large headgroups, lysophosphatidylinositol, lysophosphatidylcholine, and lysophosphatidylglycerol, all inhibit activity, while lysophosphatidylethanolamine, which has a small headgroup and is not considered to be cone shaped, has only a slight effect. Kinetic analysis of CD39-H59G in the presence of LPC produces results identical to those shown for AA in Figure 2E (data not shown), suggesting that LPC also converts CD39 to the same altered functional state as do solubilization and truncation.
The effect of LPLs is mimicked by sub-CMC concentrations of Triton X-100, an amphiphile that has no structural features in common with LPLs other than an overall cone shape. As shown in Figure 4A, Triton X-100 inhibits CD39 ATPase activity in a dose-dependent manner when added to native membranes; maximal inhibition comparable to that observed with LPLs is reached by 0.005%, below the CMC. The comparison of effects on full length, NT, CT, and soluble CD39 in Figure 4B indicates that, as with solubilization, unsaturated fatty acids, and LPLs, both transmembrane domains are required for sensitivity to nonsolubilizing concentrations of Triton X-100. The same kinetic analysis and results as described above for AA and LPC suggest that addition of Triton X-100 to native membranes also converts CD39 to the previously characterized altered functional state (data not shown).
Figure 4.
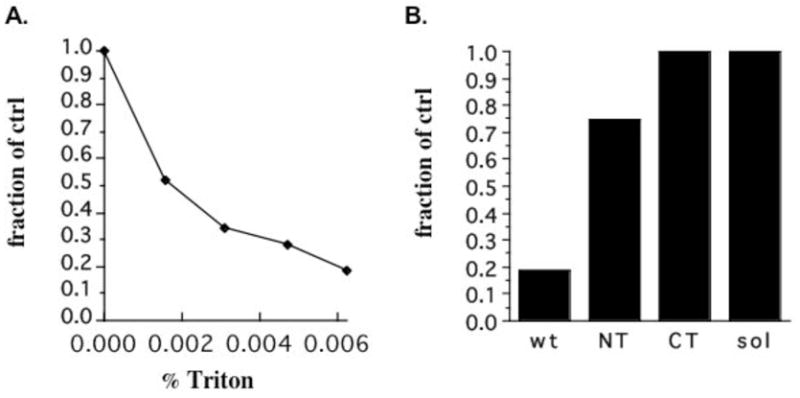
ATPase activity in the presence of Triton X-100. (A) ATPase activity of full length, membrane-bound CD39 was measured in the presence of the indicated nonsolubilizing concentrations of Triton X-100. (B) ATPase activity in the presence of 0.005% Triton X-100 was measured for full length CD39, NT, CT, and soluble CD39 and expressed as a fraction of native full length CD39 activity.
Bilayer alterations increase transmembrane helix mobility
The above results reveal that the functional state of CD39 is altered in a transmembrane domain dependent manner by a variety of structurally unrelated compounds that have little in common other than their ability to insert in the membrane and alter its mechanical properties. Consistent with Papanikolaou et al (30), we found that removal of cholesterol, an inverse cone shaped molecule, by cyclodextrin also reduces activity by approximately 80%. Together these data point toward a common mechanism by which alteration of membrane mechanical properties acts via the transmembrane helices to convert the active site to a different functional state.
If so, the same membrane alterations would also be expected to change some structural or dynamic feature of the transmembrane helices. We therefore used our previously reported set of cysteine-substituted TM1, TM2, and TM1-TM2 constructs to examine how inter- and intramolecular disulfide crosslinking patterns respond to the membrane treatments described above. As described previously (28 and references therein), relative crosslinking rates reveal the relative proximity of different cysteine pairs and are commonly used to determine helicity and interacting surfaces of transmembrane domains. The technique has further been established as a means to detect dynamic motions within a protein (38). While the method does not provide direct information on the rates and frequency of such motions, it has been established as a means to detect qualitative differences in mobility for a given protein under different conditions (38, 39).
Rather than using temperature sensitivity to distinguish among helix interfaces as in our previous report, we used crosslinking rate at 37°C to monitor changes in crosslinking propensity. As shown in Figure 5A, intermolecular TM1-TM1′ and TM2-TM2′ crosslinking rates at a series of positions near the extracellular side of the membrane correlate with the helical patterns previously identified by temperature dependence. On TM1, positions L32, V35, and G36, which are insensitive to temperature, exhibit the fastest crosslinking rates, the latter two with complete crosslinking by 30″, while L37, which is partially sensitive to temperature, exhibits an intermediate rate with crosslinking complete by 2′, and I33 and A34, both of which fail to crosslink at 4°C, are the slowest, with crosslinking complete by 5′. While generally slightly slower than TM1, relative crosslinking rates on TM2 also reflect a helical pattern; L482, the position least sensitive to temperature, is nearly completely crosslinked by 30″, while M483, a partially temperature sensitive position, is intermediate, and S481 and V484, neither of which crosslinks at 4°C, are the slowest.
Figure 5.

TM1 and TM2 crosslinking rates. (A) Full length CD39 constructs containing single cysteine substitutions at the indicated positions in TM1 or TM2 were crosslinked in isolated membranes by exposure to 0.3 mM copper phenanthroline (CuP) for 30′, 1′, 2′, or 5′ at 37°C. Crosslinking efficiency is expressed as the percent dimerized. (B) Crosslinking rates for I33C were compared at 0.03 (◆), 0.3 (●), and 3 mM (▲) CuP at 37°C.
In order to verify that the observed rates reflect cysteine-cysteine collisions rather than accessibility to crosslinking reagent, we compared crosslinking rates at a series of copper phenanthroline (CuP) concentrations. As shown in Figure 5B for position I33, the crosslinking rate plateaus between 0.03 and 0.3 mM CuP; adding a tenfold excess of CuP has no further effect on the rate. The maximal rate was reached before this point for all other positions as well, indicating that the crosslinking rates measure helix-helix collision rates, rather than helix-reagent collision or reaction rates.
To determine the effects of membrane properties on crosslinking rates, we measured rates in the presence of 20 μM OA, 0.005% Triton X-100, or following cholesterol extraction by cyclodextrin as representatives of inverse cone shaped amphiphiles, cone shaped amphiphiles, and alteration by removal rather than addition of a membrane component. As summarized in Figure 6A and B, each of these treatments increased the crosslinking rate at every slow position such that all were completely crosslinked by 30″. For both TM1 and TM2 comparison of the percent crosslinked at 30″ reveals the distinction between the primary interface and those of other orientations; OA, Triton X-100, and cyclodextrin each produce the same result of abolishing this distinction. Although we cannot determine whether the rates become equal, the fact that the rate increase at the slow positions is not accompanied by a decrease at other positions to the previously observed slow rates suggests that these treatments increase the time spent in the alternate orientations rather than shifting the helices to a different primary conformation.
Figure 6.
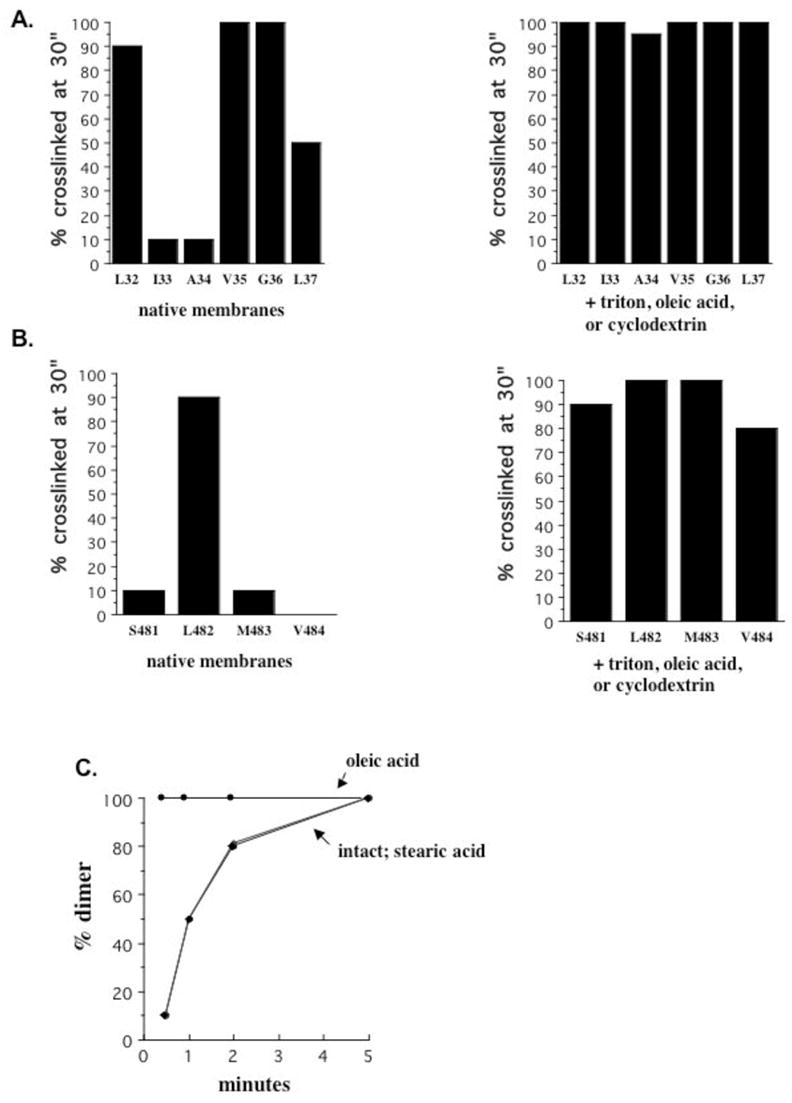
TM1 and TM2 crosslinking efficiency in membranes treated with Triton X-100, OA, or cyclodextrin. (A) Percent crosslinking at 30″ was compared for the single TM1 cysteine substituted contructs in native membranes (left panel) and in the presence of either 0.005% Triton X-100, 20 μM OA, or 3% cyclodextrin (right panel). The three treatments yielded identical results. (B) Percent crosslinking at 30″ was compared for TM2 positions as described in (A) for TM1. (C) Crosslinking rates for I33C were compared in intact membranes (◆), and in the presence of 20 μM OA (●) or its saturated counterpart stearic acid (▲). (D) Percent crosslinking at 30″ was determined at the indicated concentrations of Triton X-100 (left panel) or OA (right panel) for I33C.
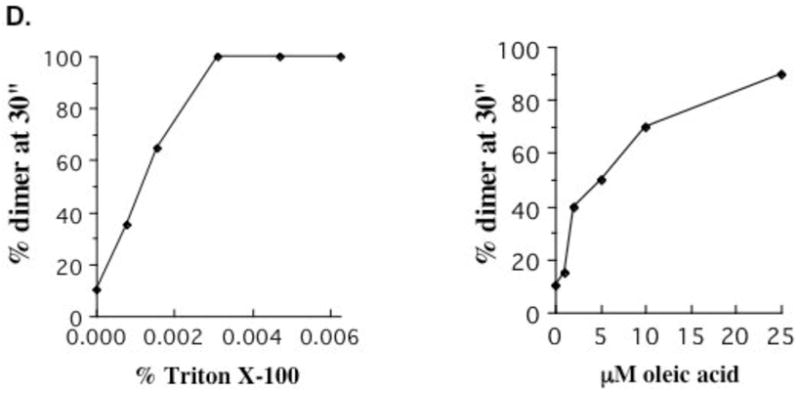
Comparison of rates in the presence of OA and its saturated counterpart stearic acid suggest a correlation between the effects on enzymatic activity and crosslinking. As shown in Figure 6C, despite its ability to insert in the membrane stearic acid has no effect on crosslinking rate at position I33, consistent with its lack of effect on activity. The correlation between effects on activity and on transmembrane helix behavior is further supported by the similar concentration dependence of the two effects. As summarized in Figure 6D, the dose dependence for increase in percent crosslinked at 30″ at position I33 is nearly identical to the dose dependence for loss of ATPase activity; the correlation is observed for both OA and Triton X-100. These results are consistent with a potential relationship between activity and the observed changes in transmembrane helix interactions.
We previously demonstrated that intermolecular crosslinking at the slower positions is due to rotational mobility within dimers rather than to collisions between dimers or formation of higher oligomers (28). If these results apply to the modified membranes in the present experiments, the increased crosslinking rates at the slower positions would be expected to result from an increase in rotational mobility of each helix. In order to test directly for an increase in rotational mobility, we examined the rate of intramolecular TM1-TM2 crosslinking between positions A34 and V484. As shown in Figure 7, the crosslinking rate is observable over the 5 minute time course in native membranes but, as for intersubunit crosslinking, the rate is increased so that crosslinking is complete by 30″ in the presence of OA, Triton X-100, or cholesterol removal. Furthermore, despite the potential for this construct to form crosslinks between dimers or among higher oligomers due to its two cysteines, no higher order crosslinking is observed under any conditions. These results demonstrate that membrane physical properties increase rotational mobility of the transmembrane helices and that, while formation of higher order oligomers is not completely ruled out, the increase in rotational mobility appears to predominate over other potential effects.
Figure 7.
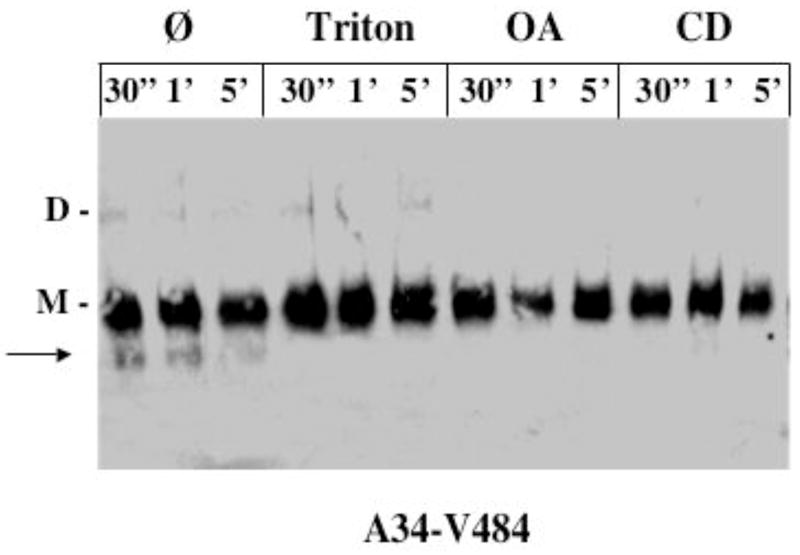
TM1-TM2 crosslinking in intact and modified membranes. CD39 containing paired cysteine substitutions at positions A34 in TM1 and V484 in TM2 was crosslinked for the indicated times in intact membranes and in membranes treated with 0.005% Triton X-100, 20 mM OA, or 3% cyclodextrin. Disappearance of the 56 kD C-terminal fragment (arrow) correlates with intrasubunit TM1-TM2 crosslinking as determined in ref 28. M, monomer; D, dimer.
Membrane alterations uncouple substrate binding and transmembrane helix dynamics
Our previous work revealed that ATP binding decreases rotational mobility of the transmembrane helices and stabilizes their primary orientation (28). In order to gain insight into how membrane physical properties might affect the relationship between substrate binding and transmembrane helix mobility, we measured the extent of crosslinking at 5 minutes in the presence of ATP and Triton X-100, OA, or cyclodextrin. For position I33, crosslinking is complete by 5 minutes in native membranes in the absence of ATP but is almost completely abolished in the presence of ATP. As summarized in Figure 8, Triton X-100, OA, or removal of cholesterol counteract the ability of ATP to inhibit crosslinking and restore crosslinking to at or near the level in the absence of ATP. As discussed below, the ability of membrane alterations to interfere with coupling between active site and transmembrane helix dynamics may have implications for the mechanism by which they regulate enzymatic function.
Figure 8.
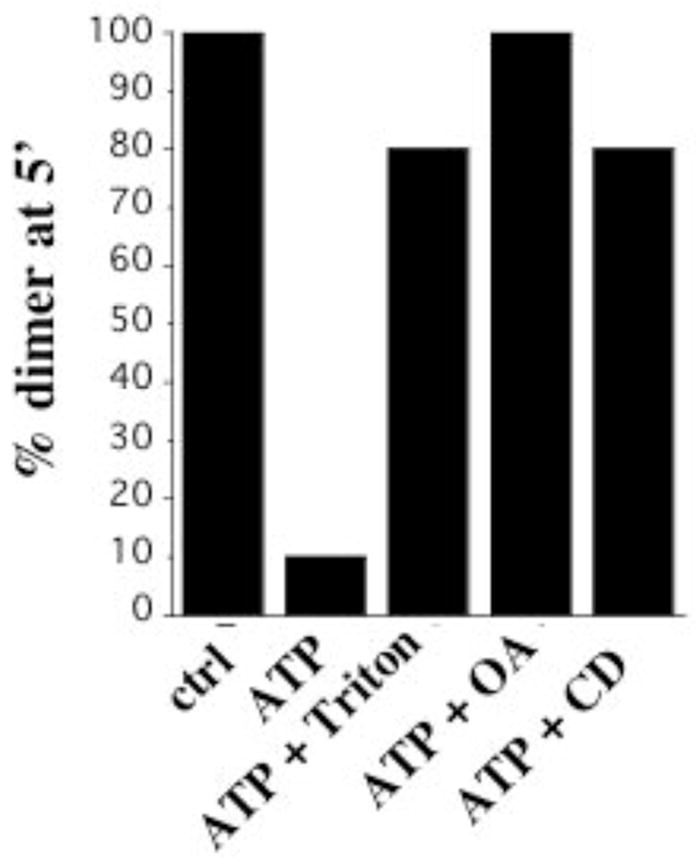
Regulation by substrate in intact and modified membranes. Percent crosslinking at 5′ was determined for I33C in intact membranes in the absence of ATP and in membranes left intact or treated with 0.005% Triton X-100, 20 μM OA, or 3% cyclodextrin in the presence of 5 mM ATP.
Removal of either helix mimics membrane alteration’s effect on other helix
In order to gain further insight into the relationship among helix mobility, helix interactions, and membrane properties, we measured intermolecular crosslinking rates for versions of CD39 lacking TM1 (NT) or TM2 (CT). As shown in Figure 9, TM1-TM1′ crosslinking rates in the CT construct lacking TM2 display none of the rate variation observed at the corresponding positions in full length CD39; instead, all positions crosslink completely by 30″. The absence of TM2 furthermore renders all positions insensitive to ATP. Similar results are observed for TM2-TM2′ crosslinking in the absence of TM1; while none of the positions achieve complete crosslinking, consistent with the previous report that a portion of NT remains monomeric as measured by sucrose density gradient sedimentation, no distinctions among positions are observed and maximal crosslinking occurs by 30″. All positions are insensitive to ATP, in contrast to the sensitivity of all but L482 in full length CD39. Furthermore, neither Triton X-100 nor OA has any effect on rates or maximal degree of crosslinking for either CT or NT. Removing one helix therefore appears to mimic the changes in crosslinking profiles induced by alterations in membrane properties. These results support the idea that helix interactions rather than diffusion in the membrane are the primary factor limiting intermolecular crosslinking rates in full length CD39. In addition, they indicate that TM1-TM2 interactions are responsible for limiting rotational mobility of each helix; in the absence of the other helix each has significantly more freedom to rotate despite its attachment to the extracellular domain.
Figure 9.
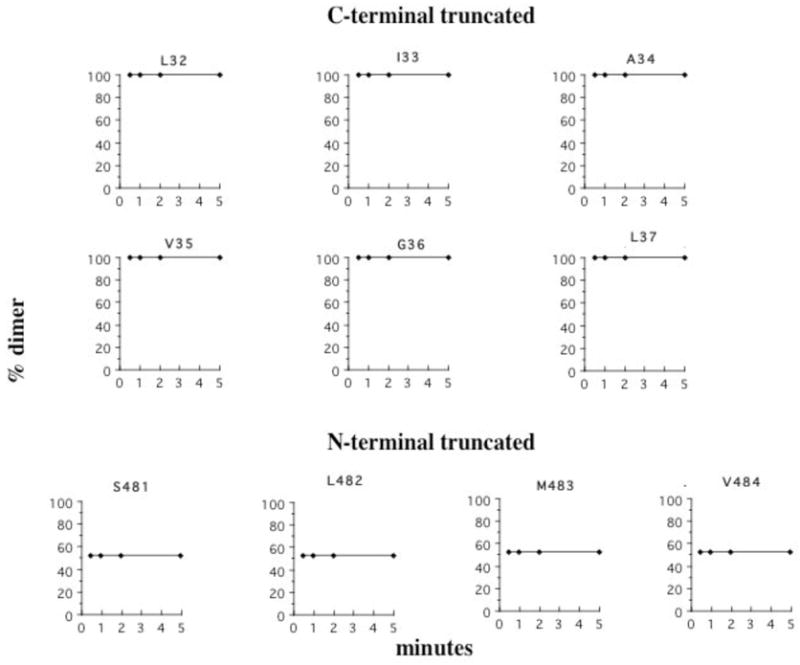
Crosslinking of constructs lacking either TM1 or TM2. CT constructs lacking TM2 and containing single cysteine substitutions at the indicated positions in TM1, and NT constructs lacking TM1 and containing single cysteine substitutions in TM2, were crosslinked for the indicated times as described for full length CD39 in Figure 4. Identical results were obtained in the presence of 5 mM ATP or in the presence of 0.005% Triton X-100 or 20 μM OA.
Discussion
The present experiments reveal that membrane mechanical properties regulate CD39 enzymatic functional state, rotational mobility of its transmembrane helices, and coupling between transmembrane helix motions and substrate binding. A variety of amphiphilic compounds that alter bilayer features such as tension, spontaneous curvature, and elasticity were all found to convert CD39 to the same functional state as does removal of transmembrane helices or detergent solubilization, as indicated by identical kinetic profiles, activities, substrate specificity, and hydrolysis mechanisms with respect to residue H59 in ACR1. Both transmembrane domains are required for sensitivity to these reagents; in contrast inhibitors that bind to the active site inhibit both full length and truncated CD39. The latter result, in conjunction with the structural diversity of the amphiphiles, supports the interpretation that these agents work via the membrane. The change in functional state is correlated with an increase in rotational mobility of both transmembrane helices; bilayer alterations increase interhelical crosslinking rates such that the helical dependence of crosslinking rates observed in native membranes is abolished on the observable time scale. Bilayer alteration furthermore counteracts the ability of ATP to restrict transmembrane helix mobility, thus uncoupling transmembrane helix dynamics from substrate binding.
While a concomitant change in monomer-oligomer equilibrium is not excluded as an explanation for the change in crosslinking rates, the change in intramolecular mobility appears to predominate as demonstrated by an increase in intramolecular TM1-TM2 crosslinking rate, lack of crosslinked dimer formation by the double cysteine substituted construct, and the fact that removal of one transmembrane helix mimics the effects of bilayer alteration. The possibility that the change in crosslinking pattern might result from local unwinding or other deviation from helicity at the ends of the transmembrane domains is also not excluded. A change in lipid packing or interaction with the various reagents might in theory promote such a change if it changes local hydrophobicity. Nevertheless, this explanation is unlikely to account for the fact that removing either transmembrane domain produces the same results without manipulation of the membrane, or for the ability to modulate this parameter with substrate or temperature without losing the overall helical crosslinking pattern. In addition, both transmembrane domains, including the regions studied here, are predicted with more than 90% confidence to be helical, independent of membrane environment. In any case, the observed change in crosslinking pattern is inconsistent with a shift to any other equally rigid conformation and therefore suggests an increase in mobility whether or not the transmembrane domains remain helical (40). Thus, the most straightforward interpretation of our data is an increase in conformational mobility, most likely predominated by but not limited to, a change in rotational mobility of TM1 and TM2.
This study suggests that the critical structural difference between the previously identified enzymatic functional states is the degree of conformational mobility. Our original work established that the enzyme reverts to an alternate functional state upon disruption of the transmembrane helices by any of a diverse set of truncation and solubilization approaches (24). Based on these results we proposed that the active site can exist in a relaxed or tense conformation and that the two transmembrane domains are required to maintain the tense, or native, conformation (41). Subsequent discovery of a high degree of TM1 and TM2 rotational mobility, of the ability of substrate to modulate mobility, and of activity reduction upon locking the helices in any single orientation, suggested that dynamic motions, rather than simple stabilization of a tense conformation, might be the key to the requirement for transmembrane domains (28). Our present results reconcile these two proposals by suggesting that, for full length, membrane-bound CD39, the tense and relaxed states correspond to moderate and large degrees of transmembrane helix mobility. For the case of interconversion between functional states by bilayer alteration, mobility is the only transmembrane domain feature we observe to be altered; both TM1 and TM2 remain present and in the membrane and do not appear to dissociate or to convert to a different primary conformation. Conversion to the relaxed state by removing TM1 or TM2 also increases rotational mobility of the remaining helix, suggesting that when not restrained by both the opposite helix and the membrane each helix is relatively unconstrained by the extracellular domain. Removing both TM1 and TM2 may thus achieve the same functional conversion by relieving the two remaining ends of the extracellular domain of restrictions on their own mobility. Thus, the native functional state appears to occur when the transmembrane domains and the membrane itself work together to establish an optimal balance between stability and mobility; the relaxed state occurs when either the transmembrane domains or the membrane are altered to allow too much mobility, allowing the intrinsic properties of the presumably more flexible extracellular domain to dominate.
The relaxed state is correlated not only with a change in enzymatic properties but also with a loss of coupling between substrate binding and helix mobility. In the native state, the transmembrane helices move relative to each other but substrate can reduce such motions; in contrast, when the enzyme is converted to the relaxed state by altering membrane properties, substrate can no longer stabilize the transmembrane helices. Previous experiments have indicated that soluble CD39 has an even higher substrate affinity than native CD39 and that both have a Km well below the concentrations used here (17, 24, 25), suggesting that the loss of coupling more likely reflects a change in the relationship between binding site and transmembrane domain dynamics than a lack of binding. One potential explanation is that altering the membrane reduces the energy barriers among helix orientations such that thermal energy is sufficient to overcome the stabilizing influence of substrate binding. Alternatively, the mode of substrate binding itself may change in the relaxed state. A change in binding mode would be consistent with the documented change in hydrolysis mechanism with respect to ACR1 (24). Since ACR1 and ACR5 are adjacent to TM1 and TM2, and since we observe that both helices are required for the substrate sensitivity of either TM1 or TM2, it might be of interest to compare the role of ACR5 in substrate binding and hydrolysis in the tense and relaxed states.
Why a membrane-induced increase in mobility would change the enzymatic properties of CD39 is unknown. If, as documented for P-glycoprotein (42, 43), the active site and transmembrane domains undergo discrete coordinated motions during nucleotide hydrolysis, the observed loss of coupling between CD39 substrate binding and helix stabilization suggests that membrane alteration would also abolish coordination between helices and active site at other stages of nucleotide hydrolysis. Enzymatic behavior may also be directly related to the degree of active site mobility, as has been reported for other enzymes as well as for other proteins that recognize a broad array of substrates (44). The native ability to hydrolyze ATP and ADP in succession without intermediate ADP release might potentially be particularly dependent on an appropriate balance between stability and flexibility.
The fact that structurally diverse amphiphiles and cholesterol removal all produce the same enzymatic and conformational results suggests that the transmembrane domains sense a mechanical property of the bilayer rather than the direct presence of a specific molecule. Each of the molecules alters several parameters such as fluidity, tension, curvature, and elasticity; in general these effects are difficult to separate and thus distinguishing exactly which parameter a given protein senses is not always possible. Nevertheless, our results in combination with previous studies suggest that CD39 is more sensitive to certain parameters than to others. The fact that cone shaped and inverse cone shaped molecules have identical effects despite their opposite influences on spontaneous curvature indicates that curvature is not the primary determinant of CD39 behavior. Previously fluidity has been proposed to regulate the related two transmembrane domain family members eNTPDase2 and 8 (45); however, the conformational changes we observe are intramolecular, and fluidity has recently been somewhat discounted as an explanation for changes in protein activity since it cannot change the equilibrium among states (46). In addition, Papanikolaou et al (30) showed that adding cholestenone to cholesterol depleted membranes does not restore CD39 activity despite the fact that it restores fluidity. However, our results parallel those of Lundbaek and colleagues, who have shown that the same variety of unsaturated fatty acids, cone-shaped molecules, and cholesterol depletion all have similar effects on voltage dependent sodium channels as well as on gramicidin channels (29, 37). Based on their study of the springlike behavior of the bilayer they proposed that while these molecules have opposite effects on curvature they all increase bilayer elasticity and consequently lower the total energy for changes in protein conformation (29). Since CD39 responds to the same array of membrane treatments and exhibits an increased propensity to change transmembrane domain conformation, bilayer elasticity may also be the predominant physical property that regulates CD39.
Our observations that CD39 is highly responsive to changes in bilayer mechanical properties suggest that its functional state can be regulated by the state of the membrane in living tissues. Bilayer properties are altered in vivo, particularly in vascular endothelial cells in which CD39 resides, directly by mechanical pressure, shear stress, and changes in osmotic pressure, by release and insertion of unsaturated fatty acids and LPC during inflammatory processes and oxidative stress, and by changes in cholesterol levels. Furthermore, extracellular nucleotides play a central role in cellular responses to and modulation of many of these processes (3–5). Mechanical pressure, shear stress, and osmotic stress trigger ATP release, and in at least the latter case the resulting extracellular ATP is required to restore osmotic balance. Signaling by extracellular nucleotides also modulates inflammatory processes and responses to oxidative stress, and the balance among extracellular ATP, ADP, and adenosine regulates blood clotting, which can be associated with cholesterol levels. The ability of CD39 to respond instantaneously to such changes in the membrane by altering its hydrolysis rate, substrate specificity, and intermediate ADP release during ATP hydrolysis might thus provide a direct feedback mechanism by which extracellular nucleotide signaling is tailored to the state of the cell.
Modulation of transmembrane helix mobility may also have implications for CD39 functions other than nucleotidase activity. Bodas et al (47) have shown that CD39 can serve as an ATP channel, and Wu et al (48) have found that the short cytoplasmic tail can bind Ran binding protein M and thereby regulate nucleotidase activity, suggesting a potential role for the transmembrane helices in relaying information between the cytoplasm and the active site. Regulation of transmembrane helix mobility according to the state of the membrane might thus regulate their ability to transport nucleotides or signals across the membrane; in the latter case in particular, our observation of uncoupling between substrate binding and helix mobility suggests that coupling between ran binding protein interaction and nucleotidase activity might also be regulated by membrane properties. Regulation of the balance between stability and mobility of the transmembrane helices according to the physical state of the membrane may therefore provide a mechanism for interdependence among the active site, the bilayer, and the internal state of the cell.
Acknowledgments
We thank members of the Guidotti lab for discussions and advice.
Abbreviations
- eNTPDase
ecto-nucleoside triphosphate diphosphoshydrolase
- ACR
apyrase conserved region
- TM1
transmembrane helix 1
- TM2
transmembrane helix 2
- AA
arachidonic acid
- OA
oleic acid
- DOHA
docosahexaenoic acid
- LPL
lysophospholipid
- LPC
lysophosphatidylcholine
- LPI
lysophosphatidylinositol
- LPG
lysophosphatidylglycerol
- LPE
lysophosphatidylethanolamine
- CuP
copper phenanthroline
- CD
cyclodextrin
- NT
N-terminal truncated CD39
- CT
C-terminal truncated CD39
Footnotes
This work was supported by Grant HL08893 from the National Institutes of Health.
References
- 1.Zimmermann H, Beaudoin AR, Bollen M, Godig JW, Guidotti G, Kirley TL, Robson SC, Sano K. In: Ecto-ATPases and Related Nucleotidases. vanDuffel L, Lemmens R, editors. 2000. pp. 1–8. [Google Scholar]
- 2.Zimmermann H, Braun N, Kegel B, Heine P. New insights into molecular structure and function of ectonucleotidases in the nervous system. Neurochem Int. 1998;32:421–425. doi: 10.1016/s0197-0186(97)00126-5. [DOI] [PubMed] [Google Scholar]
- 3.Schweibert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615:7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 4.Brake AJ, Julius D. Signaling by extracellular nucleotides. Annu Rev Cell Dev Biol. 1996;12:519–541. doi: 10.1146/annurev.cellbio.12.1.519. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. In: The P2 Nucleotide Receptors. Turner JT, Weisman GA, Fedan JS, editors. 1998. pp. 3–42. [Google Scholar]
- 6.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 7.Gayle RB, Maliszewski CR, Gimpel SD, Schoenborn MA, Caspary RG, Richards C, Brasel K, Price V, Drosopoulos JH, Islam Alyonycheva TN, Broekman MJ, Marcus AJ. Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J Clin Invest. 1998;101:1851–1859. doi: 10.1172/JCI1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus AJ, Broekma MJ, Drosopoulos JH, Islam N, Pinsky DJ, Sesti C, Levi R. Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases. J Pharmacol Exp Ther. 2003;305:9–16. doi: 10.1124/jpet.102.043729. [DOI] [PubMed] [Google Scholar]
- 9.Wang TF, Guidotti G. Golgi localization and functional expression of human uridine diphosphatase. J Biol Chem. 1998;273:11392–99. doi: 10.1074/jbc.273.18.11392. [DOI] [PubMed] [Google Scholar]
- 10.Braun N, Fengler S, Ebeling C, Servos J, Zimmermann H. Sequencing, functional expression and characterization of rat NTPDase6, a nucleoside diphosphatase and novel member of the ecto-nucleoside triphosphate diphosphohydrolase family. Biochem J. 2000;351:639–647. [PMC free article] [PubMed] [Google Scholar]
- 11.Shi JD, Kukar T, Wang CY, Li QZ, Cruz PE, Davoodi-Semiromi A, Gu Y, Lian W, Wu DH, She JX. Molecular cloning and characterization ofa novel mammalian endo-apyrase (LALP1) J Biol Chem. 2001;276:17474–78. doi: 10.1074/jbc.M011569200. [DOI] [PubMed] [Google Scholar]
- 12.Handa M, Guidotti G. Purification and cloning of a soluble ATP-diphosphohydrolase (apyrase) from potato tubers (Solanum tuberosum. Biochem Biophys Res Commun. 1996;218:916–923. doi: 10.1006/bbrc.1996.0162. [DOI] [PubMed] [Google Scholar]
- 13.Schulteam Esch J, Sevigny J, Kaczmarek E, Siegel JB, Imai M, Koziak K, Beaudoin AR, Robson SC. Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry. 1999;38:2248–2258. doi: 10.1021/bi982426k. [DOI] [PubMed] [Google Scholar]
- 14.Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc Natl Acad Sci. 1992;89:7290–94. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handa M. PhD thesis. Cambridge: Harvard University; 1997. [Google Scholar]
- 16.Smith TM, Kirley TL. Site-directed mutagenesis of a human brain ecto-apyrase: evidence that the E-type ATPases are related to the actin/heat shock 70/sugar kinase superfamily. Biochemistry. 1999;38:321–29. doi: 10.1021/bi9820457. [DOI] [PubMed] [Google Scholar]
- 17.Drosopoulos JH, Broekman MJ, Islam N, Maliszewski CR, Gayle RB, Marcus AJ. Site-directed mutagenesis of human endothelial cell ecto-ADPase/soluble CD39: requirement of glutamate 174 and serine 218 for enzyme activity and inhibition of platelet recruitment. Biochemistry. 2000;39:6936–6943. doi: 10.1021/bi992581e. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Hicks-Berger CA, Smith TM, Kirley TL. Site-directed mutagenesis of human nucleoside triphosphate diphosphohydrolase 3: the importance of residues in the apyrase conserved regions. Biochemistry. 2001;40:3943–3950. doi: 10.1021/bi002711f. [DOI] [PubMed] [Google Scholar]
- 19.Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Ann Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- 20.Chadwick BP, Frischauf AM. The CD39-like gene family: identification of three new human members (CD39L2, CD39L3, and CD39L4), their murine homologues, and a member of the gene family from Drosophila melanogaster. Genomics. 1998;50:357–367. doi: 10.1006/geno.1998.5317. [DOI] [PubMed] [Google Scholar]
- 21.Maliszewski CR, Delespesse GJ, Schoenborn MA, Armitage RJ, Fanslow WC, Nakajima T, Baker E, Sutherland GR, Poindexter K, Birks C, et al. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J Immunol. 1994;153:3574–3583. [PubMed] [Google Scholar]
- 22.Grinthal A, Guidotti G. CD39 has two transmembrane domains. Why? Purinergic Signalling. 2006;2:391–8. doi: 10.1007/s11302-005-5907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang TF, Ou Y, Guidotti G. The transmembrane domains of ectoapyrase (CD39) affect its enzymatic activity and quaternary structure. J Biol Chem. 1998;273:24814–24821. doi: 10.1074/jbc.273.38.24814. [DOI] [PubMed] [Google Scholar]
- 24.Grinthal A, Guidotti G. Substitution of His59 converts CD39 apyrase into an ADPase in a quaternary structure dependent manner. Biochemistry. 2000;39:9–16. doi: 10.1021/bi991751k. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Guidotti G. Soluble apyrases release ADP during ATP hydrolysis. Biochem Biophy Res Commun. 2001;282:90–95. doi: 10.1006/bbrc.2001.4555. [DOI] [PubMed] [Google Scholar]
- 26.Heine P, Braun N, Heilbronn A, Zimmermann H. Functional characterization of rat ecto-ATPase and ecto-ATP diphosphohydrolase after herterologous expression in CHO cells. Eur J Biochem. 1999;262:102–7. doi: 10.1046/j.1432-1327.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- 27.Failer BU, Aschrafi A, Schmalzing G, Zimmermann H. Determination of native oligomeric state and substrate specificity of rat NTPDase1 and NTPDase2 after heterologous expression in Xenopus oocytes. Eur J Biochem. 2003;270:1802–1809. doi: 10.1046/j.1432-1033.2003.03542.x. [DOI] [PubMed] [Google Scholar]
- 28.Grinthal A, Guidotti G. Dynamic motions of CD39 transmembrane domains regulate and are regulated by the enzymatic active site. Biochemistry. 2004;43:13849–58. doi: 10.1021/bi048644x. [DOI] [PubMed] [Google Scholar]
- 29.Lundbaek JA, Birn P, Hansen AJ, Sogaard R, Nielsen C, Girshman J, Bruno MJ, Tape SE, Egebjerg J, Greathouse DV, Mattice GL, Koeppe RE, Andersen OS. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of micelle-forming amphiphiles and cholesterol. J Gen Physiol. 2004;123:599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papnikolauo A, Papfotika A, Murphy C, Papmarcaki T, Tsolas O, Drab M, Kurzchalia TV, Kasper M, Christoforidis S. Cholesterol-dependent lipid assemblies regulate the activity of the ecto-nucleotidase CD39. J Biol Chem. 2005;280:26406–14. doi: 10.1074/jbc.M413927200. [DOI] [PubMed] [Google Scholar]
- 31.Coppi MV, Guidotti G. Intracellular localization of Na, K-ATPase alpha2 subunit mutants. Arch Biochem Biophys. 1997;346:312–321. doi: 10.1006/abbi.1997.0332. [DOI] [PubMed] [Google Scholar]
- 32.Ames BN. Assay of inorganic phosphate, total phosphate, and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 33.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 34.Patel AJ, Lazdunski M, Honore E. Lipid and mechano-gated 2P domain K(+) channels. Curr Opin Cell Biol. 2001;13:422–8. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 35.Casado M, Ascher P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity. J Physiol. 1998;513:317–30. doi: 10.1111/j.1469-7793.1998.317bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat Struct Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 37.Lundbaek JA, Birn P, Tape SE, Toombes GES, Sogaard R, Koeppe RE, Gruner SM, Hansen AJ, Andersen OS. Capsaicin regulates voltage-dependent sodium channels by altering bilayer elasticity. Mol Pharmacol. 2005;68:680–9. doi: 10.1124/mol.105.013573. [DOI] [PubMed] [Google Scholar]
- 38.Careaga CL, Falke JJ. Thermal motions of surface α-helices in the D-galactose chemosensory receptor. J Mol Biol. 1992;226:1219–35. doi: 10.1016/0022-2836(92)91063-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butler SL, Falke JJ. Effects of protein stabilizing agents on thermal backbone motions: a disulfide trapping study. Biochemistry. 1996;35:10595–600. doi: 10.1021/bi961107v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao B, Porollo A, Adamczak R, Jarrell M, Meller J. Enhanced recognition of protein transmembrane domains with prediction-based structural profiles. Bioinformatics. 2006;22:303–9. doi: 10.1093/bioinformatics/bti784. [DOI] [PubMed] [Google Scholar]
- 41.Grinthal A, Guidotti G. Transmembrane domains confer different substrate specificities and adenosine diphosphate hydrolysis mechanisms on CD39, CD39L1, and chimeras. Biochemistry. 2002;41:1947–56. doi: 10.1021/bi015563h. [DOI] [PubMed] [Google Scholar]
- 42.Loo TW, Clarke DM. Vanadate trapping of nucleotide at the ATP-binding sites of human multidrug resistance P-glycoprotein exposes different residues to the drug-binding site. Proc Nat Acad Sci. 2002;99:3511–3516. doi: 10.1073/pnas.022049799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loo TW, Bartlett MC, Clarke DM. Drug binding in human P-glycoprotein causes conformational changes in both nucleotide-binding domains. J Biol Chem. 2003;278:1575–1578. doi: 10.1074/jbc.M211307200. [DOI] [PubMed] [Google Scholar]
- 44.Kay LE. Protein dynamics from NMR. Nature Structural Biology: NMR supplement. 1998:513–517. doi: 10.1038/755. [DOI] [PubMed] [Google Scholar]
- 45.Mukasa T, Lee Y, Knowles AF. Either the carboxy- or the amino-terminal region of the human ecto-ATPase (E-NTPDase2) confers detergent and temperature sensitivity to the chicken ecto-ATP-diphosphohydrolase (E-NTPDase 8) Biochemistry. 2005;44:11160–70. doi: 10.1021/bi050019k. [DOI] [PubMed] [Google Scholar]
- 46.Lee AG. How lipids affect the activities of integral membrane proteins. Biochim Biophys Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Bodas E, Aleu J, Pujol G, Martin-Satue M, Marsal J, Solsona C. ATP crossing the cell plasma membrane generates an ionic current in Xenopus oocytes. J Biol Chem. 2000;272:20268–20273. doi: 10.1074/jbc.M000894200. [DOI] [PubMed] [Google Scholar]
- 48.Wu Y, Sun X, Kaczmarek E, Dwyer KM, Bianchi E, Usheva A, Robson SC. RanBPM associates with CD39 and modulates ecto-nucleotidase activity. Biochem, J. 2006 doi: 10.1042/BJ20051568. [Feb 14 Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


