Abstract
The purpose of the research was to demonstrate that comorbid health conditions disproportionately affect elderly cancer patients. Descriptive analyses and stacked area charts were used to examine the prevalence and severity of comorbid ailments by age of 27,506 newly diagnosed patients treated at one of eight cancer centers between 1998 and 2003. Hypertension was the most common ailment in all patients, diabetes was the second most prevalent ailment in middle-aged patients, and previous solid tumor(s) were the second most prevalent ailment in patients aged 74 and older. Although the prevalence and severity of comorbid ailments including dementia and congestive heart failure increased with age, some comorbidities such as HIV/AIDS and obesity decreased. Advances in cancer interventions have increased survivorship, but the impact of the changing prevalence and severity of comorbidities at different ages has implications for targeted research into targeted clinical and psychosocial interventions.
Keywords: Cancer, comorbidities, epidemiology
1.1 Introduction
As the population in the United States and other industrialized nations ages, cancer will increasingly constitute an important public health concern since cancer incidence and mortality disproportionately affects elderly individuals.[1,2] Yancik and Ries [3] that elderly patients currently account for 60% of incident tumors and account for 70% of cancer mortality. Research suggests that in the next two decades, almost 20% of the population will be aged 65 or older, and almost 70% of all malignancies will occur in these individuals.[2,4] Among the major tumor sites —lung, colon, breast, and prostate — at least half of newly diagnosed patients are over 65 years of age.[5]
Comorbid health conditions such as heart disease, pulmonary disease, diabetes, and arthritis are commonly present in elderly patients.[3,6,7] The diagnosis of cancer in the senior adult population is often made amidst the diagnosis or treatment of other medical conditions and geriatric syndromes (i.e. cognitive impairment, depression, polypharmacy secondary to multiple comorbidities).[8] In one study, it was found that four out of five older Americans have at least one significant medical condition.[9] These illnesses may present as single conditions or as combinations of conditions. The variety of comorbid conditions and their individual severity, as well as the cumulative impact of these conditions, have the potential to uniquely impact the cancer patient's treatment and prognosis.[8,10-14]
In this study, the interdependent nature of age and comorbidity in newly diagnosed cancer patients was analyzed. Specifically, the question of how the prevalence and severity of individual comorbid ailments change with the changing age of the cancer patient was investigated. Understanding the prevalence of specific comorbid illnesses across the aging spectrum guides future research and development of targeted interdisciplinary team assessments and interventions. These assessments and interventions are aimed at recognizing and managing comorbidities and geriatric syndromes in cancer patients, thereby improving the quantity and quality of cancer survivorship.
2.1 Methods
This was an observational study of newly diagnosed cancer patients treated at one of eight participating cancer care facilities between January 1998 and July 2003. Each of the facilities is an American College of Surgeons-approved cancer program and maintains a cancer registry according to the guidelines of the Commission on Cancer. Of these facilities, four are Community Hospital Comprehensive Cancer Programs (COMP) located in different cities in Florida, Missouri, and North Dakota; one is a Teaching Hospital Cancer Program (THCP) located in Washington, D.C.; one is a Community Hospital Cancer Program (CHCP) located in Missouri, one is an ACOS-designated Affiliate Hospital Cancer Program (AFCP) in Texas, and one is an NCI-Designated Comprehensive Cancer Center Program (NCIP) located in Missouri.
Demographic, tumor, treatment, and follow-up information were gathered according to the guidelines of the American College of Surgeons Commission on Cancer and published in the Registry Operations and Data Standards (ROADS) (1996 through 2002) and Facility Oncology Registry Data Standards (FORDS) (2002-current) manuals. Medical comorbidity information was gathered prospectively from the medical record by trained cancer registrars at the time of routine cancer registry data extraction. Comorbid conditions were defined according to the Adult Comorbidity Evaluation-27 (ACE-27) index.[15] The ACE-27 is a validated comorbidity index developed especially for adult cancer patients. The ACE-27 captures different comorbid conditions and grades the degree of organ decompensation (Mild, Moderate, or Severe) for each comorbid condition, except obesity (BMI >=38), which is scored as Moderate. Severe decompensation for each medical comorbidity reflects end-organ disease and is thought to be irreversible. Mild comorbid ailments can be treated well with conservative measures and result in little morbidity. Moderate ailments require treatment to prevent future morbidity and mortality. Since cancer patients often have multiple concurrent medical comorbidities, an overall comorbidity score (Mild, Moderate, or Severe) is assigned. The overall comorbidity score is based upon either the severity of the most severe individual condition or assigned the Severe category when two or more Moderate individual comorbid conditions are present in different organ systems. Participating cancer registrars all successfully completed a validated web-based comorbidity education program prior to coding comorbidity. This web-based program was developed with an educational grant from the National Cancer Institute and is intended to train cancer registrars and other health care professionals. The ACE-27 index, coding instructions, online calculator, and web-based education program can be viewed at http://cancercomorbidity.wustl.edu. Population-based national data from the National Center for Health Statistics [16] was used to compare selected comorbid ailments within our cohort with national norms.
Data analysis included descriptive statistics of demographics, tumor stage and site, overall comorbidity score, and individual comorbid ailments. SAS for Windows 8.2 was used to analyze frequencies for the different combinations of categories. For ease of presentation, we grouped patients into four different age categories: 18-53, 54-64, 65-73, and 74-102. To examine the changing prevalence of each individual comorbid ailment in cancer patients with age, we used restricted cubic spline functions in logistic regression models (S+ version 7.0, Insightful Inc, Seattle WA). These functions are smooth and flexible, and well suited to capture non-linear relationships.[17] Comorbidity categories with less than 200 patients were analyzed with splines with 3 knots (2 degrees of freedom). More frequent comorbidity categories were analyzed with more flexible functions (200-499 patients: 4 knots, 3 df; more than 500 patients: 5 knots, 4 df). Results are shown in stacked area charts. Stacked area charts display different categories of data stacked to illustrate relative size.
3.1 Results
3.1.1 Description of Study Population
Participating centers collected information for 29,216 cancer patients. Of this cohort, 112 (0.38%) patients under the age of 18 were excluded, 1,179 (4.0%) patients were excluded for having unknown comorbidity information, and 419 (1.4%) patients were excluded for having an unknown primary tumor site. The study population therefore includes 27,506 cancer patients who were all 18 years of age or older at the time of diagnosis. Consecutive patients, defined by diagnosis at reporting facility regardless of where the first course of treatment occurred or by diagnosis elsewhere but all or part of first course of treatment at participating center, were included. For 23,650 (86%) patients, the index cancer was their first cancer.
The proportion of this adult cohort that were age 65 or older was 47% (13,029) which is significantly higher than the proportion of American who are older than 65 (17%). The majority (85%) of patients were white and the exact proportion was slightly higher than the national estimate (82%).(Table 1) There were nearly equal numbers of men and women. Of the total population, 2898 (11%) had Severe overall level of comorbidity, while 14,964 (54%) of patients were determined to have either Mild or Moderate overall comorbidity severity level. The overall comorbidity severity score increases with increasing age (Figure 1). The mean number of comorbid conditions also increases as patients age. Patients in the youngest age group had, on average, less than one (0.7) comorbid ailment while patients in the oldest age group had, on average, 1.8 comorbid ailments. A subgroup analysis also demonstrates that with increasing age, the proportion of patients who present with mild comorbidities decreases while the proportion of patients who present with moderate and severe comorbidities increases. The degree of tumor spread and site of involvement showed results similar to those of national statistics.[18,19]
Table 1. Description of Population and Clinical Characteristics.
| Variable | Characteristic | N (%) |
|---|---|---|
| Age | 18-53 | 7129 (25.9) |
| 54-64 | 7348 (26.7) | |
| 65-73 | 6680 (24.3) | |
| 74-102 | 6349 (23.1) | |
| Race | White | 23288 (84.8) |
| Black | 3785 (13.8) | |
| Other | 363 (1.3) | |
| Gender | Male | 13958 (50.7) |
| Female | 13547 (49.3) | |
| Comorbidity | None | 9644 (35.1) |
| Mild | 10131 (36.8) | |
| Moderate | 4833 (17.6) | |
| Severe | 2898 (10.5) | |
| Tumor Stage | In situ/ Local | 12080 (43.9) |
| Regional | 6041 (21.9) | |
| Distant metastases | 4386 (15.9) | |
| Tumor Site | Prostate | 4623 (16.8) |
| Breast | 4479 (16.3) | |
|
| ||
| Lung | 3793 (13.8) | |
| Colorectal | 2755 (10.0) | |
| Gynecological | 2130 (7.7) | |
| Oral Cavity/ Larynx | 1523 (5.5) | |
| Skin (excluding basal cell) | 893 (3.2) | |
| Kidney | 769 (2.8) | |
| Nervous System | 514 (1.9) | |
| Musculoskeletal | 413 (1.5) | |
Figure 1.
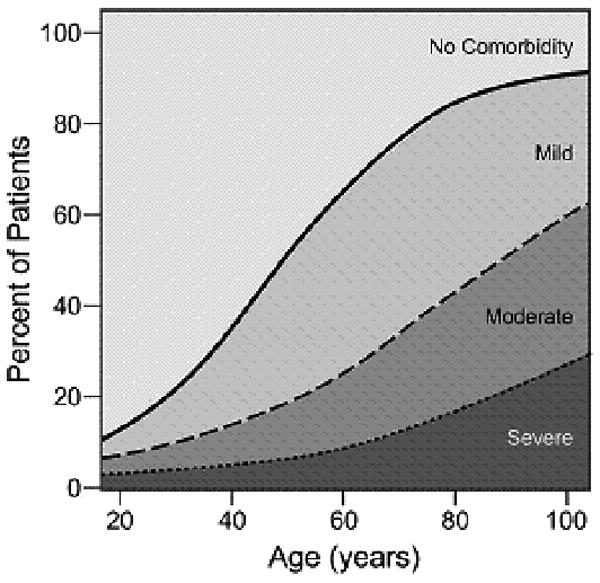
Prevalence of overall comorbidity by severity across the age spectrum
3.1.2 Comorbidity Prevalence Analysis
Hypertension was the most common comorbid ailment with 36% of the cohort affected overall, with the frequency increasing with increasing age -- 19% of patients aged 18 to 53 years old to 46% of patients aged 74 to 102 years old.(Table 2) The next most common comorbid ailments were secondary solid tumor(s) (12%), respiratory disease (12%), diabetes mellitus (12%), and angina/coronary artery disease (10%).
Table 2. Prevalence of Each Comorbid Ailment in Different Age Cohorts.
| Comorbid Ailment | 18-53
N (%) |
54-64
N (%) |
65-73
N (%) |
74-102
N (%) |
|---|---|---|---|---|
| Myocardial Infarct | 113 (1.6) | 346 (4.7) | 524 (7.8) | 673 (10.6) |
| Angina/ Artery Disease | 148 (2.1) | 560 (7.6) | 924 (13.8) | 1129 (17.8) |
| Congestive Heart Failure | 54 (0.8) | 150 (2.0) | 200 (3.0) | 487 (7.7) |
| Hypertension | 1349 (18.9) | 2588 (35.2) | 2959 (44.3) | 2946 (46.4) |
| Arrhythmias | 69 (1.0) | 204 (2.8) | 363 (5.4) | 760 (12.0) |
| Venous Disease | 43 (0.6) | 75 (1.0) | 65 (1.0) | 82 (1.3) |
| Peripheral Arterial Disease | 19 (0.3) | 93 (1.3) | 164 (2.5) | 212 (3.3) |
| Respiratory Disease | 429 (6.0) | 758 (10.3) | 996 (14.9) | 1075 (16.9) |
| Hepatic Disease | 157 (2.2) | 122 (1.7) | 85 (1.3) | 65 (1.0) |
| Gastro-Intestinal Disease | 171 (2.4) | 256 (3.5) | 299 (4.5) | 348 (5.5) |
| Pancreatic Disease | 13 (0.2) | 13 (0.2) | 13 (0.2) | 10 (0.2) |
| Renal Disease | 60 (0.8) | 77 (1.0) | 115 (1.7) | 169 (2.7) |
| Diabetes Mellitus | 403 (5.7) | 824 (11.2) | 1033 (15.5) | 958 (15.1) |
| Stroke | 54 (0.8) | 162 (2.2) | 277 (4.1) | 502 (7.9) |
| Dementia | 3 (0.0) | 3 (0.0) | 29 (0.4) | 268 (4.2) |
| Paralysis | 8 (0.1) | 10 (0.1) | 3 (0.0) | 3 (0.0) |
| Neuromuscular Disease | 47 (0.7) | 45 (0.6) | 60 (0.9) | 81 (1.3) |
| Psychiatric Disorder | 365 (5.1) | 324 (4.4) | 207 (3.1) | 256 (4.0) |
| Rheumatologic Disease | 74 (1.0) | 127 (1.7) | 145 (2.2) | 147 (2.3) |
| HIV/AIDS | 140 (2.0) | 29 (0.4) | 11 (0.2) | 1 (0.0) |
| Previous Solid Tumor(s) | 438 (6.1) | 659 (9.0) | 939 (14.1) | 1284 (20.2) |
| Leukemia and Myeloma | 3 (0.0) | 14 (0.2) | 27 (0.4) | 16 (0.3) |
| Lymphoma | 11 (0.2) | 17 (0.2) | 21 (0.3) | 27 (0.4) |
| Alcohol Abuse | 188 (2.6) | 197 (2.7) | 122 (1.8) | 65 (1.0) |
| Illicit Drug Abuse | 123 (1.7) | 31 (0.4) | 16 (0.2) | 2 (0.0) |
| Obesity (BMI >38) | 275 (3.9) | 246 (3.3) | 157 (2.4) | 102 (1.6) |
Estimates for the prevalence of hypertension in our cohort are lower than national estimates.[20] For example, males between the ages of 65 and 74 in our study had a prevalence of hypertension of 42%, while the estimate from the national survey was 58%. Women between the ages of 65 and 74 in our study had a prevalence of 47%, while the frequency was 74% in the national survey. It should also be noted that our finding of less than 3% of the study population having experienced alcohol abuse is at odds with the national estimates of 8% of the population self-reported as “heavy” drinkers[16] or 36% of adults seeking care at an outpatient medical practice of an urban university teaching hospital who met criteria for a history of alcohol abuse or dependence. [21] In addition, our finding of less than 4% of the study cohort reported as morbidly obese (BMI >38) differs from national estimates of obesity.[22] Dementia was documented in only 4% of patients age 74-102.
The different comorbid ailments showed different prevalence patterns across the age spectrum. Most comorbid ailments exhibited expected relationship to age. For example, the prevalence of neurological conditions, such as stroke, increased with age while the prevalence of illicit drug abuse and obesity decreased with age. Diabetes was the second most prevalent ailment in patients aged 54-73, and previous solid tumor(s) were the second most prevalent ailment in patients aged 74 and greater. The proportion of patients with mild, moderate, and, especially, severe dementia increases dramatically among patients aged 74 and older when compared to patients aged 65-73. In addition, the proportion of patients in the oldest age group with hypertension and diabetes decreased in comparison with patients aged 65-73. This finding suggests that the benefit of intervention from geriatricians may be greater in younger senior adult patients with mild and moderate forms of the diseases.
The relationship between comorbid ailments and tumor sites was explored and several interesting findings emerged (Table 3). As mentioned above, hypertension was the most common comorbid ailment regardless of tumor site. For patients with lung cancer, respiratory disease (28%) and previous solid tumor (18%) were the most common comorbid ailment. Women with breast cancer were much less likely to have cardiac comorbidities of angina (4%) and myocardial infarction (3%), than prostate, lung, or colorectal patients. This may be because the breast cancer patients under observation were younger than their male counterparts with prostate, colorectal or lung cancer. It has been suggested that women develop cardiovascular disease later than men.(23)
Table 3. Most Prevalent Comorbid Ailment by Tumor Site.
| Primary Tumor Site | N (%) | Primary Tumor Site | N (%) |
|---|---|---|---|
| Prostate | 4623 (100) | Lung | 3793 (100) |
|
| |||
| Hypertension | 1694 (36.6) | Hypertension | 1428 (37.7) |
| Angina | 588 (12.7) | Respiratory Disease | 1081 (28.5) |
| Diabetes | 458 (9.9) | Previous Solid Tumor | 681 (18.0) |
| Respiratory Disease | 363 (7.9) | Angina | 539 (14.2) |
| Myocardial Infarct | 308 (6.7) | Diabetes | 421 (11.1) |
| Previous Solid Tumor | 281 (6.1) | Myocardial Infarct | 375 (9.9) |
| Stroke | 136 (2.9) | Stroke | 202 (5.3) |
| Stomach/Intestinal Disease | 121 (2.6) | Congestive Heart Failure | 194 (5.1) |
| Psychiatric Disease | 99 (2.1) | Stomach/Intestinal Disease | 191 (5.0) |
| Congestive Heart Failure | 84 (1.8) | Psychiatric Disease | 182 (4.8) |
|
| |||
| Breast | 4479 (100) | Colorectal | 2755 (100) |
|
| |||
| Hypertension | 1544 (34.5) | Hypertension | 1140 (41.4) |
| Previous Solid Tumor | 554 (12.4) | Diabetes | 429 (15.6) |
| Diabetes | 464 (10.4) | Previous Solid Tumor | 393 (14.3) |
| Respiratory Disease | 366 (8.2) | Angina | 332 (12.1) |
| Psychiatric Disease | 260 (5.8) | Respiratory Disease | 321 (11.7) |
| Angina | 190 (4.2) | Myocardial Infarct | 221 (8.0) |
| Obesity | 173 (3.9) | Congestive Heart Failure | 150 (5.4) |
| Myocardial Infarct | 137 (3.1) | Stroke | 146 (5.3) |
| Stroke | 125 (2.8) | Stomach/Intestinal Disease | 129 (4.7) |
| Stomach/Intestinal Disease | 108 (2.4) | Psychiatric Disease | 112 (4.1) |
As cancer patients age, the number and severity of comorbid conditions increased with most patients over the age of 80 suffering from at least one comorbid ailment. The graphical display of the association between changing prevalence and severity of comorbid ailments across the age spectrum is shown for six common comorbid ailments (Figures 2A-2F). Many different patterns emerge from the data. For example, the prevalence of some comorbid ailments increases sharply with increasing age, like dementia (Figure 2A) and congestive heart failure (Figure 2B). However, the prevalence of severe comorbidity is greater in the case of dementia than in congestive heart failure, where the increase is driven by an increase in cases of mild comorbidity. The prevalence of previous solid tumor(s) (Figure 2C) increases more gradually with age. On the other hand, HIV/AIDS (Figure 2D) decreases in prevalence as patients age. Some diseases, such as diabetes mellitus (Figure 2E) and hypertension (Figure 2F) increase during the young adult age range, plateau during late middle age, and begin declining with advanced age. The prevalence of both diabetes and hypertension are primarily driven by mild severity of disease.
Figure 2(2.A - 2.F).
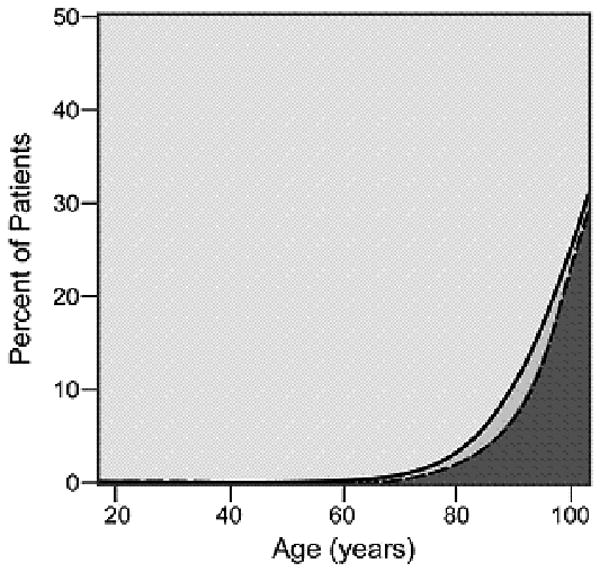
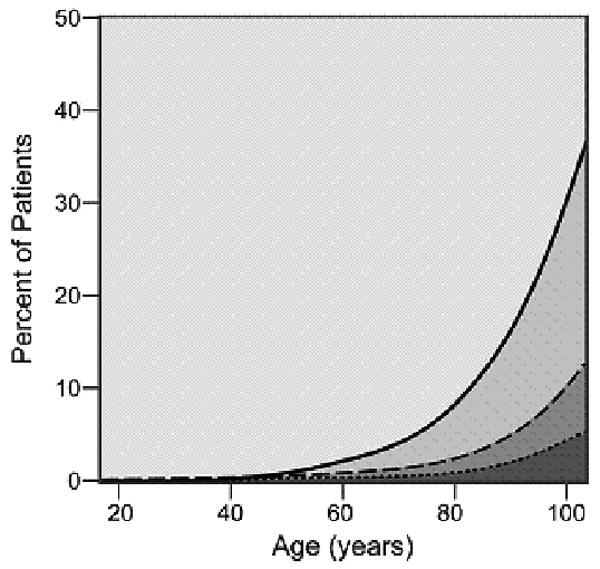
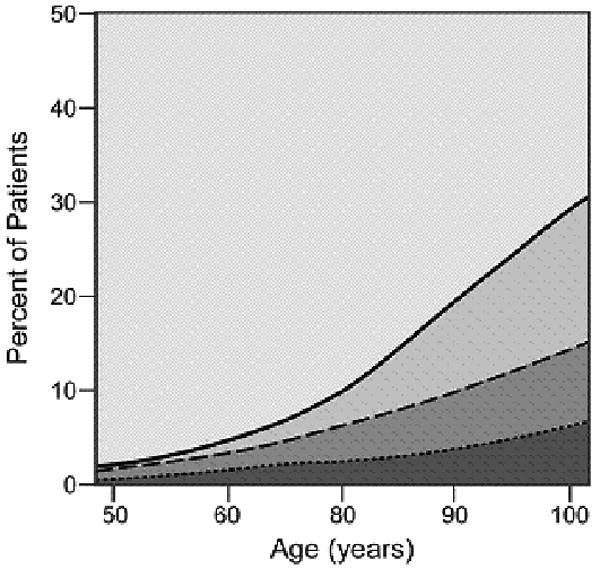
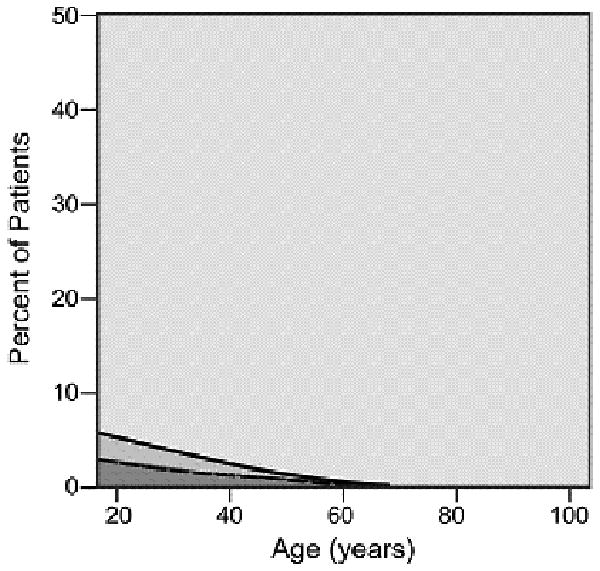
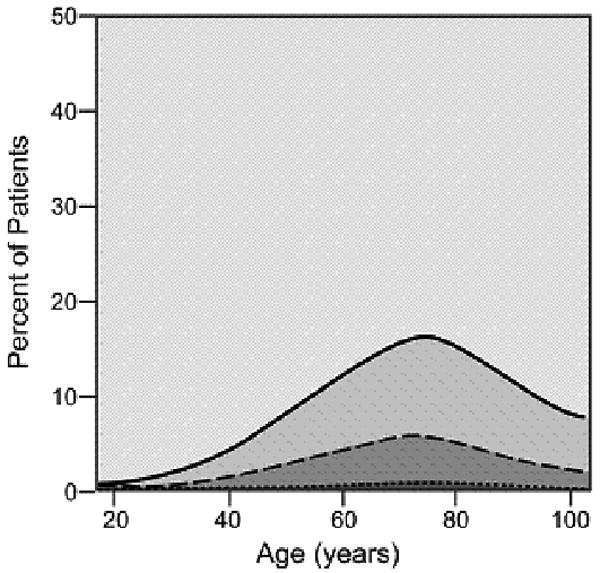
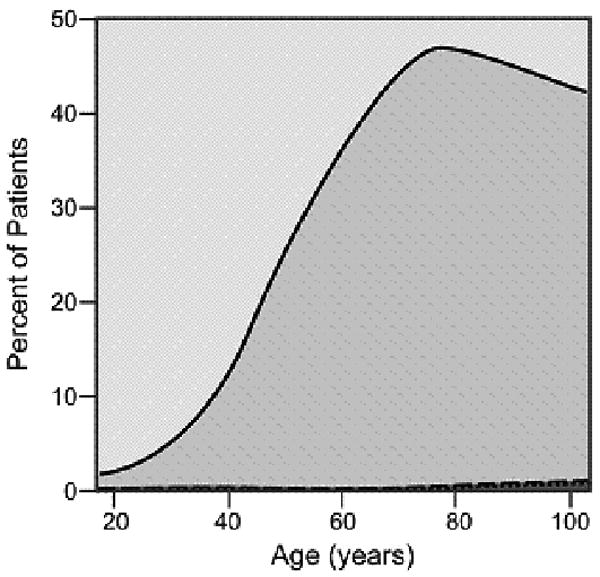
Prevalence of various comorbid ailments by severity across the age spectrum.
- 2.A Dementia
- 2.B. Congestive Heart Failure
- 2.C Previous Solid Tumor(s)
- 2.D HIV/AIDS
- 2.E Diabetes Mellitus
- 2.F Hypertension
4.1 Discussion
This study of over 27,000 newly diagnosed cancer patients documents the remarkable patterns of prevalence and severity of comorbidities as patients age. In this cohort, over half of the cancer patients had overall Mild or Moderate comorbidity severity. Patients with this degree of comorbidity may benefit from interdisciplinary assessments designed to recognize and treat pre-existing and incident comorbid illnesses and geriatric syndromes during cancer therapy. In addition, 10% of the patients in our cohort presented with an overall comorbidity score of Severe, indicating at least 2 moderate comorbidities or at least 1 comorbidity with irreversible end-organ damage. Patients with Severe comorbidity may benefit from a targeted assessment of functional status and early discussions regarding goals of care. Natural collaborators in the care of this patient population would be palliative care physicians and nurses.
Not surprisingly, it was noted the varying prevalence of certain comorbid illnesses based on patient age. Conditions such as HIV/AIDS, obesity, and illicit drug abuse were more prevalent in younger patients, while conditions such as dementia and congestive heart failure increased in prevalence and severity across the age spectrum. This information can be used to select screening questionnaires and functional and psychosocial assessments, as well as develop research protocols that address the most prevalent comorbid conditions for each patient age group. Finally, this study demonstrates that certain comorbid ailments are more prevalent in patients with certain tumor types. These results can guide patient selection for clinical care protocols and research studies that address specific comorbid illnesses and subsequent risk for experiencing an adverse event during cancer treatment (i.e., chemo-brain, functional decline, polypharmacy), thereby increasing efficiency and likelihood of impacting outcomes.
Dementia was rarely diagnosed in patients aged 65 and over, and if documented, the majority of cases received an ACE-27 Index Score of Severe for this comorbid illness. Epidemiology studies estimate the prevalence of dementia in persons aged 65 and older to be approximately 6-10% [24], while experts estimate the prevalence of mild cognitive impairment (impaired cognition for age that does not meet criteria for dementia) to be much higher. In a recent longitudinal cohort study of 1315 urban community-dwelling senior adults, 24% of subjects age 65-75 years and 33% of subjects age greater than 75 had mild cognitive impairment (MCI).[25] In clinical settings, MCI is unrecognized in the vast majority of patients and dementia is often not recognized until patients develop signs and symptoms in the advanced stages of disease. The data reported here support these findings. We suspect a substantial number of senior cancer patients have unrecognized cognitive impairment that may impact clinical and psychosocial outcomes without intervention. Small pilot studies documenting results of brief cognitive screens in the inpatient and outpatient settings note up to 27% of senior cancer patients are experiencing cognitive impairment (i.e., MCI, dementia, and/or delirium).[26,27] These results underscore the need for targeted screens to enhance recognition of cognitive impairment and interventions designed to promote patient safety (i.e., reduce risk for medication errors, delirium, falls) and compliance with cancer treatment, as noted by Extermann et al.[28] This remains an area for future research.
Previous reports have suggested that certain comorbidities are greater in cancer patients than in peer-matched cancer-free individuals.[29] Fried et al. [9] noted that the variation and severity of existing conditions increases with age among the elderly. Guralnik[30] noted that the increase in the co-occurrence of medical conditions increased risk for disability. Interestingly, we found the prevalence of hypertension considerably lower in our cohort than national estimates. This may be due to selection bias if in fact patients referred to a cancer center for treatment were considered by their primary care physician to be “healthy” and/or compliant enough to undergo cancer treatment. Conversely, this may also reflect true differences between cancer patients and a collection of respondents selected to be representative of the nation. Since it is difficult to imagine a biological effect that could account for almost a 30% difference in the prevalence of hypertension it probably reflects a combination of inadequacies in the medical record and selection bias. Although we did not investigate the co-occurrence or clustering of comorbid conditions, our study of singular conditions supports other research that investigated the changing prevalence of comorbidities in older patients.
The prevalence of newly diagnosed cancer patients with previous malignant tumors increased as patients age (6% in patients age 18-53; 20% in patients age 74 and over). These results are similar to a recent descriptive study from our institution of hospitalized cancer patients with a mean age 74.1 ± 5.9 years; 17% of these patients had another malignancy prior to their current cancer.27 Additionally, Janssen-Heijnen et al. [31], noted that the prevalence of previous cancer diagnoses increased with advanced age. Such findings are likely related to the fact that aging, in addition to prior exposure to chemotherapy and radiation, often facilitates carcinogenesis.[2,32,33]
Previous research[34] reported that the prevalence of hypertension and diabetes among women is highest in the older age groups. The results in this study support the findings of Yancik et al.[35] and Barbieri[36] that the prevalence of hypertension and diabetes actually begins to decline in the oldest age cohorts. One possible explanation is natural attrition of those with these comorbidities succumbing to their illness or related cardiovascular disease before reaching advanced age and acquiring a cancer diagnosis. To our knowledge, findings that HIV/AIDS was virtually non-existent in older age cohorts have not been fully examined in other cancer patient populations. This finding may be a reflection of the relative age-dependence of the risk behaviors associated with disease contraction.[37] We expect that future studies will show an increase in the prevalence of HIV/AIDS in the older age cohorts. Statistics show that 10-15% of all reported new HIV infections occur among people over the age of 50, with a quarter of these among people over the age of 60.[38] In addition, the advent and widespread use of anti-retroviral drugs for the treatment of HIV infection has led to a decrease in the morbidity and mortality among infected patients. As a result, more patients are surviving into older age.[39] Patients with HIV/AIDS may develop functional impairments similar to older adults without HIV.[40] An interdisciplinary team approach (i.e., physician, social worker, pharmacist, counselor) often utilized by providers caring for patients with HIV/AIDS may serve as a model for care for these patients who develop a malignancy.
The data in this study are instrumental in guiding clinical decision-making and developing targeted assessments and streamlined care protocols to address pre-existing conditions as well as risk factors for incident comorbidities and geriatric syndromes secondary to cancer treatment. A few small pilot studies have been conducted to explore the impact of interdisciplinary team models of care and interventions addressing comorbid illnesses and geriatric syndromes in cancer patients. Utilizing an interdisciplinary team consisting of a nurse, nurse practitioner, pharmacist, dietitian, social worker, and geriatric oncologist, Extermann and colleagues[28] identified an average of 5 stable comorbidities and 6 active medical problems (comorbidities and/or geriatric syndromes not maximally treated) during initial assessment of newly diagnosed elderly breast cancer patients with a mean age of 79. This interdisciplinary team intervention had a direct influence on the cancer treatment (i.e., ensuring compliance with therapy, enrolling in a clinical trial), and significantly improved quality of life scores over the 6-month study period.
Advances in cancer interventions have increased survivorship[41], but the impact of comorbidities, as well as their changing prevalence at different ages, has several implications for quality of life, treatment choice, duration of survival, and research into targeted clinical and psychosocial interventions. Comorbidities may mask symptoms of malignancies, or may highlight an existing or developing neoplasm.[42] As part of a comprehensive geriatric assessment of senior cancer patients, comorbidity assessment with the ACE-27 Index has the potential to more fully inform physicians about prognosis, treatment, and quality of life. Under-treated comorbid conditions and geriatric syndromes can combine with the index cancer and cancer treatment (chemotherapy, radiation, surgery) to decrease a patient's functional status and quality of life. Recognition of functional, psychosocial, and cognitive impairments is often missed using standard oncology performance assessment scales.[26] In a series of 800 patients aged 70 and over presenting to the Senior Adult Oncology Program at the H. Lee Moffitt Cancer Center, a comprehensive geriatric assessment detected at least one comorbid illness among 94% of the patients, 17% had dementia, and 56% were dependent in at least one instrumental activity of daily living (i.e., using the telephone, transportation, shopping, cooking, cleaning, laundry, medications, or finances).[4] A substantial proportion (45-70%) of older adult cancer patients presenting to cancer clinics for treatment have dependencies in basic and instrumental activities of daily living. Without interventions designed to recognize disabilities and/or improve mobility and functional performance as well as stabilize comorbid conditions, patients who may have been able to receive maximal cancer treatment may not tolerate standard therapy.[8,11] For example, chemotherapy in addition to pre-existing congestive heart failure and chronic bronchitis, may create a significant impact on a patient's ability to perform activities of daily living. An intervention to recognize early signs of functional decline and implement treatment with physical and occupational therapy may result in improved outcomes in such cases.
There are several limitations of this research. First, the ACE-27 comorbid assessment does not capture functional impairments or quality of life related to the comorbidity. Second, there may be under reporting of certain conditions, like hypertension, obesity, and alcohol abuse due to social stigma or legal ramifications related to the diagnosis. In addition, the criteria for classification of alcohol abuse on the ACE-27 is likely more stringent than common classification systems. Third, the impact of cancer treatment on the comorbid ailment was not considered. Fourth, the development of new comorbid ailments after the time of diagnosis was not considered.
The knowledge gained from this study of prevalence and severity of comorbid illnesses across the age spectrum adds to existing literature regarding factors impacting cancer survivorship. These data also highlight the need for further research targeting enhanced recognition and treatment of comorbidities and geriatric syndromes in older cancer patients.
Footnotes
Conflict of Interest Statement: None of the authors have any conflicts of interest. This research was supported by a grant from the National Cancer Institute (R01 CA10479-01). Study sponsors had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yancik R, Ries LA. Cancer in older persons: an international issue in an aging world. Semin Oncol. 2004;31(2):128–136. doi: 10.1053/j.seminoncol.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Balducci L. Epidemiology of cancer and aging. J Oncol Manag. 2005;14(2):47–50. [PubMed] [Google Scholar]
- 3.Yancik R, Ries LA. Aging and cancer in America. Demographic and epidemiologic perspectives. Hemato Oncol Clin North Am. 2000;14(1):17–23. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 4.Balducci L, Extermann M. Cancer and aging. An evolving panorama. Hematol Oncol Clin North Am. 2000;14(1):1–16. doi: 10.1016/s0889-8588(05)70274-4. [DOI] [PubMed] [Google Scholar]
- 5.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 6.Extermann M. Measurement and impact of comorbidity in older cancer patients. Crit Rev Oncol Hematol. 2000;35(3):181–200. doi: 10.1016/s1040-8428(00)00090-1. [DOI] [PubMed] [Google Scholar]
- 7.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453–471. doi: 10.1016/s0959-8049(99)00319-6. [DOI] [PubMed] [Google Scholar]
- 8.Yancik R, Ganz PA, Varricchio CG, et al. Perspectives on comorbidity and cancer in older patients: approaches to expand the knowledge base. J Clin Oncol. 2001;19(4):1147–1151. doi: 10.1200/JCO.2001.19.4.1147. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Wallace RB. The complexity of chronic illness in the elderly: From clinic to community. In: Wallace RB, Woolson RE, editors. The Epidemiologic Study of the Elderly. New York: Oxford University Press Inc; 1992. pp. 10–19. [Google Scholar]
- 10.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. J Clin Oncol. 2006;24(15):2304–2310. doi: 10.1200/JCO.2005.03.1567. [DOI] [PubMed] [Google Scholar]
- 11.Geraci JM, Escalante CP, Freeman JL, et al. Comorbid disease and cancer: the need for more relevant conceptual models in health services research. J Clin Oncol. 2005;23(30):7399–7404. doi: 10.1200/JCO.2004.00.9753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. Int J Cancer. 2001;103(6):792–802. doi: 10.1002/ijc.10882. [DOI] [PubMed] [Google Scholar]
- 13.Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22(15):3099–3103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 14.Janssen-Heijnen ML, Houterman S, Lemmens VEPP, et al. Prognostic impact of increasing age and co-morbidity in cancer patients: A population-based approach. Crit Rev Oncol Hematol. 2005;55:231–240. doi: 10.1016/j.critrevonc.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Piccirillo JF, Creech CM, Zequeira R, et al. Inclusion of comorbidity into oncology data registries. J Reg Manag. 1999;26(2):66–70. [Google Scholar]
- 16.National Center for Health Statistics. Health, United States, 2004 With Chartbook on Trends in the Health of Americans. Hyattsville MA: 2004. [PubMed] [Google Scholar]
- 17.Harrell FEJ, Lee KL, Pollock BG. Regression Models in Clinical Studies: Determining Relationships Between Predictors and Response. J Natl Cancer Inst. 1988;80(15):1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 18.Jemal A, Murray T, Ward E, et al. Cancer Statistics, 2005. CA Cancer J Clin. 2005 Jan-Feb;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]; CA Cancer J Clin. 2005;55(4):259. Erratum in. [Google Scholar]
- 19.Gloeckler R, Reichman ME, Lewis DR, et al. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8(6):541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. Health, United States 2006 With Chartbook on Trends in the Health of Americans. Hyattsville MD: 2006. [PubMed] [Google Scholar]
- 21.Buchsbaum DG, Buchanan RG, Centor RM, et al. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med. 1991;115(10):774–777. doi: 10.7326/0003-4819-115-10-774. [DOI] [PubMed] [Google Scholar]
- 22.Albright CL. Risk associated with obesity and its related lifestyle factors: what about cancer? Hawaii Med J. 2003;62(11):256–259. [PubMed] [Google Scholar]
- 23.Castelli WP. Cardiovascular disease in women. Am J Obstet Gynecol. 1988;158(6 Pt 2):1553–1557. doi: 10.1016/0002-9378(88)90189-5. [DOI] [PubMed] [Google Scholar]
- 24.Hendrie HC. Epidemiology of dementia and Alzheimer's disease. Am J Geriatr Psychiatry. 1998;6(2) 1:S3–18. doi: 10.1097/00019442-199821001-00002. [DOI] [PubMed] [Google Scholar]
- 25.Manly JJ, Bell-McGinty S, Tang MX, et al. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62(11):1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 26.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20(2):494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 27.Flood KL, Carroll MB, Le CV, et al. Geriatric syndromes in elderly patients admitted to an oncology-acute care for elders unit. J Clin Oncol. 2006;24(15):2298–2303. doi: 10.1200/JCO.2005.02.8514. [DOI] [PubMed] [Google Scholar]
- 28.Extermann M, Meyer J, McGinnis M, et al. A comprehensive geriatric intervention detects multiple problems in older breast cancer patients. Critical Reviews in Oncology-Hematology. 2004;49(1):69–75. doi: 10.1016/s1040-8428(03)00099-4. [DOI] [PubMed] [Google Scholar]
- 29.Yancik R, Havlik RJ, Wesley MN, et al. Cancer and comorbidity in older patients: a descriptive profile. Ann Epidemiol. 1996;6(5):399–412. doi: 10.1016/s1047-2797(96)00063-4. [DOI] [PubMed] [Google Scholar]
- 30.Guralnik JM, LaCroix AZ, Everett DF, et al. Aging in the Eighties: The Prevalence of Comorbidity and its Association with Disability. Vital and Health Statistics of the National Center for Health Statistics. 1989;170:1–8. [Google Scholar]
- 31.Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Age and co-morbidity in cancer patients: a population-based approach. Cancer Treat Res. 2005;124:89–107. doi: 10.1007/0-387-23962-6_5. [DOI] [PubMed] [Google Scholar]
- 32.Rowe JW, Minaker KL. Geriatric Medicine. In: Finch CE, Schneider EL, editors. Handbook of The Biology of Aging, 2 Ed. New York: Van Nostrand Reinhold Company; 1985. pp. 932–959. [Google Scholar]
- 33.Anisimov VN. The relationship between aging and carcinogenesis: a critical appraisal. Crit Rev Oncol Hematol. 2003;45(3):277–304. doi: 10.1016/s1040-8428(02)00121-x. [DOI] [PubMed] [Google Scholar]
- 34.Ogle KS, Swanson GM, Woods N, et al. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88(3):653–663. doi: 10.1002/(sici)1097-0142(20000201)88:3<653::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 35.Yancik R, Wesley MN, Ries LA, et al. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–892. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 36.Barbieri M, Rizzo MR, Manzella D, et al. Age-related insulin resistance: is it an obligatory finding? The lesson from healthy centenarians. Diabetes Met Res Rev. 2001;17(1):19–26. doi: 10.1002/dmrr.178. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control. Supplement to HIV/AIDS Surveillance: Demographics and Behavioral Data. Vol. 2. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. pp. 1–28. HIV/AIDS Behavioral Surveillance Project 1997-2000. [Google Scholar]
- 38.AIDS Info Net. Older People, HIV, and AIDS. [April 30, 2005]; Available at: www.aidsinfonet.org/articles.php.
- 39.Butt AA, Dascomb KK, DeSalvo KB, et al. Human immunodeficiency virus infection in elderly patients. South Med J. 2001;94(4):397–400. [PubMed] [Google Scholar]
- 40.Selwyn PA, Rivard M, Kappell D, et al. Palliative care for AIDS at a large urban teaching hospital: program description and preliminary outcomes. J Palliat Med. 2003;6(3):461–474. doi: 10.1089/109662103322144844. [DOI] [PubMed] [Google Scholar]
- 41.Rowland JH, Yancik R. Cancer survivorship: the interface of aging, comorbidity, and quality care. J Natl Cancer Inst. 2006;98(8):504–505. doi: 10.1093/jnci/djj154. [DOI] [PubMed] [Google Scholar]
- 42.Repetto L, Comandini D, Mammoliti S. Life expectancy, comorbidity and quality of life: the treatment equation in the older cancer patients. Crit Rev Oncol Hematol. 2001;37(2):147–152. doi: 10.1016/s1040-8428(00)00104-9. [DOI] [PubMed] [Google Scholar]


