Abstract
NAD(P)H:quinone oxidoreductase 1 is a proposed target in pancreatic cancer. We describe the synthesis of a series of indolequinones, based on the 5- and 6-methoxy-1,2-dimethylindole-4,7-dione chromophores with a range of phenolic leaving groups at the (indol-3-yl)methyl position. The ability of these indolequinones to function as mechanism-based inhibitors of purified human NQO1 was evaluated, as was their ability to inhibit both NQO1 and cell growth in human pancreatic MIA PaCa-2 tumor cells. The inhibition of rhNQO1 was related to the pKa of the leaving group: compounds with poorer phenolic leaving groups were poor inhibitors whereas those with more acidic leaving groups were more efficient inhibitors. These inhibition data also correlated with the inhibition NQO1 in MIA PaCa-2 cells. However, the data demonstrate that NQO1 inhibition does not correlate with growth inhibitory activity, at least in the MIA PaCa-2 cell line, suggesting that targets in addition to NQO1 need to be considered to explain the potent growth inhibitory activity of this series of indolequinones in human pancreatic cancer cells.
Introduction
The enzyme NQO1 (EC 1.6.99.2), also known as DT-diaphorase, is an obligate 2-electron reductase characterized by its ability to use either NADH or NADPH as cofactor. NQO1 catalyzes the 2-electron reduction of quinones and hence can protect cells against the toxic effects of quinones.1–5 However NQO1 is also involved in the reductive activation of anticancer agents such as mitomycin C and other cytotoxic quinones that operate by the so-called bioreductive mechanism,4,6–8 and continues to generate interest because of its elevated levels in many tumors and tumor cell lines.3 During our long-standing interest in NQO1, we have undertaken a detailed study on the substrate specificity of the enzyme, and the correlation of quinone structure with rate of metabolism by the enzyme and toxicity towards human tumor cell lines.9–13
As part of our studies on quinone substrates for NQO1, we have recently developed a highly specific, potent mechanism-based (suicide substrate) inhibitor 5-methoxy-1,2-dimethyl-3-(4-nitrophenoxymethyl)indole-4,7-dione 1, a compound also known as ES936, and have fully characterized its interaction with the enzyme using biochemistry, mass spectrometry and crystallography.14 In cellular studies using human breast and colon cancer cells lines, it inhibits the enzyme at low nanomolar concentrations.15 We rationalize the action of 1 by the mechanism shown in Scheme 1, whereby 2-electron reduction of the quinone by NQO1 gives the corresponding hydroquinone 2. In this compound, the indole nitrogen lone pair is no longer conjugated with the quinone carbonyl group, and hence “normal” indole reactivity takes over, resulting in the elimination of the aryloxy group, in the case of 1 4-nitrophenoxide, from the 3-indolylmethyl position to generate a highly electrophilic iminium ion 3. Since this occurs in the enzyme active site, the generation of 3 leads to irreversible alkylation of the enzyme, shown by our mass spectrometric studies to be at Tyr-127 or Tyr-129,11 and hence its inhibition.
Scheme 1.
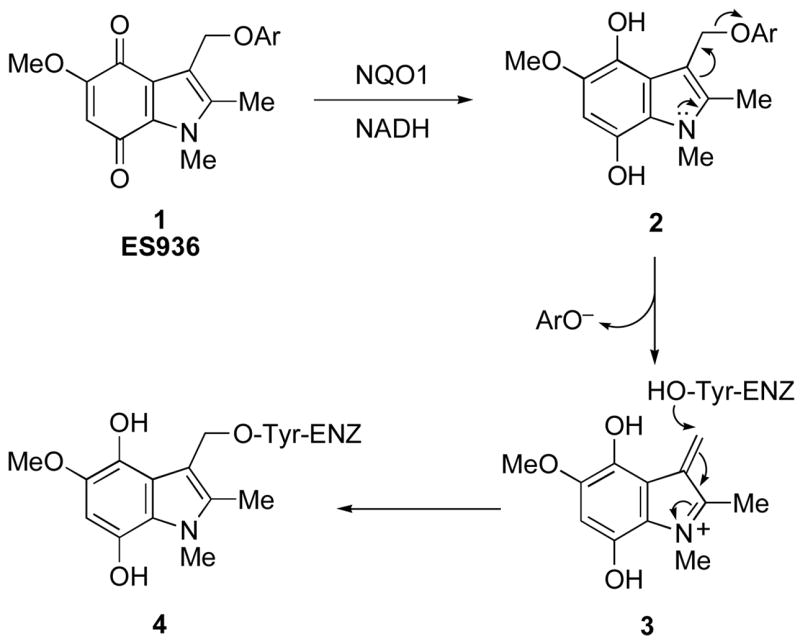
[Ar = 4-nitrophenyl] Proposed mechanism-based inhibition of NQO1 by the 3-(4-nitrophenoxy)methylindolequinone 1.
It was of considerable interest when it was recently reported that inhibition of NQO1 in pancreatic tumor cells using dicumarol resulted in inhibition of cell growth of the malignant in vitro phenotype of cells.16 Although it only accounts for less than 10% of all cancers, pancreatic cancer has one of the worst prognoses. It is rapidly fatal; data from Cancer Research UK show that one-year survival rates are less than 15% (five year survival ca. 2%). Surgery and/or radiation are the common treatments, since to date chemotherapy has made little or no impact. It was proposed that the effect of dicumarol on pancreatic tumor cell growth was mediated by inhibition of NQO1, causing a rise in superoxide levels.16 We have recently shown that NQO1 can directly scavenge superoxide, and in cells containing high levels of the enzyme (i.e. most solid tumors) scavenging by NQO1 competes with superoxide dismutase (SOD).17 These data provide support for the hypothesis that inhibition of NQO1 could lead to increased levels of superoxide, and, given the low levels of SOD in pancreatic cancer cell lines, renders the contribution of NQO1, the levels of which are known to be elevated, potentially extremely important in regulating superoxide in these cells. Although the interaction of dicumarol with NQO1 has been characterized crystallographically,18 the major problem with this inhibitor, however, is its lack of selectivity, since it is known to inhibit many other enzymes.6 The indolequinone 1, on the other hand is a mechanism-based inhibitor of NQO1, and we have confirmed that, like dicumarol, it is a potent inhibitor of human pancreatic cancer cell growth in vitro. The compound is also effective in vivo; MIA PaCa-2 xenografts in mice grow significantly slower after treatment with 1.19 However, in the case of 1, our studies showed that the cytotoxicity was independent of superoxide generation, and therefore raises the question of whether NQO1 inhibition is the only mechanism operating in pancreatic cancer cells.
In order to gain additional insights, we initiated a much wider study of quinone based inhibitors of NQO1, and we now describe the synthesis of a number of new analogs of 1, their biological evaluation as mechanism-based inhibitors of NQO1 and their toxicity towards pancreatic tumor cell lines. Some preliminary data on six of these quinones have been included in our previous publications.11,19,20
Results and Discussion
Chemistry
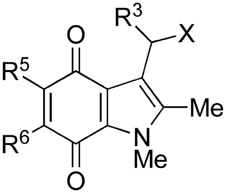
The structures of the new NQO1 inhibitors are shown in Figure 1. 5-Methoxy-1,2-dimethyl-3-(4-nitrophenoxymethyl)indole-4,7-dione 1 was prepared as previously described,9 and nine further analogs 5–13 were synthesized, in which the 4-nitrophenoxy leaving group (4-nitrophenol pKa = 7.2) was replaced by a range of other phenolic leaving groups. These were chosen to explore both the effect of leaving group ability, as evidenced by the pKa of the corresponding phenol, and the steric effects of the 3-aryloxymethyl group. We were also conscious of the need to identify possible alternative electron-withdrawing groups on the phenolic moiety, since nitro (NO2) groups are not without problems in potential drug molecules due to their metabolism. Hence the choice of 2,4,6-trifluorophenoxy group since 2,4,6-trifluorophenol has a similar pKa to 4-nitrophenol (7.5 and 7.2 respectively). Of these nine compounds, the phenoxy-,21 the 2,4-dinitrophenoxy-,22 and the 2-fluoro-4-nitrophenoxy-compounds,225, 8 and 9, had been prepared previously. In general, two methods were used to prepare the new 3-aryloxylmethylindolequinones, both starting from the known 3-hydroxymethyl-5-methoxy-1,2-dimethylindole-4,7-dione.23 Firstly, the primary alcohol was treated with thionyl chloride, and the resulting chloride reacted with a phenol in the presence of a base, usually potassium carbonate. The 3-aryloxylmethylindolequinones 6, 7 and 10 were prepared in this manner. Alternatively, the 3-hydroxymethyl compound was reacted with the phenol under the conditions of the Mitsunobu reaction in the presence of a triarylphosphine and a dialkyl azodicarboxylate. The indolequinones 11–13 were thus obtained: in the case of 2-hydroxypyridine, both N- and O-linked products were formed. The last analog in this series of compounds was the 3-[1-(4-nitrophenoxy)ethyl]indolequinone 14;24 in principle the additional methyl group should stabilize the intermediate iminium ion and thereby affect rates of fragmentation of the hydroquinone 2 (Scheme 1).
Figure 1.
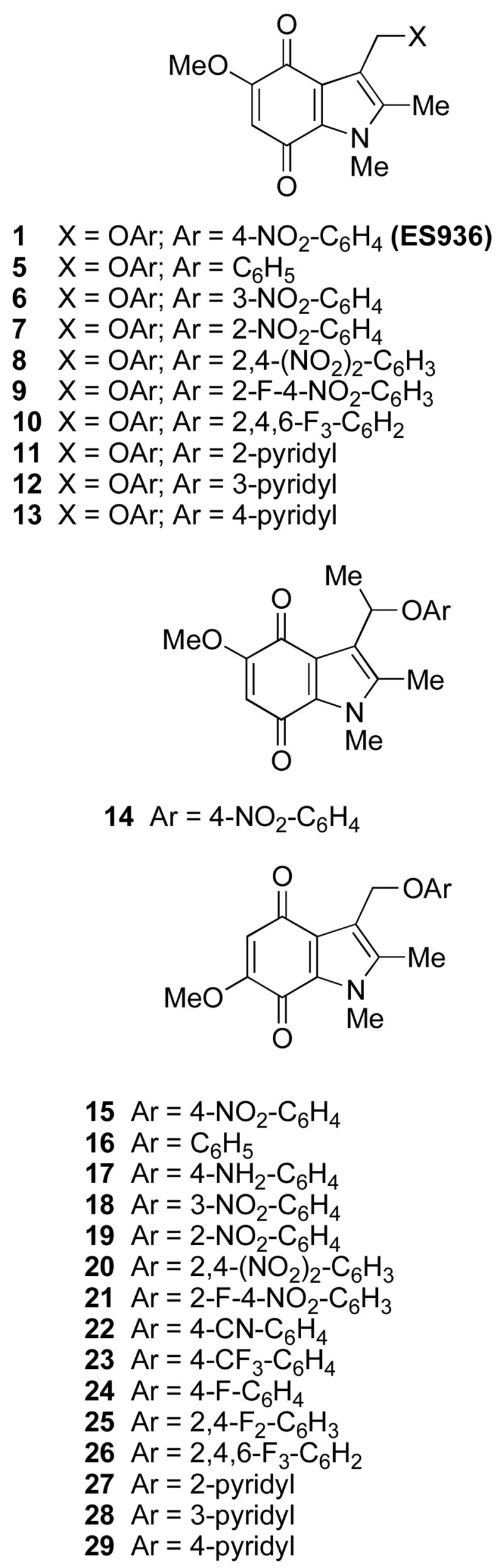
Structures of NQO1 inhibitors.
We have also investigated an isomeric series of NQO1 inhibitors based on the 6-methoxyindole-4,7-quinone ring system. Although this may appear a trivial change, the switch of the electron-releasing methoxy group from the 5- to the 6-position has an effect on the electronic properties of the quinone ring system, and hence on the reductive elimination of phenoxide as required for NQO1 inhibition (cf. Scheme 1). Hence it was by no means obvious that such analogs would be potent inhibitors since the position of the methoxy group affects the electrophilicity of the alkylating species formed upon loss of the leaving group – the 6-methoxy group, but not the 5-methoxy, can donate electron density toward the key exocyclic carbon. The synthesis of the 6-methoxyindolquinone series is summarized in Scheme 2. Thus 2-benzyloxy-4-methoxybenzaldehyde 3025 was condensed with methyl azidoacetate, and the resulting azidocinnamate 31 was cyclized to the indole 32 in a Hemetsberger indole synthesis.26–28 Treatment of the ester 32 with lithium aluminum hydride under forcing conditions resulted in reduction to the 2-methylindole 33 in satisfactory yield. Formylation under Vilsmeier-Haack conditions gave the indole-3-carbaldehyde 34 in excellent yield and this was followed by N-methylation to give the indole 35. Thereafter the indole-3-carboxaldehyde was progressed to the corresponding 3-hydroxymethylindolequinone 37 as outlined in Scheme 2.
Scheme 2.
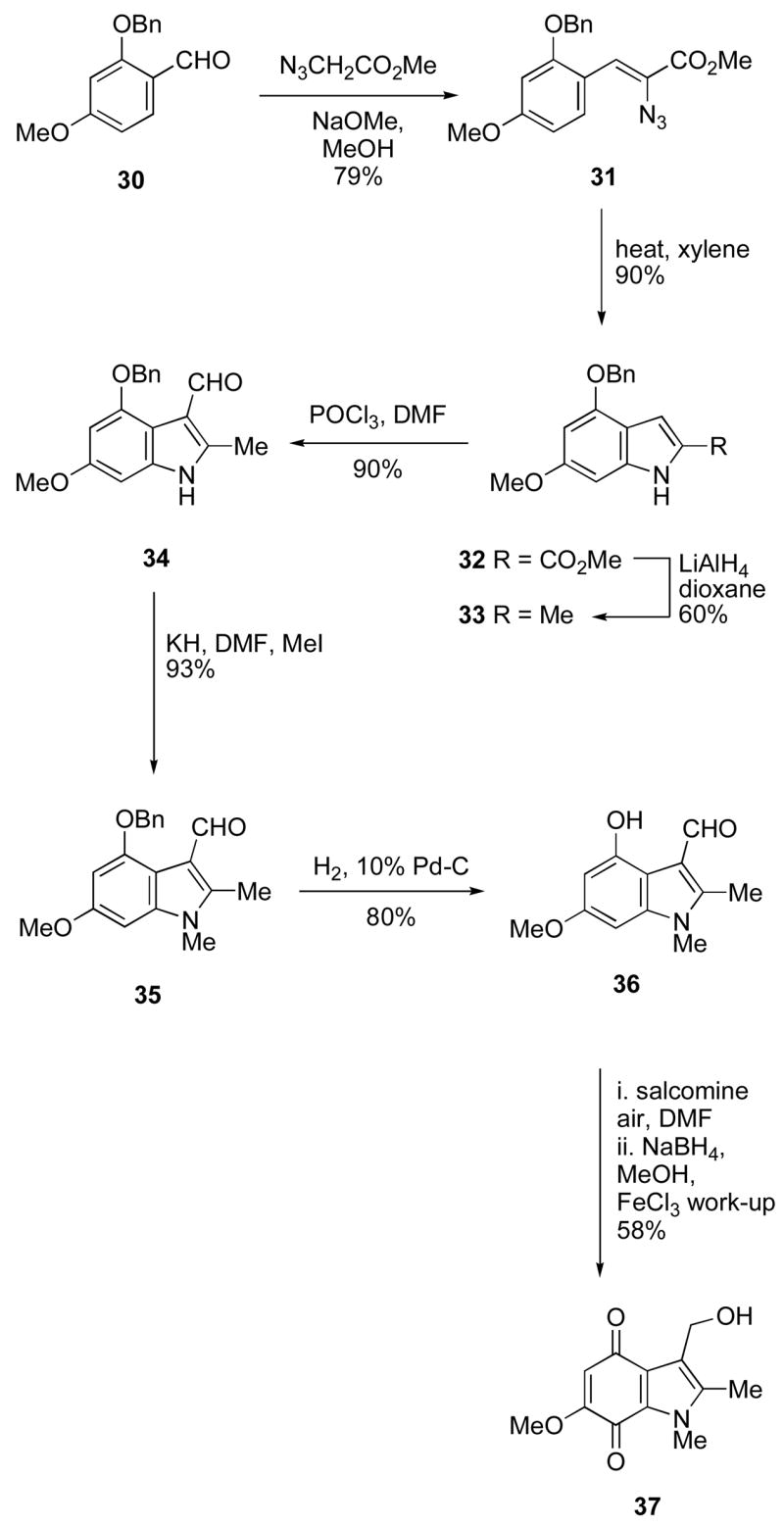
Preparation of 3-hydroxymethyl-6-methoxy-1,2-dimethylindole-4,7-dione.
The attachment of phenolic leaving groups to the 3-hydroxymethyl-6-methoxyindolequinone 37 was for the most part accomplished by conversion to the 3-chloromethylindole followed by reaction with the corresponding phenol in the presence of base as described above for the isomeric 5-methoxy series. The indolequinones 15, 16, 18, 19, 21–26, and 28 were all prepared in this manner. The 2,4-dinitrophenoxy compound 20 was prepared by reaction of the hydroxymethyl compound 37 with 2,4-dinitrofluorobenzene, and the 2- and 4-pyridyl derivatives 27 and 29 were obtained using the Mitsunobu reaction (Scheme 3). The 3-(4-aminophenoxy)methyl compound 17 was prepared from 4-azidophenol29 by coupling to the hydroxymethyl compound 37 followed by reduction of the azide group.
Scheme 3.
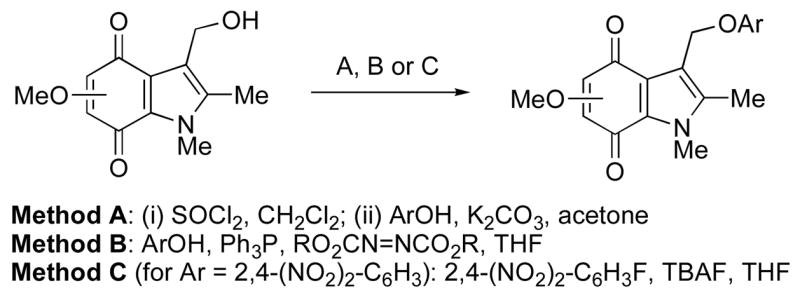
Preparation of 3-aryloxymethylindolequinones from corresponding 5- or 6-methoxy-3-hydroxymethyl derivatives.
Biology
All 26 indolequinones 1 and 5–29 were assayed for their ability to inhibit the quinone reductase NQO1 using a series of assays employing both purified recombinant human NQO1 and NQO1-rich cells. Initially, experiments were carried out to determine whether members of this series of indolequinones were mechanism-based inhibitors of NQO1 (Table 1, Figure 2). For these experiments indolequinones were incubated with rhNQO1 in the absence and presence of NADH. At the concentrations of indolequinones used in this assay only minor inhibition of NQO1 activity was observed in the absence of NADH indicating that inhibition of NQO1 by these indolequinones occurred following catalytic turnover of the indolequinone by NQO1. Representative examples of NADH-dependent inhibition of NQO1 by indolequinones are shown in Figure 2.
Table 1.
Inhibition of hNQO1 by indolequinones 1 and 5–29, and inhibition of cell growth in the MIA PaCa-2 cell line
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | R3 | R5 | R6 | X | Mechanism-based inhibitiona | Partition ratiob | >90% Inhibition of NQO1 in MIA PaCa-2 cellsb/nM | IC50 MIA PaCa-2 4 h/nMc | IC50 MIA PaCa-2 72 h/nMc |
| 1 | H | OMe | H | OAr Ar=4-NO2-C6H4 | yes | 3.5 | 10–100 | 629±17 | 508±5 |
| 15 | H | H | Ome | OAr Ar=4-NO2-C6H4 | yes | 3.7 | 10–100 | 638±15 | 355±3 |
| 5 | H | OMe | H | OAr Ar=C6H5 | yes | 4000 | nd | 1385±24 | 962±18 |
| 16 | H | H | Ome | OAr Ar=C6H5 | yes | 3800 | nd | 4563±26 | 409±30 |
| 17 | H | H | OMe | OAr Ar =4-NH2-C6H4 | no | nd | nd | 504±4 | 160±4 |
| 6 | H | OMe | H | OAr Ar=3-NO2-C6H4 | yes | 2.3 | 10–100 | 352±16 | 258±21 |
| 18 | H | H | OMe | OAr Ar=3-NO2-C6H4 | yes | 3.5 | 10–100 | 351±25 | 257±22 |
| 7 | H | OMe | H | OAr Ar=2-NO2-C6H4 | yes | 1.0 | nd | 345±20 | 104±18 |
| 19 | H | H | OMe | OAr Ar=2-NO2-C6H4 | yes | 1.0 | 10–100 | 363±9 | 178±4 |
| 8 | H | OMe | H | OAr Ar=2,4-(NO2)2-C6H3 | see text | nd | nd | NC | NC |
| 20 | H | H | OMe | OAr Ar=2,4-(NO2)2-C6H3 | see text | nd | nd | NC | NC |
| 9* | H | OMe | H | OAr Ar=2-F-4-NO2-C6H3 | yes | 0.8 | nd | 4654±48 | 3556±31 |
| 21 | H | H | OMe | OAr Ar=2-F-4-NO2-C6H3 | yes | 0.8 | 10–100 | 529±5 | 417±5 |
| 22 | H | H | OMe | OAr Ar=4-CN-C6H4 | yes | 37 | nd | 463±6 | 192±3 |
| 23 | H | H | OMe | OAr Ar=4-CF3-C6H4 | yes | 652 | 5000–10000 | 496±3 | 311±5 |
| 24 | H | H | OMe | OAr Ar=4-F-C6H4 | yes | >100000 | 5000–10000 | 905±25 | 493±32 |
| 25 | H | H | OMe | OAr Ar=2,4-F2-C6H4 | yes | 21.3 | 10–100 | 255±5 | 75±4 |
| 10 | H | OMe | H | OAr Ar=2,4,6-F3-C6H4 | yes | 1.9 | nd | 427±5 | 86±3 |
| 26 | H | H | OMe | OAr Ar=2,4,6-F3-C6H4 | yes | 1.7 | 10–100 | 452±4 | 212±3 |
| 11 | H | OMe | H | OAr Ar=2-pyridyl | yes | nd | <10 | NC | NC |
| 27 | H | H | OMe | OAr Ar=2-pyridyl | yes | 1.1 | 10–100 | 9579±48 | 2393±28 |
| 12 | H | OMe | H | OAr Ar=3-pyridyl | yes | 6.1 | 10–100 | 904±26 | 562±24 |
| 28 | H | H | OMe | OAr Ar=3-pyridyl | yes | nd | nd | 2475±40 | 1824±19 |
| 13 | H | OMe | H | OAr Ar=4-pyridyl | yes | 1.3 | nd | 2007±16 | 2172±25 |
| 29 | H | H | OMe | OAr Ar=4-pyridyl | yes | 0.9 | 10–100 | 2560±7 | 3271±15 |
| 14 | Me | OMe | H | OAr Ar=4-NO2-C6H4 | yes | 9.1 | nd | 1829±14 | 1696±23 |
classification of mechanism-based inhibition refers to dependence (or not) on NADH;
nd not determined;
NC denotes no convergence. IC50 value cannot be determined as profile is a straight line.
Compound 9 was found to be unstable in DMSO and was tested immediately for NQO1 mechansim-based inhibition.
Figure 2.
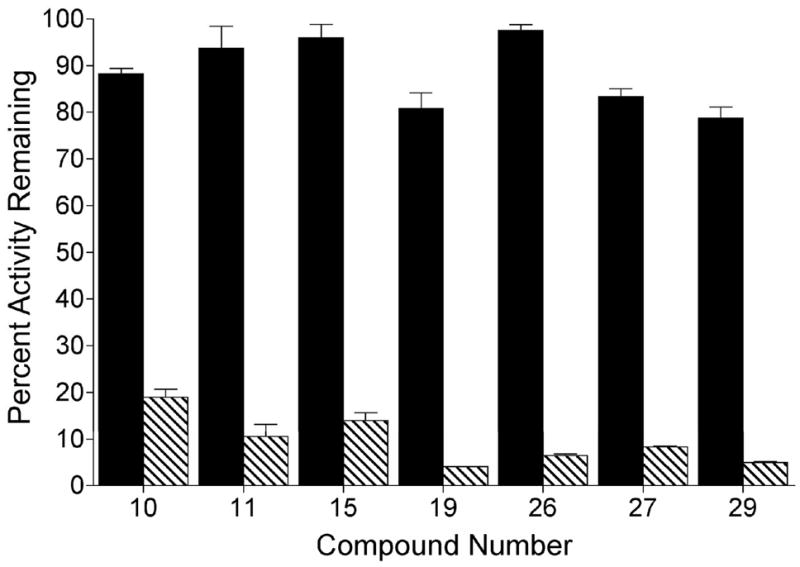
Mechanism based inhibition of NQO1 by representative indolequinones. NQO1 activity was assayed following the incubation of indolequinone (100 nM) with rhNQO1 (1 μg) in the absence (solid bars) and presence (striped bars) of 0.2 mM NADH. Incubations (0.5 mL) were performed for 5 min at 32 °C. Results are the mean ± standard deviation of three separate determinations.
The dependence on NADH for inhibition was taken as preliminary evidence for mechanism-based inactivation of NQO1,14 and compounds showing such properties were studied further. An important term used to describe the efficiency of inactivation for mechanism-based enzyme inhibitors is the partition ratio. The partition ratio is defined as the number of catalytic cycles required to inhibit one molecule of enzyme. In these experiments partition ratios were determined by incubating defined molar ratios of indolequinone and rhNQO1 in the presence of NADH and measuring the resultant NQO1 activity. Partition ratios for the inhibition of NQO1 by this series of indolequinones are shown in Table 1. Many of the indolequinones in this study had partition ratios near 1, indicating very efficient inactivation of NQO1, and 13 out of 26 indolequinones in this series had partition ratios less than 5. The 2,4-dinitrophenoxy derivatives 8 and 20 displayed poor solubility characteristics and precipitated out of solution, so partition ratios could not be determined.
The ability of these indolequinones to inhibit NQO1 in cells was examined using the human pancreatic cancer cell line MIA PaCa-2 (NQO1 activity; 1000 nmol DCPIP/min/mg). For these studies MIA PaCa-2 cells were treated with indolequinones (1–10,000 nM) for 1 hour, the cells were then washed free of drug and NQO1 activity was measured in cell sonicates. Results from these experiments clearly demonstrated that these indolequinones could enter cells and inactivate NQO1 (Figure 3 and Table 1). For most indolequinones in this study greater than 95% of NQO1 activity could be inhibited at indolequinone concentrations between 10–100 nM (Table 1). However, indolequinones with large partition ratio such as 23 or 24 required much higher concentrations to achieve 95% inhibition of NQO1 (Figure 3, Table 1).
Figure 3.
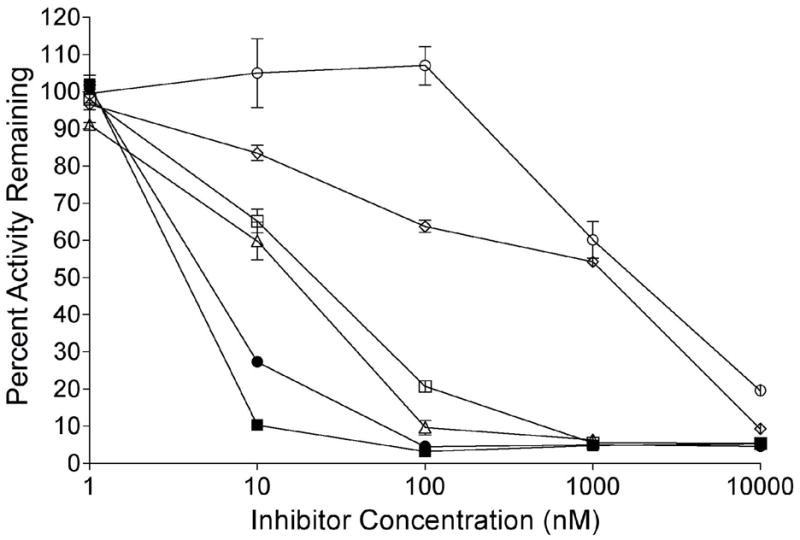
Inhibition of NQO1 activity in MIA PaCa-2 cells treated with indolequinones. NQO1 activity was measured in MIA PaCa-2 cells following treatment with indolequinones (1 nM-10 μM) for 1 h. Results are the mean ± standard deviation of three separate determinations. Open circles, 24; open diamonds, 23; open squares, 22; open triangles, 12; closed circles, 6; closed squares, 19.
Finally, the ability of these indolequinones to induce cytotoxicity was measured in MIA PaCa-2 cells using the MTT growth inhibition assay (Table 1). Since previous studies with mechanism-based inhibitors 1,15, 23 and 29 have demonstrated near complete inhibition of NQO1 catalytic activity after 4 hours, this time point, as well as a longer time point of 72 hours, were selected for growth inhibition studies in the MIA PaCa-2 cells. The IC50 values are reported for this extended series of compounds for both 4 and 72 hour treatments with the appropriate indolequinone (Table 1). The growth inhibitory potency of these compounds after 4 hours of incubation indicates that compounds 1, 15, 17, 6, 18, 7, 19, 21, 22, 23, 25, 10 and 26 were effective inhibitors of the growth of MIA PaCa-2 cells (IC50 4 h < 650 nM) while compounds 5, 16, 8, 20, 9, 24, 11, 27, 12, 28, 13, 29 and 14 were relatively ineffective at inducing growth inhibition (IC50 4 h > 900 nM).
The phenol and pyridine-based leaving groups derived from each of the indolequinones tested were also examined for growth inhibitory potency. The compounds tested included phenol, 2-, 3- and 4-nitrophenol, 2-fluoro-4-nitrophenol, 4-fluorophenol, 2,4-difluorophenol, 2,4,6-trifluorophenol, 4-trifluoromethylphenol, 4-cyanophenol, 4-aminophenol, 2-, 3- and 4-hydroxypyridine. All compounds were relatively non-toxic (data shown in Supporting Information). For example, all IC50 values obtained by MTT analysis were greater than 12,900 nM after 72 h of incubation of these compounds with MIA PaCa-2 cells. These data indicate that growth inhibitory properties of the indolequinones are unlikely to be related to the liberation of phenols or pyridines from the parent molecule.
Interestingly, the indolequinones 27, 12, 28, 13, 29 and 14 were potent mechanism-based inhibitors of NQO1 activity, however displayed poor growth inhibitory profiles. This finding indicates dissociation of NQO1 inhibitory activity and the ability to induce growth inhibition in MIA PaCa-2 cells for these indolequinone compounds and implies a function via an NQO1-independent mechanism to inhibit cell proliferation.
In addition, compounds 5, 16 and 24, which are very poor inhibitors of NQO1 as indicated by high partition ratios demonstrated decreases in IC50 value after 72 hours relative to 4 hours of incubation with cells and the change with compound 4 was dramatic from 4563 nM to 409 nM. This observation also argues for an NQO1-independent mechanism of toxicity in MIA PaCa-2 cells.
Discussion
In order to make a more detailed comparison of the efficiency of NQO1 inactivation, the partition ratios of the indolequinones were examined. Within the series of analogs of 1 bearing a 5-methoxy group, there was a loose correlation of the partition ratio with the leaving group ability of the aryloxy group at the 3-indolylmethyl position, as evidenced by the pKa of the corresponding phenol. Certainly, compound 9 with the most acidic phenol leaving group, 2-fluoro-4-nitrophenol (pKa = 5.7), has the lowest partition ratio, whilst the higher are exhibited by the indolequinones 5 and 24 which have the poorest leaving group (phenol pKa = 9.9, 4-fluorophenol pKa = 9.9). It is also noteworthy that a nitrophenolate leaving group is not essential since the indolequinone 10 bearing a 2,4,6-trifluorophenoxy group (2,4,6-trifluorophenol pKa = 7.5) is a highly efficient inhibitor. Likewise, the compounds 12 and 13 with 3-hydroxypyridine and 4-hydroxypyridine (4-pyridone) leaving groups (3-hydroxypyridine pKa = 8.51, 4-hydroxypyridine pKa = 5.2) were also efficient inhibitors of NQO1. Finally, compound 14 bearing the additional methyl group at the 3-indolylmethyl position is a poorer inhibitor and less cytotoxic than its des-methyl analog 1, notwithstanding the fact that the additional methyl group should stabilize the intermediate iminium ion and thereby affect rates of fragmentation of the hydroquinone 2 (Scheme 1).
Although it not obvious that the isomeric series of NQO1 inhibitors based on the 6-methoxyindole-4,7-quinone ring system would be inhibitors of NQO1 due to the electronic effect of switching of the methoxy group from the 5- to the 6-position, the indolequinones 15, 16 and 18–29 all inactivated the enzyme. In fact, the 6-methoxy analog 15 of 1 is equipotent with the 5-methoxy isomer. Likewise, in the five pairs of compounds where the leaving groups are phenoxide (compounds 5 and 16), 2-nitrophenoxide (7 and 19), 2-fluoro-4-nitrophenoxide (compounds 9 and 21), 2,4,6-trifluorophenoxide (compound 10 and 26), or the 4-pyridyloxy (compounds 13 and 29), the 5- and 6-methoxy isomers exhibit very similar biological profiles as inhibitors of NQO1 as evidenced by their similar partition ratios. Therefore, surprisingly, the position of the electron releasing methoxy group appears to have little effect on the ability of the indolequinones to undergo reduction by NQO1, followed by elimination of the aryloxy group from the 3-indolylmethyl position with the ensuing inactivation of the enzyme.
As with the 5-methoxy series of indolequinones, there also appears to be a relationship between the pKa of the aryloxy leaving group at the 3-indolylmethyl position and the efficiency of enzyme inhibition, as measured by the partition ratio, in the 6-methoxyindolequinones. This is nicely illustrated by the series of fluorophenoxy compounds 24–26, where the compounds become more potent inhibitors as the pKa of the leaving group decreases as the degree of fluorine substitution increases (4-fluorophenol pKa = 9.9; 2,4-difluorophenol pKa = 8.7; 2,4,6-trifluorophenol pKa = 7.5).
Next, the inhibition of NQO1 in cellular systems by representative indolequinones was examined to verify inhibition of NQO1, the proposed intracellular target of these compounds and assure that quinones were taken up into cells. Importantly, there was a general relationship between the partition ratio measured using purified NQO1 and the ability of the indolequinones to inhibit NQO1 in MIA PaCa-2 cells. Hence it was shown that the indolequinones 1, 6, 15, 21, 25, 26, 27, 29 all caused >95% inhibition of enzyme activity in the human pancreatic MIA PaCa-2 solid tumor cell line after 1 hour. The data for compounds 1, 15, 29, 5, 6, 13 and 29 were included in our previous paper,20 and the data for the remaining compounds are shown in Figure 3.
In previous studies, we showed that 1 inhibited the growth of human pancreatic MIA PaCa-2 cancer cells, and therefore it was of interest to evaluate the this indolequinone series in the same cell line. Although greater than 95% inhibition of NQO1 occurs after a 1 hour exposure to indolequinones, MIA PaCa-2 cells were treated with indolequinones for 4 hours in order to maintain a prolonged period of NQO1 inhibition, since once these inhibitors are removed NQO1 activity slowly returns due to the synthesis of new NQO1 protein. A longer exposure period (72 hours) was also used for comparative purposes and the IC50 values are reported for both 4 and 72 hour treatments in Table 1. The 26 indolequinones tested exhibited IC50 values in the range ca. 0.1–9.5 μM (100–9500 nM) with the 2- and 3-nitrophenyl (6, 7, 18, 19), and the di- and tri-fluorophenyl (10, 25, 26) derivatives being among the most potent growth inhibitors, and the pyridyloxy derivatives (13, 27–29) the least effective. Hence it is clear that potent NQO1 inhibitors such as the pyridyloxy derivatives do not exhibit the highest levels of cytotoxicity. Conversely, relatively poor inhibitors such as the 4-aminophenoxy 17 and 4-trifluoromethylphenoxy 23 compounds demonstrated potent cell growth inhibition at both time periods. In addition, the relatively poor inhibitors, the phenoxy derivatives 5 and 16 and the 4-fluorophenoxy 24 compound, demonstrated potentiated cell growth inhibition with increasing drug exposure. These data on a large set of indolequinones reinforce our earlier observations,20 that NQO1 inhibition can be divorced from cell growth inhibition in the human pancreatic MIA PaCa-2 cell line at least.
In summary, we have examined a series of 26 indolequinones, most of them previously unreported, and evaluated their capacity to inhibit NQO1 in cell-free and cell-based systems, together with their ability to inhibit cell proliferation in the human pancreatic MIA PaCa-2 cancer cell line. We have characterized new mechanism-based inhibitors of NQO1 and shown that their partition ratios reflect their ability to inhibit NQO1 in tumor cells. Our data demonstrate that NQO1 inhibition does not correlate with growth inhibitory activity, at least in the MIA PaCa-2 cell line, suggesting that targets in addition to NQO1 need to be considered to explain the potent activity of this series of indolequinones in human pancreatic cancer cells.
Experimental Section
Chemistry
General experimental details
Commercially available reagents were used throughout without purification unless otherwise stated. Light petroleum refers to the fraction with bp 40–60 °C and was distilled before use. Ether refers to diethyl ether. Reactions were routinely carried out under a nitrogen or argon atmosphere. Analytical thin layer chromatography was carried out on aluminum-backed plates coated with Merck Kieselgel 60 GF254, and visualized under UV light at 254 and/or 360 nm. Chromatography was carried out using Merck Kieselgel 60 H silica or Matrex silica 60. Fully characterized compounds were chromatographically homogeneous.
UV/Vis spectra were recorded on a Phillips PU 8700 series spectrophotometer. Infrared spectra were recorded in the range 4000–600 cm−1 using Nicolet Magna FT-550, Perkin Elmer 1600 series, or Avatar 320 FT-IR spectrometers. NMR spectra were carried out on Jeol EX270, Bruker AC300, AV400 and DRX500 instruments (operating at 1H frequencies of 270, 300, 400 and 500 MHz respectively; corresponding 13C frequencies are 67.5, 75, 100 and 125 MHz). Chemical shifts are quoted in ppm with TMS as internal standard. J values are recorded in Hz. In the 13C spectra, signals corresponding to CH, CH2 or CH3 groups, as assigned from DEPT, are noted; all others are C. High and low resolution mass spectra were recorded on Micromass GCT TDF, Bruker MicroTof or VG Autospec spectrometers, or at the EPSRC Mass Spectrometry Center (Swansea).
General Method A. Coupling of 3-(hydroxymethyl)indolequinones with substituted phenols via the 3-chloromethylindole
The following general procedure was used unless otherwise stated: To a stirred solution of the 5-methoxy or 6-methoxy 3-hydroxymethyl-1,2-dimethylindole-4,7-dione(0.1–0.2 mmol, 1 equiv) in dichloromethane (5 mL) at 0 °C was added thionyl chloride (50 equiv) dropwise. The reaction mixture was stirred at room temperature for 1 h. The solvent and excess thionyl chloride were removed in vacuo and the crude product was used directly in the next step without further purification. A mixture of the crude 3-chloromethylindolequinone, the phenol (3 equiv), and potassium carbonate (3 equiv) was stirred in DMF (5 mL) for 16 h. The solvent was removed in vacuo and the crude product dissolved in dichloromethane (50 mL), washed with NaOH (2 M; 20 mL), HCl (2 M; 20 mL), water (20 mL), dried (MgSO4), filtered and the filtrate evaporated in vacuo. The crude product was purified by chromatography.
General Method B. Coupling of 3-(hydroxymethyl)indolequinones with substituted phenols via the Mitsunobu reaction
The following general procedure was used unless otherwise stated: the dialkyl azodicarboxylate (4 equiv) was added to a solution of the 5-methoxy or 6-methoxy 3-(hydroxymethyl)-1,2-dimethylindole-4,7-dione (100 mg, 0.4 mmol), triarylphosphine (3 equiv) and the phenol (3 equiv) in THF (15 mL). The solution was stirred for 1 h. The solvent was removed in vacuo and the residue dissolved in ethyl acetate and washed with sodium hydroxide (1 M), hydrochloric acid (1 M), and water, dried (MgSO4) and concentrated. The residue was purified by column chromatography.
5-Methoxy-1,2-dimethyl-3-(4-nitrophenoxymethyl)indole-4,7-dione 1 (ES936)
Prepared as previously described.9
5-Methoxy-1,2-dimethyl-3-(phenoxymethyl)indole-4,7-dione 5
Prepared as previously described.21
5-Methoxy-1,2-dimethyl-3-(3-nitrophenoxymethyl)indole-4,7-dione 6
Using Method A, 3-(hydroxymethyl)-5-methoxy-1,2-dimethylindole-4,7-dione23 (30 mg, 0.13 mmol), thionyl chloride (0.75 mL, 10.3 mmol), 3-nitrophenol (53 mg, 0.38 mmol) and potassium carbonate (53 mg, 0.38 mmol) gave after chromatography (elution with 20% ethyl acetate/light petroleum) the title compound (33 mg, 44%) as an orange crystalline solid; mp 219–227 °C; δ 7.82 (2 H, m, ArH), 7.43 (1 H, t, J 8.4, ArH), 7.29 (1 H, m, ArH), 5.64 (1 H, s, 6-H), 5.36 (2 H, s, CH2), 3.91 (3 H, s, Me), 3.82 (3 H, s, Me), 2.32 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 178.8 (C), 178.2 (C), 160.0 (C), 159.1 (C), 149.2 (C), 138.0 (C), 130.0 (CH), 129.0 (C), 121.6 (CH), 121.4 (C), 115.9 (overlapping CH and C), 109.9 (CH), 106.8 (CH), 61.1 (CH2), 56.5 (Me), 32.4 (Me), 9.9 (Me); MS (ESI) 379 (M+Na+, 100%), 218 (71); Found: M+Na+, 379.0902. C18H16N2O6 + Na requires 379.0906.
5-Methoxy-1,2-dimethyl-3-(2-nitrophenoxymethyl)indole-4,7-dione 7
Using Method A, 3-(hydroxymethyl)-5-methoxy-1,2-dimethylindole-4,7-dione(30 mg, 0.13 mmol), thionyl chloride (1.2 mL, 16.5 mmol), 2-nitrophenol (124.3 mg, 0.89 mmol) and potassium carbonate (123.5 mg, 0.89 mmol) gave after chromatography (elution with ethyl acetate) the title compound (24.6 mg, 54%) as an orange crystalline solid; mp 204–205 °C; 1H NMR (400 MHz; CDCl3) δ 7.78 (1 H, dd, J 1.6, 8.0, ArH); 7.50 (1 H, dt, J 1.2, 8.4, ArH), 7.41 (1 H, dd, J 0.6, 8.4, ArH), 7.00 (1 H, dt, J 0.6, 8.0, ArH), 5.63 (1 H, s, 6-H), 5.50 (2 H, s, CH2), 3.89 (3 H, s, Me), 3.82 (3 H, s, Me), 2.26 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 178.6 (C), 178.5 (C), 159.5 (C), 151.6 (C), 140.5 (C), 139.0 (C), 134.2 (CH), 128.7 (C), 125.4 (CH), 121.2 (C), 120.7 (CH), 116.4 (CH), 115.9 (C), 106.8 (CH), 62.1 (CH2), 56.2 (Me), 32.4 (Me), 9.9 (Me); MS (ESI) 379 (M+Na+, 100); Found: M+Na+, 379.0883. C18H16N2O6 + Na requires 379.0906.
5-Methoxy-1,2-dimethyl-3-(2,4-dinitrophenoxymethyl)indole-4,7-dione 8
Prepared as previously described.22
3-(2-Fluoro-4-nitrophenoxymethyl)-5-methoxy-1,2-dimethylindole-4,7-dione 9
Prepared as previously described.22
5-Methoxy-1,2-dimethyl-3-(2,4,6-trifluorophenoxymethyl)indole-4,7-dione 10
Using Method A, 3-(hydroxymethyl)-5-methoxy-1,2-dimethylindole-4,7-dione(30 mg, 0.13 mmol), thionyl chloride (0.85 mL, 11.7 mmol), 2,4,6-trifluorophenol (56.7 mg, 0.38 mmol), and potassium carbonate (52.9 mg, 0.38 mmol) gave after chromatography (elution with ethyl acetate) the title compoundtitle compound (28.9 mg, 62%) as an orange-red crystalline solid; mp 201–202 °C; 1H NMR (400 MHz; CDCl3) δ 6.67 (2 H, t, J 8.0, ArH), 5.62 (1 H, s, 6-H), 5.33 (2 H, s, CH2), 3.93 (3 H, s, Me), 3.81 (3 H, s, Me), 2.35 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 177.8 (C), 176.7 (C), 156.3 (dt, JCF 245, 14, CF), 155.5 (ddd, JCF 250, 14, 8, CF), 155.0 (C), 137.9 (C), 130.8 (td, JCF 14, 6, C), 127.7 (C), 120.5 (C), 115.2 (C), 105.6 (C), 99.5 (ddd, JCF 27, 27, 8, CH), 64.9 (CH2), 55.4 (Me), 31.4 (Me), 8.5 (Me); MS (ESI) 388 (M+Na+, 100%), 363 (4); Found: M+Na+, 388.0773. C18H14F3NO4 + Na requires 388.0773; Anal. (C18H14F3NO4) C, H, N.
5-Methoxy-1,2-dimethyl-3-(pyridin-2-yloxymethyl)indole-4,7-dione 11
Using Method B, 2-hydroxypyridine (25 mg, 0.26 mmol), triphenylphosphine (101 mg, 0.39 mmol), diethyl azodicarboxylate (0.047 mL, 0.30 mmol) and 3-hydroxymethyl-5-methoxy-1,2-dimethylindole-4,7-dione (30 mg, 0.13 mmol) gave after chromatography (ethyl acetate elution) the title compound (20 mg, 50%) as an orange solid, mp 138–140 °C; 1H NMR (400 MHz; CDCl3) δ 8.18 (1 H, ddd, J 0.8, 2.0, 5.2, PyrH), 7.54 (1 H, ddd, J 2.0, 7.2, 8.4, PyrH), 6.86 (1 H, ddd, J 0.8, 5.2, 6.8, PyrH), 6.72 (1 H, dt, J 0.8, 8.4, PyrH), 5.61 (1 H, s, 6-H), 5.51 (2 H, s, CH2), 3.90 (3 H, s, Me), 3.81 (3 H, s, Me), 2.32 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 178.9 (C), 177.7 (C), 163.7 (C), 159.8 (C), 146.8 (C), 138.5 (CH), 138.0 (CH), 121.9 (C), 117.2 (2 C), 116.8 (CH), 111.2 (CH), 106.6 (CH), 58.0 (CH2), 56.4 (Me), 32.4 (Me), 9.7 (Me); MS (ESI) 335 (M+Na+, 100%), 218 (82); Found: M+Na+, 335.1002. C17H16N2O4 + Na requires 335.1008.
Also formed was 5-methoxy-1,2-dimethyl-3-(2-pyridon-1-ylmethyl)indole-4,7-dione (8.4 mg, 21% yield) as an orange solid, mp 226–232 °C; 1H NMR (400 MHz; CDCl3) δ 7.95 (1 H, dd, J 2.0, 6.8, PyrH), 7.26 (1 H, ddd, J 2.0, 6.4, 8.8, PyrH), 6.50 (1 H, d, J 8.8, PyrH), 6.10 (1 H, td, J 1.2, 6.8, PyrH), 5.62 (1 H, s, 6-H), 5.22 (2 H, s, CH2), 3.88 (3 H, s, Me), 3.82 (3 H, s, Me), 2.47 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 177.5 (2 C), 158.5 (C), 138.6 (CH), 138.4 (C), 138.3 (CH), 131.2 (C), 128.1 (C), 120.4 (CH), 119.5 (C), 115.2 (C), 105.8 (CH), 104.9 (CH), 55.5 (Me), 41.4 (CH2), 31.6 (Me), 9.1 (Me); MS (ESI) 335 (M+Na+, 100%), 218 (50); Found: M+Na+, 335.1006. C17H16N2O4 + Na requires 335.1008.
5-Methoxy-1,2-dimethyl-3-(3-pyridyloxymethyl)indole-4,7-dione 12
Using Method B, 3-hydroxypyridine (40.8 mg, 0.43 mmol), triphenylphosphine (169 mg, 0.64 mmol), diethyl azodicarboxylate (0.079 mL, 0.50 mmol) and 3-hydroxymethyl-5-methoxy-1,2-dimethylindole-4,7-dione (50 mg, 0.21 mmol) gave after chromatography (ethyl acetate elution) the title compound (34 mg, 51%) as an orange solid, mp 158–162 °C; 1H NMR (400 MHz; CDCl3) δ 8.38 (1 H, bs, PyrH), 8.25 (1 H, d, J 0.8, PyrH), 7.45 (1 H, dd, J 2.0, 8.4, PyrH), 7.30 (1 H, dd, J 4.0, 8.4, PyrH), 5.66 (1 H, s, 6-H), 5.39 (2 H, s, CH2), 3.93 (3 H, s, Me), 3.84 (3 H, s, Me), 2.34 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 178.7 (C), 178.2 (C), 159.6 (C), 154.8 (C), 141.9 (CH), 138.4 (CH), 138.1 (C), 128.9 (C), 124.1 (CH), 121.8 (CH), 121.3 (C), 116.3 (C), 106.8 (CH), 60.8 (CH2), 56.5 (Me), 32.4 (Me), 9.9 (Me); MS (ESI) 313 (MH+, 100%), 218 (76); Found: MH+, 313.1174. C17H16N2O4 + H requires 313.1188.
5-Methoxy-1,2-dimethyl-3-(pyridin-4-yloxymethyl)indole-4,7-dione 13
Using Method B, 4-hydroxypyridine (40.8 mg, 0.43 mmol), triphenylphosphine (169 mg, 0.64 mmol), diethyl azodicarboxylate (0.079 mL, 0.50 mmol) and 3-hydroxymethyl-5-methoxy-1,2-dimethylindole-4,7-dione (50 mg, 0.21 mmol) gave after chromatography (ethyl acetate elution) the title compound (25.5 mg, 38%) as an orange solid, mp 195–201 °C; 1H NMR (400 MHz; CDCl3) δ 8.40 (2 H, br s, PyrH), 6.89 (2 H, d, J 4.8, PyrH), 5.62 (1 H, s, 6-H), 5.34 (2 H, s, CH2), 3.89 (3 H, s, Me), 3.80 (3 H, s, Me), 2.29 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 178.7 (C), 178.3 (C), 164.5 (C), 159.6 (C), 151.1 (CH), 138.1 (C), 128.9 (C), 121.2 (C), 115.9 (C), 110.6 (CH), 106.8 (CH), 60.1 (CH2), 56.5 (Me), 32.4 (Me), 9.9 (Me); MS (ESI) 218 (MH+, 100%), 313 (9); Found: MH+, 313.1190. C17H16N2O4 + H requires 313.1188.
5-Methoxy-1,2-dimethyl-3-[1-(4-nitrophenoxy)ethyl]indole-4,7-dione 14
Prepared as previously described.24
6-Methoxy-1,2-dimethyl-3-(4-nitrophenoxymethyl)indole-4,7-dione 15
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (0.045 g, 0.19 mmol), thionyl chloride (0.70 mL, 9.57 mmol), 4-nitrophenol (0.080 g, 0.57 mmol) and potassium carbonate (0.079 g, 0.57 mmol) gave after chromatography (gradient elution with 30–60% ethyl acetate/light petroleum) the title compound (0.057 g, 85%) as a yellow/orange crystalline solid; mp 213–215 °C (decomp) (from methanol); 1H NMR (300 MHz; CDCl3) δ 8.19 (2 H, d, J 9.3, ArH), 7.06 (2 H, d, J 9.3, ArH), 5.68 (1 H, s, 5-H), 5.43 (2 H, s, CH2), 3.92 (3 H, s, Me), 3.83 (3 H, s, Me), 2.34 (3 H, s, Me); 13C NMR (75 MHz; CDCl3) δ 184.9 (C), 171.8 (C), 163.9 (C), 160.4 (C), 142.0 (C), 140.8 (C), 127.5 (C), 126.3 (CH), 124.3 (C), 116.4 (C), 115.3 (CH), 107.1 (CH), 61.3 (CH2), 57.1 (Me), 33.1 (Me), 10.6 (Me); MS (FI) 356 (M+, 10%), 219 (28), 139 (100); Found: M+, 356.1006. C18H16N2O6 requires 356.1008; Anal. (C18H16N2O6) H, N. C: calcd, 60.67; found, 60.11.
6-Methoxy-1,2-dimethyl-3-(phenoxymethyl)indole-4,7-dione 16
To a solution of 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (0.04 g, 0.17 mmol) in dichloromethane (5 mL) at 0 °C was added thionyl chloride (0.62 mL, 8.51 mmol) dropwise and the reaction mixture was stirred at room temperature for 1 h. The solvent was removed in vacuo and the crude product was used directly in the next step without further purification.
A solution of the crude chloride in DMF (10mL) was added dropwise to a stirring suspension of phenol (0.032 g, 0.34 mmol) and sodium hydride (60% in oil; 0.014 g, 0.34 mmol) in DMF (10 mL) at 0 °C. After the addition the mixture was allowed to stir at room temperature for 5 h. The reaction mixture was quenched with a saturated solution of ammonium chloride (10 mL), washed with NaOH (2 M; 10 mL), HCl (2 M; 10 mL), water (2 × 20 mL), and dried (MgSO4). The solvent was removed in vacuo and the crude product purified by chromatography (30% ethyl acetate/light petroleum) to give the title compound (23 mg, 47%) as a yellow crystalline solid; mp 173–175 °C (decomp) (from ethyl acetate/light petroleum); 1H NMR (500 MHz; DMSO-d) δ 7.29 (2 H, m, ArH), 6.99 (2 H, m, ArH), 6.94 (1 H, m, ArH), 5.77 (1 H, s, 5-H), 5.20 (2 H, s, CH2) 3.87 (3 H, s, Me), 3.78 (3 H, s, Me), 2.29 (3 H, s, Me); 13C NMR (125 MHz; DMSO-d) δ 184.4 (C), 171.0 (C), 160.3 (C), 158.9 (C), 141.5 (C), 129.9 (CH), 126.9 (C), 123.7 (C), 121.0 (CH), 116.7 (C), 115.0 (CH), 106.9 (CH), 59.9 (CH2), 57.1 (Me), 32.9 (Me), 10.0 (Me); MS (ES) 334 (M+Na+, 100%), 218 (11); Found: M+Na+, 334.1056. C18H17NO4 + Na requires 334.1055.
3-(4-Aminophenoxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 17
To a solution of DMSO (10 mL) were added 4-iodophenol (1 g, 4.55 mmol), sodium azide (355 mg, 5.46 mmol), copper(I) iodide (87 mg, 0.46 mmol), L-proline (105 mg, 0.91 mmol) and sodium hydroxide (36.4 mg, 0.91 mmol). The reaction mixture was degassed and then stirred under an argon atmosphere at 60 ° C for 20 h. On cooling the reaction mixture was diluted with water (10 mL) and extracted into ethyl acetate (2 × 25 mL). The solvent was removed in vacuo to give 4-azidophenol as a brown/red oil (300 mg, 49%) (lit.,29 pale red oil) that was taken through to the next step without further purification.
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (60 mg, 0.26 mmol), thionyl chloride (0.93 mL, 12.77 mmol), 4-azidophenol (103 mg, 0.77 mmol) and potassium carbonate (106 mg, 0.77 mmol) gave after column chromatography (gradient elution with 20–30% ethyl acetate/light petroleum) 3-(4-azidophenoxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione (50 mg, 59%) as an impure yellow crystalline solid (ca. 5% impurity); mp 182–184 °C (not recrystallized); 1H NMR (500 MHz; CDCl3) δ 6.99–6.97 (2 H, m, ArH), 6.94–6.92 (2 H, m, ArH), 5.65 (1 H, s, 5-H), 5.35 (2 H, s, CH2), 3.90 (3 H, s, Me), 3.81 (3 H, s, Me), 2.32 (3 H, s, Me); 13C NMR (125 MHz; CDCl3) δ 184.5 (C), 171.4 (C), 160.0 (C), 155.9 (C), 140.4 (C), 138.3 (C), 132.6 (C), 127.1 (C), 124.0 (C), 120.0 (CH), 116.3 (CH), 106.8 (CH), 60.7 (CH2), 56.6 (Me), 32.7 (Me), 10.2 (Me); MS (ES) 375 (M+Na+, 100%), 218 (65); Found: M+Na+, 375.1060. C18H16N4O4 + Na requires 375.1069.
To a solution of 3-(4-azidophenoxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione (25 mg, 0.07 mmol) in THF/water (4 mL; 9:1) was added polymer supported triphenylphosphine (0.047 g, 0.14 mmol). The reaction mixture was stirred at room temperature for 48 h and then diluted with dichloromethane (25 mL), washed with water (10 mL), dried (MgSO4) and purified by column chromatography (gradient elution with 30–60% ethyl acetate/light petroleum) to give a solid. Further purification by preparative reverse phase HPLC (250 × 21.2 mm Polaris C18 column; gradient elution 5–95% MeCN/water) gave the title compound (6 mg, 26%) as a red crystalline solid; mp 211–213 °C (from MeCN/hexane); 1H NMR (400 MHz; CDCl3) δ 6.86–6.82 (2 H, m, ArH), 6.65–6.62 (2 H, m, ArH), 5.64 (1 H, s, 5-H), 5.23 (2 H, s, CH2), 3.90 (3 H, s, Me), 3.81 (3 H, s, Me), 3.42 (2 H, bs, NH2), 2.31 (3 H, s, Me); 13C NMR (125 MHz; CDCl3) δ 184.4 (C), 171.3 (C), 159.8 (C), 151.6 (C), 140.4 (C), 140.2 (C), 126.9 (C), 124.0 (C), 118.1 (C), 116.3 (CH), 116.2 (CH), 106.7 (CH), 61.0 (CH2), 56.5 (Me), 32.6 (Me), 10.2 (Me); MS (ES) 349 (M+Na+, 100%), 327 (45); Found: M+Na+, 349.1156. C18H18N2O4 + Na requires 349.1164.
6-Methoxy-1,2-dimethyl-3-(3-nitrophenoxymethyl)indole-4,7-dione 18
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (40 mg, 0.17 mmol), thionyl chloride (0.62 mL, 8.51 mmol), 3-nitrophenol (71 mg, 0.51 mmol) and potassium carbonate (71 mg, 0.51 mmol) gave after chromatography (elution with 30% ethyl acetate/light petroleum) the title compound (0.047 g, 78%) as a yellow crystalline solid; mp 203–205 °C (from ethyl acetate/light petroleum); 1H NMR (500 MHz; DMSO-d) δ 7.82–7.79 (2 H, m, ArH), 7.58 (1 H, t, J 8.0, ArH), 7.44 (1 H, ddd, J 8.0, 2.5, 1.5, ArH), 5.76 (1 H, s, 5-H), 5.32 (2 H, s, CH2) 3.86 (3 H, s, Me), 3.77 (3 H, s, Me), 2.30 (3 H, s, Me); 13C NMR (125 MHz; DMSO-d) δ 183.8 (C), 170.6 (C), 159.8 (C), 159.0 (C), 148.8 (C), 141.1 (C), 130.7 (C), 126.6 (C), 123.3 (C), 122.2 (CH), 115.6 (CH), 115.2 (C), 108.8 (CH), 106.4 (CH), 60.5 (CH2), 56.7 (Me), 32.5 (Me), 9.5 (CH3); MS (ES) 379 (M+Na+, 100%), 218 (16); Found: M+Na+, 379.0906. C18H16N2O6 + Na requires 379.0901; Anal. (C18H16N2O6) H, N. C: calcd, 60.67; found, 59.99.
6-Methoxy-1,2-dimethyl-3-(2-nitrophenoxymethyl)indole-4,7-dione 19
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (20 mg, 0.21 mmol), thionyl chloride (0.78 mL, 10.64 mmol), 2-nitrophenol (89 mg, 0.64 mmol) and potassium carbonate (88 mg, 0.64 mmol) gave after column chromatography (elution with 30% ethyl acetate/light petroleum) the title compound (33 mg, 44%) as an orange crystalline solid; mp 222–224 °C (from ethyl acetate); 1H NMR (500 MHz; CDCl3) δ 7.78 (1 H, dd, J 8.1, 1.7, ArH), 7.49 (1 H, ddd, J 8.5, 7.4, 1.7, ArH), 7.39 (1 H, dd, J 8.5, 0.9, ArH), 7.00, (1 H, m, ArH), 5.66 (1 H, s, 5-H), 5.53 (2 H, s, CH2), 3.91 (3 H, s, Me), 3.82 (3 H, s, Me), 2.38 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 185.1 (C), 171.6 (C), 160.4 (C), 151.9 (C), 141.6 (C), 140.7 (C), 134.5 (CH), 127.3 (C), 125.7 (CH), 124.1 (C), 120.9 (CH), 116.6 (C), 116.3 (CH), 106.9 (CH), 62.1 (CH2), 57.0 (Me), 33.0 (Me), 10.6 (Me); MS (ES) 379 (M+Na+, 100%); Found: M+Na+,379.0901. C18H16N2O6 + Na requires 379.0906; Anal. (C18H16N2O6) H, N. C: calcd, 60.67; found, 60.07.
6-Methoxy-1,2-dimethyl-3-(2,4-dinitrophenoxymethyl)indole-4,7-dione 20
To a solution of 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (40 mg, 0.17 mmol) and 2,4-dinitrofluorobenzene (128 μl, 1.02 mmol) in THF (5 mL) at 0 °C was added a solution of TBAF (1 M in THF; 1.02 mmol). The reaction mixture was stirred for 18 h at room temperature. The solvent was removed in vacuo and the crude product was purified by chromatography (elution with dichloromethane then 50% dichloromethane/ethyl acetate) to give the title compound (54 mg, 79%) as a yellow crystalline solid; mp 184–186 °C (from ethyl acetate/light petroleum); 1H NMR (400 MHz; DMSO-d) δ 8.68 (1 H, d, J 2.8, ArH), 8.37 (1 H, dd, J 2.8, 9.2, ArH), 7.60 (1 H, d, J 9.2, ArH), 5.69 (1 H, s, 5-H), 5.67 (2 H, s, CH2) 3.91 (3 H, s, Me), 3.84 (3 H, s, Me), 2.38 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 184.9 (C), 171.3 (C), 160.1 (C), 156.0 (C), 141.4 (C), 140.1 (C), 139.3 (C), 129.0 (CH), 127.0 (C), 123.6 (C), 121.7 (CH), 115.9 (CH), 114.7 (C), 106.6 (CH), 62.5 (CH2), 56.8 (Me), 32.8 (Me), 10.3 (Me); MS (ES) 424 (M+Na+, 100%); Found: M+Na+, 424.0735. C18H15N3O8 + Na requires 424.0756; Anal. (C18H15N3O8) H. C: calcd, 53.87; found, 53.43; N: calcd, 10.47; found, 9.75.
3-(2-Fluoro-4-nitrophenoxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 21
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (0.040 g, 0.17 mmol), thionyl chloride (0.62 mL, 8.51 mmol), 2-fluoro-4-nitrophenol (0.080 g, 0.51 mmol) and potassium carbonate (0.071 g, 0.51 mmol) gave after chromatography (elution with 20% ethyl acetate/light petroleum) the title compound (0.040 g, 63%) as a yellow crystalline solid; mp 229–231 °C (from chloroform/hexane); 1H NMR (400 MHz; CDCl3) δ 8.04 (1 H, ddd, J 8.8, 2.8, 1.6, ArH), 7.96 (1 H, dd, J 10.8, 2.8, ArH), 7.34 (1 H, dd, J 8.8, 8.4, ArH), 5.68 (1 H, s, 5-H), 5.53 (2 H, s, CH2) 3.92 (3 H, s, Me), 3.83 (3 H, s, Me), 2.36 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 184.6 (C), 171.4 (C), 160.0 (C), 151.5 (d, JCF 249, CF), 152.2 (d, JCF 11.3, C), 140.9 (C), 140.7 (C), 127.2 (C), 123.9 (C), 121.0 (d, JCF 3.8, CH), 115.6 (C), 114.1 (CH), 112.3 (d, JCF 23.8, CH), 106.7 (CH), 61.8 (CH2), 56.7 (Me), 32.7 (Me), 10.2 (Me); MS (ES) 397 (M+Na+, 79%), 218 (100); Found: M+Na+, 397.0788. C18H15FN2O6 + Na requires 397.0812; Anal. (C18H15FN2O6) C, H. N: calcd, 7.44; found, 6.92.
3-(4-Cyanophenoxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 22
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (60 mg, 0.26 mmol), thionyl chloride (0.93 mL, 12.77 mmol), 4-hydroxybenzonitrile (91.1 mg, 0.77 mmol) and potassium carbonate (106 mg, 0.77 mmol) gave after column chromatography (gradient elution with 20–30% ethyl acetate/light petroleum) the title compound (53 mg, 62%) as a yellow crystalline solid; mp 184 °C (from ethyl acetate); 1H NMR (400 MHz; CDCl3) δ 7.57 (2 H, dd, J 7.0, 1.8, ArH), 7.05 (2 H, dd, J 7.0, 1.8, ArH), 5.67 (1 H, s, 5-H), 5.38 (2 H, s, CH2), 3.92 (3 H, s, Me), 3.83 (3 H, s, Me), 2.32 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 184.4 (C), 171.4 (C), 161.7 (C), 159.9 (C), 140.3 (C), 134.0 (CH), 127.1 (C), 123.9 (C), 119.2 (C), 116.2 (C), 115.6 (CH), 106.7 (CH), 104.1 (C), 60.5 (CH2), 56.6 (Me), 32.7 (Me), 10.2 (Me); MS (ES) 359 (M+Na+, 80%), 218 (100); Found: M+Na+, 359.1017. C19H16N2O4 + Na requires 359.1007; Anal. (C19H16N2O4) H, N. C: calcd, 67.85; found, 66.67.
6-Methoxy-1,2-dimethyl-3-[4-(trifluoromethyl)phenoxymethyl]indole-4,7-dione 23
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (60 mg, 0.26 mmol), thionyl chloride (0.93 mL, 12.77 mmol), 4-trifluoromethylphenol (124 mg, 0.77 mmol) and potassium carbonate (106 mg, 0.77 mmol) gave after column chromatography (gradient elution with 20–40% ethyl acetate/hexane) the title compound (75 mg, 78%) as an orange crystalline solid; mp 150 °C (from ethyl acetate/hexane); 1H NMR (400 MHz; CDCl3) δ 7.52 (2 H, d, J 8.8, ArH), 7.05 (2 H, d, J 8.8, ArH), 5.66 (1 H, s, 5-H), 5.37 (2 H, s, CH2), 3.91 (3 H, s, Me), 3.82 (3 H, s, Me), 2.32 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 184.4 (C), 171.4 (C), 160.9 (C), 159.9 (C), 140.3 (C), 127.1 (C), 126.8 (q, JCF 3.7, CH), 124.4 (q, JCF 271, CF3), 123.0 (q, JCF 32.7, C), 123.9 (C), 116.7 (C), 114.8 (CH), 106.7 (CH), 60.4 (CH2), 56.6 (Me), 32.6 (Me), 10.2 (Me); MS (ES) 402 (M+Na+, 49%), 218 (100); Found: M+Na+, 402.0930. C19H16F3NO4 +Na requires 402.0929; Anal. (C19H16F3NO4) C, H, N.
3-(4-Fluorophenoxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 24
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (60 mg, 0.21 mmol), thionyl chloride (0.78 mL, 10.6 mmol), 4-fluorophenol (71.6 mg, 0.64 mmol) and potassium carbonate (88 mg, 0.64 mmol) gave after column chromatography (gradient elution with 20–40% ethyl acetate/hexane) the title compound (30 mg, 43%) as a yellow crystalline solid; mp 171 °C (from ethyl acetate); 1H NMR (400 MHz; CDCl3) δ 6.96–6.93 (4 H, m, ArH), 5.65 (1 H, s, 5-H), 5.28 (2 H, s, CH2), 3.91 (3 H, s, Me), 3.82 (3 H, s, Me), 2.32 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 184.4 (C), 171.4 (C), 159.9 (C), 157.4 (d, JCF 238, CF), 154.6 (C), 140.3 (C), 127.0 (C), 124.0 (C), 117.4 (C), 116.0 (d, JCF 8.1, CH), 115.8 (d, JCF 23.0, CH), 106.7 (CH), 60.9 (CH2), 56.6 (Me), 32.6 (Me), 10.2 (Me); MS (ES) 352 (M+Na+, 100%), 218 (56); Found: M+Na+, 352.0959. C18H16FNO4 + Na requires 352.0961.
3-(2,4-Difluorophenoxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 25
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (60 mg, 0.26 mmol), thionyl chloride (0.93 mL, 12.77 mmol), 2,4-difluorophenol (99.5 mg, 0.77 mmol) and potassium carbonate (106 mg, 0.77 mmol) gave after column chromatography (gradient elution with 20–40% ethyl acetate/hexane) the title compound (42 mg, 47%) as a yellow crystalline solid; mp 173 °C (from ethyl acetate); 1H NMR (400 MHz; CDCl3) δ 7.11 (1 H, td, J 9.2, 5.4, ArH), 6.82 (1 H, ddd, J 11.2, 8.3, 2.8, ArH), 6.79–6.73 (1 H, m, ArH), 5.65 (1 H, s, 5-H), 5.34 (2 H, s, CH2), 3.91 (3 H, s, Me), 3.81 (3 H, s, Me), 2.35 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 184.4 (C), 171.4 (C), 159.9 (C), 156.7 (dd, JCF 242, 10.3, CF), 152.9 (dd, JCF 249, 12.1, CF), 142.8 (dd, JCF 10.8, 3.3, C), 140.7 (C), 127.0 (C), 124.0 (C), 117.0 (dd, JCF 9.5, 2.9, CH), 116.9 (C), 110.4 (dd, JCF 22.4, 3.8, CH), 106.6 (CH), 104.7 (dd, 2J 26.7, 2J 22.3, CH), 62.3 (CH2), 56.6 (Me), 32.6 (Me), 10.1 (Me); MS (ES) 370 (M+Na+, 80%), 218 (100); Found: M+Na+, 370.0863. C18H15F2NO4 + Na requires 370.0867; Anal. (C18H15F2NO4) C, H, N.
6-Methoxy-1,2-dimethyl-3-(2,4,6-trifluorophenoxymethyl)indole-4,7-dione 26
Using Method A, 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (60 mg, 0.26 mmol), thionyl chloride (0.93 mL, 12.77 mmol), 2,4,6-trifluorophenol (113 mg, 0.77 mmol) and potassium carbonate (106 mg, 0.77 mmol) gave after column chromatography (gradient elution with 20–40% ethyl acetate/hexane) the title compound (70 mg, 75%) as a yellow crystalline solid; mp 221 °C (from ethyl acetate/hexane); 1H NMR (400 MHz; CDCl3) δ 6.67 (2 H, t, J 8.2, ArH), 5.60 (1 H, s, 5-H), 5.32 (2 H, s, CH2), 3.92 (3 H, s, Me), 3.79 (3 H, s, Me), 2.35 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 183.9 (C), 171.5 (C), 159.7 (C), 157.3 (dt, JCF 246, 14.3, CF), 156.5 (ddd, JCF 250, 14.8, 7.7, CF), 141.0 (C), 131.9 (dd, JCF 15.3, 5.0, C), 127.0 (C), 124.2 (C), 116.5, (C), 106.6 (CH), 100.6 (t, JCF 26.7, CH), 65.9 (CH2), 56.5 (Me), 32.6 (Me), 9.7 (Me); MS (ES) 388 (M+Na+, 57%), 218 (100); Found: M+Na+, 388.0766. C18H14F3NO4 + Na requires 388.0773; Anal. (C18H14F3NO4) C, H, N.
6-Methoxy-1,2-dimethyl-3-(pyridin-2-yloxymethyl)indole-4,7-dione 27
Using Method B, diethyl azodicarboxylate (96 μl, 0.61 mmol), 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (40 mg, 0.17 mmol), tri(2-furyl)phosphine (122 mg, 0.51 mmol) and 2-hydroxypyridine (49.5 mg, 0.51 mmol) gave after column chromatography (gradient elution with 10–40% ethyl acetate/light petroleum) the title compound (22 mg, 41%) as an orange crystalline solid; mp 178–180 °C (from ethyl acetate/light petroleum); 1H NMR (500 MHz; CDCl3) δ 8.19 (1 H, dd, J 5.0, 1.5, ArH), 7.57–7.54 (1 H, m, ArH), 6.88 (1H, ddd, J 7.0, 5.0, 1.0, ArH), 6.74 (1 H, d, J 8.5, ArH) 5.66 (1 H, s, 5-H), 5.54 (2 H, s, CH2), 3.92 (3 H, s, Me), 3.81 (3 H, s, Me), 2.35 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 183.9 (C), 171.5 (C), 163.6 (C), 159.7 (C), 146.8 (CH), 140.3 (C), 138.5 (CH), 127.3 (C), 124.5 (C), 117.5 (C), 116.8 (CH), 111.1 (CH), 106.8 (CH), 57.9 (CH2), 56.5 (Me), 32.6 (Me), 10.0 (Me); MS (ES) 335 (M+Na+, 72%), 218 (100%); Found: M+Na+, 335.1006. C17H16N2O4 + Na requires 335.1007; Anal. (C17H16N2O4) H, N. C: calcd, 65.38; found, 62.00.
3-Methoxy-1,2-dimethyl-3-(3-pyridyloxymethyl)indole-4,7-dione 28
To a solution of 3-hydroxypyridine (26.9 mg, 0.277 mmol) in DMF (3 mL) was added sodium hydride (60% dispersion in oil) (11 mg, 0.28 mmol) at 0 °C. This solution was stirred at room temperature for 45 min. The crude chloride (0.213 mmol) (prepared as in Method A) in DMF (4 mL) was added dropwise at 0 °C and the reaction mixture stirred at room temperature for a further 3 h. The solvent was concentrated in vacuo and the crude product dissolved in dichloromethane (50 mL), washed with water (2 × 25 mL) and NaOH (25 mL) dried (MgSO4) and the solvent removed in vacuo. The crude product was purified by column chromatography (gradient elution with 30–80% ethyl acetate/light petroleum) to give the title compound (28 mg, 42%) as a yellow crystalline solid; mp 179–181 °C (from ethyl acetate/light petroleum); 1H NMR (400 MHz; CDCl3) δ 8.36 (1 H, bs, ArH), 8.21 (1 H, apparent bd, ArH), 7.36 (1H, ddd, J 8.4, 1.6, 1.2, ArH), 7.22 (1 H, dd, J 8.4, 4.8, ArH) 5.67 (1 H, s, 5-H), 5.38 (2 H, s, CH2), 3.92 (3 H, s, Me), 3.82 (3 H, s, Me), 2.33 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 184.4 (C), 171.4 (C), 159.9 (C), 154.7 (C), 142.1 (CH), 140.4 (C), 138.6 (CH), 127.1 (C), 124.0 (C), 123.9 (CH), 121.4 (CH), 116.6 (C), 106.7 (CH), 60.5 (CH2), 56.6 (Me), 32.6 (Me), 10.2 (Me); MS (EI) 313 (MH+, 100%); Found: MH+, 313.1178. C17H16N2O4 + H requires 313.1188.
6-Methoxy-1,2-dimethyl-3-(pyridin-4-yloxymethyl)indole-4,7-dione 29
Using Method B, diethyl azodicarboxylate (96 μl, 0.61 mmol), 3-(hydroxymethyl)-6-methoxy-1,2-dimethylindole-4,7-dione 37 (40 mg, 0.17 mmol), tri(2-furyl)phosphine (122 mg, 0.51 mmol) and 4-hydroxypyridine (49.5 mg, 0.51 mmol) gave after column chromatography (gradient elution with 10–40% ethyl acetate/light petroleum) the title compound (0.022 g, 41%) as an orange crystalline solid; mp 161–163 °C (from ethanol/light petroleum); 1H NMR (400 MHz; CDCl3) δ 8.42 (2 H, d, J 5.0, ArH), 6.92 (2 H, d, J 5.0, ArH), 5.67 (1 H, s, 5-H), 5.39 (2 H, s, CH2), 3.92 (3 H, s, Me), 3.83 (3 H, s, Me), 2.32 (3 H, s, Me); 13C NMR (100 MHz; CDCl3) δ 184.5 (C), 171.4 (C), 164.7 (C), 160.0 (C), 150.8 (CH), 140.4 (C), 127.2 (C), 123.9 (C), 116.1 (C), 110.7 (CH), 106.7 (CH), 60.2 (CH2), 56.6 (Me), 32.7 (Me), 10.2 (Me); MS (ES) 313 (MH+,17%), 218 (100); Found: MH+, 313.1177. C17H16N2O4 + H requires 313.1188.
Biology
Materials
NADH, FAD, 2,6-dichlorophenol-indophenol (DCPIP), 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and bovine serum albumin were obtained from Sigma Chemical (St. Louis MO). rhNQO1 was purified from E. coli using Cibacron blue affinity chromatography as previously described,30 and had a specific activity of 800 μmol DCPIP/min/mg protein. For all biological assays stock concentrations of indolequinones (5 mM) were prepared in dimethyl sulfoxide and stored in the dark at 32 °C.
Cell lines
MIA PaCa-2 human pancreatic carcinoma cells were obtained from ATCC (Manassas VA). MIA PaCa-2 cells were grown in Dulbecco’s modified Eagle’s medium adjusted to contain 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10% (v/v) fetal bovine serum, 2.5% (v/v) horse serum, 100 units/mL penicillin and 100 μg/mL streptomycin in a humidified incubator containing 5% carbon dioxide at 37 °C.
NQO1 inhibition studies
The mechanism-based inactivation of rhNQO1 by this indolequinone series was assayed using the following methods. In these reactions (0.5 mL final volume) rhNQO1 (2 μg/mL) was incubated with 0.1–5.0 μM of the indolequinone in the absence and presence of 0.2 mM NADH in 50 mM potassium phosphate buffer, pH 7.4 containing 125 mM NaCl and 1 mg/mL bovine serum albumin at 32 °C. After 5 min, a 50 μL aliquot was removed and diluted 100-fold in stop buffer (50 mM potassium phosphate buffer, pH 7.4 containing 250 mM sucrose, 5 μM FAD and 0.1% (v/v) Tween-20) and NQO1 activity was measured using the reduction of DCPIP. Briefly, 960 μL was transferred to a cuvet with 0.2 mM NADH. The reaction was started with the addition of 40 μM DCPIP (final volume 1 mL) and the linear decreased in absorbance was monitored at 600 nm for 1 min at 32 °C.
Partition ratios for the inactivation of rhNQO1 by the indolequinones, that demonstrated mechanism-based inhibition, were determined essentially as described above, except that the compounds and rhNQO1 were incubated in the presence of 0.2 mM NADH for 15 min, with defined molar ratios of indolequinone to rhNQO1 (range 0.2:1 to 1,250:1).
Inactivation of NQO1 in MIA PaCa-2 cells in culture, by these indolequinones, was determined using the following procedure. MIA PaCa-2 cells (1 × 106) were plate in 60 mm plates with 5 mL of complete medium. The following day the growth medium was replaced with the indolequinone containing complete medium for 1 h, after which the medium as removed, the cells washed with 10 mL of phosphate buffered saline and the cells were then scraped into 0.5 mL of 50 mM potassium phosphate buffer, pH 7.4 containing 250 mM sucrose and 5μM FAD and briefly sonicated on ice. NQO1 activity was determined in MIA PaCa-2 sonicates using the reduction of DCPIP. Briefly, sonicates (20 μL) were added to 50 mM potassium phosphate buffer, pH 7.4 containing 1 mg/mL bovine serum albumin and 0.2 mM NADH. The reaction was started with the addition of 40 μM DCPIP (final volume 1 mL) and the linear decreased in absorbance was monitored at 600 nm for 1 min at 32 °C.
Growth inhibition assays
Growth inhibition in the human pancreatic MIA PaCa-2 cancer cell line was measured using the MTT colorimetric assay.20 In these studies, the MIA PaCa-2 cells were seeded at 2 × 103 cells/well in 96-well plates, in triplicate, for each indolequinone, and allowed to attach for 16 h. Medium was removed by aspiration and the MIA PaCa-2 cells were treated with the appropriate indolequinone (6.25–3200 nM) in complete medium for 4 and 72 h time periods. The medium was removed and MTT (50 μg) in medium (50 μL) was added to each well and incubated for a further 4 h. Cell viability was determined by measuring the cellular reduction of MTT to the crystalline formazan product, dissolved by the addition of DMSO (100 μL). Optical density was determined at 550 nm using a Molecular Devices Thermomax microplate reader. The IC50 values were defined as the concentration of indolequinone that resulted in 50% reduction in cell number compared to the DMSO treated control, determined from semi-log plots of percentage of control versus indolequinone concentration.
Supplementary Material
General experimental details, experimental procedures for compounds 31–37, elemental analyses, full characterization data for compounds 6, 7, 10–13 and 15–29, HPLC data, and cytotoxicity data on phenols and hydroxypyridines. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We acknowledge support from the FORCE cancer charity (PhD studentship to M.A.C.), the Association for International Cancer Research (PhD studentship to A.C.), and National Institute of Health grant RO1 CA114441.
Abbreviations
- rhNQO1
recombinant human NAD(P)H:quinone oxidoreductase 1
- DCPIP
2,6-dichlorophenol-indophenol
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- TBAF
tetra-n-butylammonium fluoride
References
- 1.Ernster L. DT Diaphorase. Method Enzymol. 1967;10:309–317. [Google Scholar]
- 2.Ernster L. DT Diaphorase: a historical review. Chem Scripta. 1987;27A:1–13. [Google Scholar]
- 3.Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem-Biol Interact. 2000;129:77–97. doi: 10.1016/s0009-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 4.Beall HD, Winski SL. Mechanisms of action of quinone-containing alkylating agents I: NQO1-directed drug development. Front Biosci. 2000;5:D639–D648. doi: 10.2741/beall. [DOI] [PubMed] [Google Scholar]
- 5.Ross D. Quinone reductases multitasking in the metabolic world. Drug Metab Rev. 2004;36:639–654. doi: 10.1081/dmr-200033465. [DOI] [PubMed] [Google Scholar]
- 6.Ross D, Siegel D, Beall H, Prakash AS, Mulcahy RT, Gibson NW. DT-diaphorase in activation and detoxicification of quinones. Cancer Metast Rev. 1993;12:83–101. doi: 10.1007/BF00689803. [DOI] [PubMed] [Google Scholar]
- 7.Ross D, Beall H, Traver RD, Siegel D, Phillips RM, Gibson NW. Bioactivation of quinones by DT-diaphorase, molecular, biochemical, and chemical studies. Oncol Res. 1994;6:493–500. [PubMed] [Google Scholar]
- 8.Ross D, Beall HD, Siegel D, Traver RD, Gustafson DL. Enzymology of bioreductive drug activation. Br J Cancer. 1996;74(Suppl XXVII):S1–S8. [PMC free article] [PubMed] [Google Scholar]
- 9.Beall HD, Winski S, Swann E, Hudnott AR, Cotterill AS, O’Sullivan N, Green SJ, Bien R, Siegel D, Ross D, Moody CJ. Indolequinone antitumor agents: correlation between quinone structure, rate of metabolism by recombinant human NQO1 and in vitro cytotoxicity. J Med Chem. 1998;41:4755–4766. doi: 10.1021/jm980328r. [DOI] [PubMed] [Google Scholar]
- 10.Winski SL, Swann E, Hargreaves RHJ, Butler J, Moody CJ, Ross D. Relationship between NAD(P)H:quinone oxidoreductase 1 (NQO1) levels in a series of stably transfected cell lines and susceptibility to antitumor quinones. Clin Cancer Res. 1999;5:179. doi: 10.1016/s0006-2952(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 11.Winski SL, Swann E, Hargreaves RHJ, Dehn DL, Butler J, Moody CJ, Ross D. Relationship between NAD(P)H:quinone oxidoreductase 1 (NQO1) levels in a series of stably transfected cell lines and susceptibility to antitumor quinones. Biochem Pharmacol. 2001;61:1509–1516. doi: 10.1016/s0006-2952(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 12.Swann E, Barraja P, Oberlander AM, Gardipee WT, Hudnott AR, Beall HD, Moody CJ. Indolequinone antitumor agents: correlation between quinone structure and rate of metabolism by recombinant human NAD(P)H: quinone oxidoreductase. Part 2. J Med Chem. 2001;44:3311–3319. doi: 10.1021/jm010884c. [DOI] [PubMed] [Google Scholar]
- 13.Fryatt T, Pettersson HI, Gardipee WT, Bray KC, Green SJ, Slawin AMZ, Beall HD, Moody CJ. Novel quinolinequinone antitumor agents: structure-metabolism studies with NAD(P)H:quinone oxidoreductase (NQO1) Bioorg Med Chem. 2004;12:1667–1687. doi: 10.1016/j.bmc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Winski SL, Faig M, Bianchet MA, Siegel D, Swann E, Fung K, Duncan MW, Moody CJ, Amzel M, Ross D. Characterization of a mechanism based inhibitor of NAD(P)H: quinone NQO1 (DT-diaphorase) by biochemical, X ray crystallographic and mass spectrometric approaches. Biochemistry. 2001;40:15135–15142. doi: 10.1021/bi011324i. [DOI] [PubMed] [Google Scholar]
- 15.Dehn DL, Siegel D, Swann E, Moody CJ, Ross D. Biochemical, cytotoxic, and genotoxic effects of ES936, a mechanism-based inhibitor of NAD(P)H: quinone oxidoreductase 1, in cellular systems. Mol Pharmacol. 2003;64:714–720. doi: 10.1124/mol.64.3.714. [DOI] [PubMed] [Google Scholar]
- 16.Cullen JJ, Hinkhouse MM, Grady M, Gaut AW, Liu JR, Zhang YP, Weydert CJD, Domann FE, Oberley LW. Dicumarol inhibition of NADPH: Quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide- mediated mechanism. Cancer Res. 2003;63:5513–5520. [PubMed] [Google Scholar]
- 17.Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. NAD(P)H:quinone oxidoreductase 1: Role as a superoxide scavenger. Mol Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 18.Asher G, Dym O, Tsvetkov P, Adler J, Shaul Y. The crystal structure of NAD(P)H quinone oxidoreductase 1 in complex with its potent inhibitor dicoumarol. Biochemistry. 2006;45:6372–6378. doi: 10.1021/bi0600087. [DOI] [PubMed] [Google Scholar]
- 19.Dehn DL, Siegel D, Zafar KS, Reigan P, Swann E, Moody CJ, Ross D. 5-Methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione, a mechanism-based inhibitor of NAD(P)H:quinone oxidoreductase 1, exhibits activity against human pancreatic cancer in vitro and in vivo. Mol Cancer Ther. 2006;5:1702–1709. doi: 10.1158/1535-7163.MCT-06-0105. [DOI] [PubMed] [Google Scholar]
- 20.Reigan P, Colucci MA, Siegel D, Chilloux A, Moody CJ, Ross D. Development of indolequinone mechanism-based inhibitors of NAD(P)H:quinone oxidoreductase 1 (NQO1): NQO1 inhibition and growth inhibitory activity in human pancreatic MIA PaCa-2 cancer cells. Biochemistry. 2007;46:5941–5950. doi: 10.1021/bi700008y. [DOI] [PubMed] [Google Scholar]
- 21.Naylor MA, Swann E, Everett SA, Jaffar M, Nolan J, Robertson N, Lockyer SD, Patel KB, Dennis MF, Stratford MRL, Wardman P, Adams GE, Moody CJ, Stratford IJ. Indolequinone anti-tumor agents: reductive activation and elimination from (5-methoxy-1,2-dimethyl-4,7-dioxo-indol-3-yl)methyl derivatives and hypoxia-selectivity in vitro. J Med Chem. 1998;41:2720–2731. doi: 10.1021/jm970744w. [DOI] [PubMed] [Google Scholar]
- 22.Swann E, Moody CJ, Stratford MRL, Patel KB, Naylor MA, Vojnovic B, Wardman P, Everett SA. Rates of reductive elimination of substituted nitrophenols from the (indol–3–yl)methyl position of indolequinones. J Chem Soc, Perkin Trans 2. 2001:1340–1345. [Google Scholar]
- 23.Cotterill AS, Moody CJ, Mortimer RJ, Norton CL, O’Sullivan N, Stephens MA, Stradiotto NR, Stratford IJ, Swann E. Cyclopropamitosenes, novel bioreductive anticancer agents. Synthesis, electrochemistry and biological activity of 7-substituted cyclopropamitosenes and related indolequinones. J Med Chem. 1994;37:3834–3843. doi: 10.1021/jm00048a019. [DOI] [PubMed] [Google Scholar]
- 24.Everett SA, Naylor MA, Barraja P, Swann E, Patel KB, Stratford MRL, Hudnott AR, Vojnovic B, Locke RJ, Wardman P, Moody CJ. Controlling the rates of reductively activated elimination from the (indol–3–yl)methyl position of indolequinones. J Chem Soc, Perkin Trans 2. 2001:843–860. [Google Scholar]
- 25.Farkas L, Gottsege A, Nogradi M, Antus S. Synthesis of natural isoflavanones ferreririn, dalbergioidin, and ougenin. J, Chem Soc, C. 1971:1994–2000. [Google Scholar]
- 26.Hemetsberger H, Knittel D, Weidmann H, Enazides 3. Thermolysis of alpha-azidocinnamates - synthesis of indole carboxylates. Monatsh Chem. 1970;101:161–165. [Google Scholar]
- 27.MacKenzie AR, Moody CJ, Rees CW. Synthesis of the Bacterial Coenzyme Methoxatin. J Chem Soc Chem Commun. 1983:1372–1373. [Google Scholar]
- 28.Bolton RE, Moody CJ, Rees CW, Tojo G. Vinyl Azides in Heterocyclic Synthesis. Part 8. Synthesis of the Naturally Occurring Phosphodiesterase Inhibitors PDE-I and PDE-II. J Chem Soc, Perkin Trans 1. 1987:931–935. [Google Scholar]
- 29.Cai Q, Zhu W, Zhang H, Zhang YD, Ma DW. Preparation of N-aryl compounds by amino acid-promoted Ullmann-type coupling reactions. Synthesis. 2005:496–499. [Google Scholar]
- 30.Beall HD, Mulcahy RT, Siegel D, Traver RD, Gibson NW, Ross D. Metabolism of bioreductive antitumor compounds by purified rat and human DT-diaphorases. Cancer Res. 1994;54:3196–3201. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General experimental details, experimental procedures for compounds 31–37, elemental analyses, full characterization data for compounds 6, 7, 10–13 and 15–29, HPLC data, and cytotoxicity data on phenols and hydroxypyridines. This material is available free of charge via the Internet at http://pubs.acs.org.


