Abstract
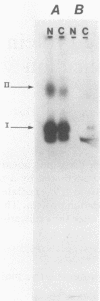
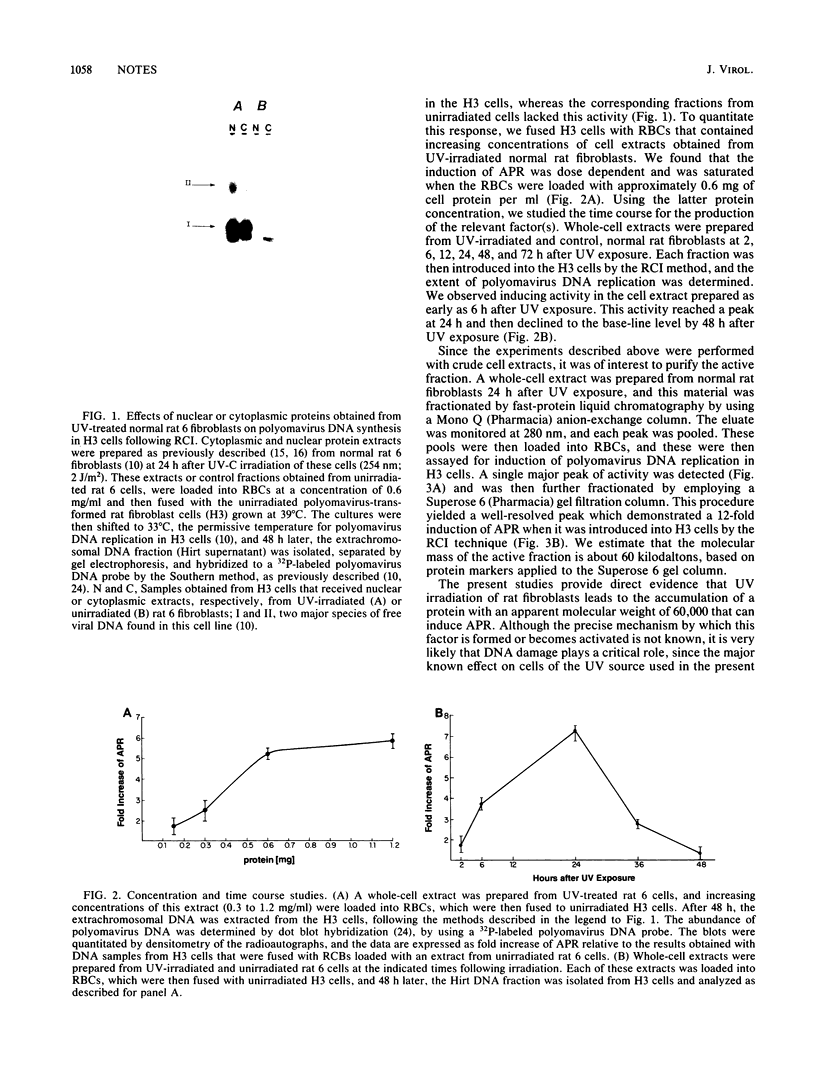
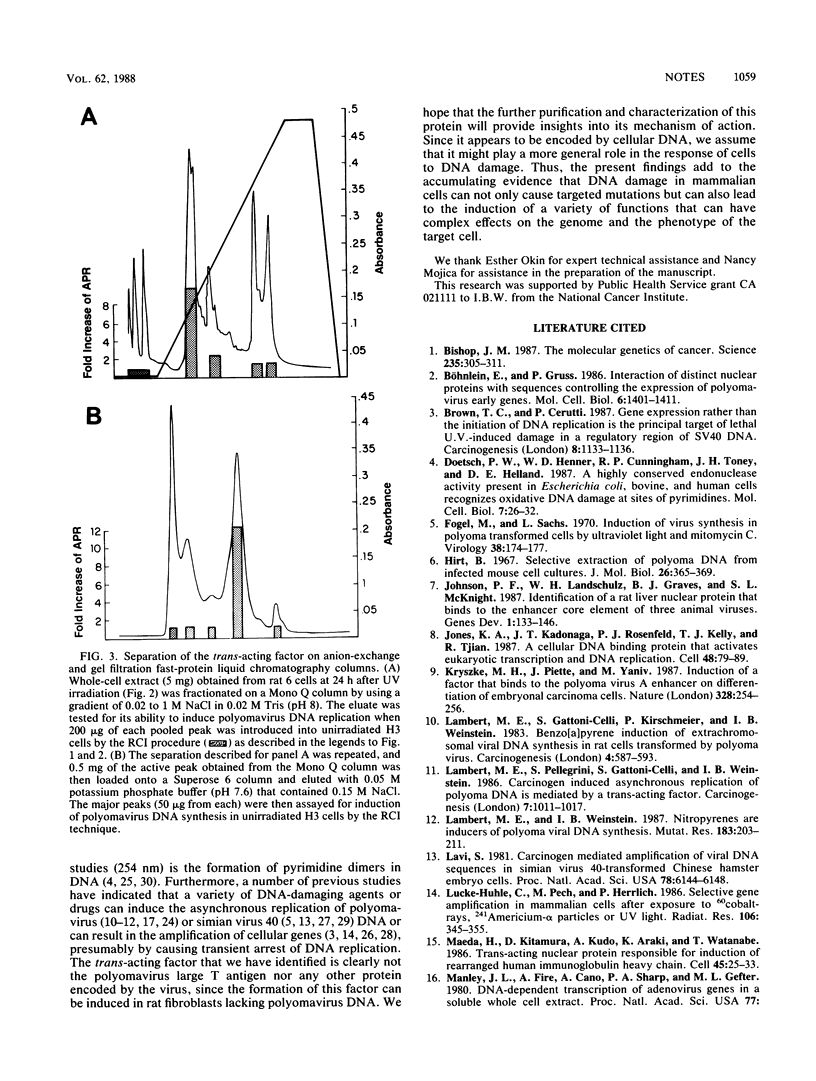
Previous studies provided indirect evidence that the ability of a variety of DNA-damaging agents to induce asynchronous polyomavirus DNA replication in the H3 rat fibroblast cell line is mediated by a trans-acting factor. Using an erythrocyte insertion technique to introduce protein fractions from UV-irradiated cells into unirradiated H3 cells, we have now obtained evidence that this factor is a 60-kilodalton protein. These findings provide evidence that DNA damage in mammalian cells induces a factor that can alter the replication of a viral DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Brown T. C., Cerutti P. Gene expression rather than the initiation of DNA replication is the principal target of lethal u.v.-induced damage in a regulatory region of SV40 DNA. Carcinogenesis. 1987 Aug;8(8):1133–1136. doi: 10.1093/carcin/8.8.1133. [DOI] [PubMed] [Google Scholar]
- Böhnlein E., Gruss P. Interaction of distinct nuclear proteins with sequences controlling the expression of polyomavirus early genes. Mol Cell Biol. 1986 May;6(5):1401–1411. doi: 10.1128/mcb.6.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch P. W., Henner W. D., Cunningham R. P., Toney J. H., Helland D. E. A highly conserved endonuclease activity present in Escherichia coli, bovine, and human cells recognizes oxidative DNA damage at sites of pyrimidines. Mol Cell Biol. 1987 Jan;7(1):26–32. doi: 10.1128/mcb.7.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel M., Sachs L. Induction of virus synthesis in polyoma transformed cells by ultraviolet light and mitomycin C. Virology. 1970 Jan;40(1):174–177. doi: 10.1016/0042-6822(70)90391-0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Johnson P. F., Landschulz W. H., Graves B. J., McKnight S. L. Identification of a rat liver nuclear protein that binds to the enhancer core element of three animal viruses. Genes Dev. 1987 Apr;1(2):133–146. doi: 10.1101/gad.1.2.133. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Kadonaga J. T., Rosenfeld P. J., Kelly T. J., Tjian R. A cellular DNA-binding protein that activates eukaryotic transcription and DNA replication. Cell. 1987 Jan 16;48(1):79–89. doi: 10.1016/0092-8674(87)90358-8. [DOI] [PubMed] [Google Scholar]
- Kryszke M. H., Piette J., Yaniv M. Induction of a factor that binds to the polyoma virus A enhancer on differentiation of embryonal carcinoma cells. Nature. 1987 Jul 16;328(6127):254–256. doi: 10.1038/328254a0. [DOI] [PubMed] [Google Scholar]
- Lambert M. E., Gattoni-Celli S., Kirschmeier P., Weinstein I. B. Benzo[a]pyrene induction of extrachromosomal viral DNA synthesis in rat cells transformed by polyoma virus. Carcinogenesis. 1983;4(5):587–593. doi: 10.1093/carcin/4.5.587. [DOI] [PubMed] [Google Scholar]
- Lambert M. E., Pellegrini S., Gattoni-Celli S., Weinstein I. B. Carcinogen induced asynchronous replication of polyoma DNA is mediated by a trans-acting factor. Carcinogenesis. 1986 Jun;7(6):1011–1017. doi: 10.1093/carcin/7.6.1011. [DOI] [PubMed] [Google Scholar]
- Lambert M. E., Weinstein I. B. Nitropyrenes are inducers of polyoma viral DNA synthesis. Mutat Res. 1987 May;183(3):203–211. doi: 10.1016/0167-8817(87)90001-0. [DOI] [PubMed] [Google Scholar]
- Lavi S. Carcinogen-mediated amplification of viral DNA sequences in simian virus 40-transformed Chinese hamster embryo cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6144–6148. doi: 10.1073/pnas.78.10.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lücke-Huhle C., Pech M., Herrlich P. Selective gene amplification in mammalian cells after exposure to 60Co gamma rays, 241Am alpha particles, or uv light. Radiat Res. 1986 Jun;106(3):345–355. [PubMed] [Google Scholar]
- Maeda H., Kitamura D., Kudo A., Araki K., Watanabe T. Trans-acting nuclear protein responsible for induction of rearranged human immunoglobulin heavy chain gene. Cell. 1986 Apr 11;45(1):25–33. doi: 10.1016/0092-8674(86)90534-9. [DOI] [PubMed] [Google Scholar]
- Manor H., Neer A. Effects of cycloheximide on virus RNA replication in an inducible line of polyoma-transformed rat cells. Cell. 1975 Jul;5(3):311–318. doi: 10.1016/0092-8674(75)90106-3. [DOI] [PubMed] [Google Scholar]
- Marsden H. S., Campbell M. E., Haarr L., Frame M. C., Parris D. S., Murphy M., Hope R. G., Muller M. T., Preston C. M. The 65,000-Mr DNA-binding and virion trans-inducing proteins of herpes simplex virus type 1. J Virol. 1987 Aug;61(8):2428–2437. doi: 10.1128/jvi.61.8.2428-2437.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott M. A., Dice J. F. Microinjection of cultured cells using red-cell-mediated fusion and osmotic lysis of pinosomes: a review of methods and applications. Biosci Rep. 1984 Jun;4(6):451–466. doi: 10.1007/BF01122221. [DOI] [PubMed] [Google Scholar]
- McKnight S., Tjian R. Transcriptional selectivity of viral genes in mammalian cells. Cell. 1986 Sep 12;46(6):795–805. doi: 10.1016/0092-8674(86)90061-9. [DOI] [PubMed] [Google Scholar]
- Nomura S., Oishi M. UV Irradiation induces an activity which stimulates Simian virus 40 rescue upon cell fusion. Mol Cell Biol. 1984 Jun;4(6):1159–1162. doi: 10.1128/mcb.4.6.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Watanabe T. Microinjection of macromolecules into normal murine lymphocytes by cell fusion technique. I. Quantitative microinjection of antibodies into normal splenic lymphocytes. J Immunol. 1982 Mar;128(3):1090–1096. [PubMed] [Google Scholar]
- Roberts J. M., Weintraub H. Negative control of DNA replication in composite SV40-bovine papilloma virus plasmids. Cell. 1986 Aug 29;46(5):741–752. doi: 10.1016/0092-8674(86)90350-8. [DOI] [PubMed] [Google Scholar]
- Ronai Z. A., Lambert M. E., Johnson M. D., Okin E., Weinstein I. B. Induction of asynchronous replication of polyoma DNA in rat cells by ultraviolet irradiation and the effects of various inhibitors. Cancer Res. 1987 Sep 1;47(17):4565–4570. [PubMed] [Google Scholar]
- Rosenstein B. S., Chao C. C., Ducore J. M. The use of metabolic inhibitors to compare the excision repair of pyrimidine dimers and nondimer DNA damages in human skin fibroblasts exposed to 254-nm and sunlamp-produced greater than 310-nm ultraviolet radiation. Environ Mutagen. 1986;8(3):335–343. doi: 10.1002/em.2860080303. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schlehofer J. R., Ehrbar M., zur Hausen H. Vaccinia virus, herpes simplex virus, and carcinogens induce DNA amplification in a human cell line and support replication of a helpervirus dependent parvovirus. Virology. 1986 Jul 15;152(1):110–117. doi: 10.1016/0042-6822(86)90376-4. [DOI] [PubMed] [Google Scholar]
- Stark G. R. DNA amplification in drug resistant cells and in tumours. Cancer Surv. 1986;5(1):1–23. [PubMed] [Google Scholar]
- Zamansky G. B. Varying sensitivity of human skin fibroblasts to polychromatic ultraviolet light. Mutat Res. 1986 Mar;160(1):55–60. doi: 10.1016/s0027-5107(96)90009-3. [DOI] [PubMed] [Google Scholar]
- van der Lubbe J. L., Abrahams P. J., van Drunen C. M., van der Eb A. J. Enhanced induction of SV40 replication from transformed rat cells by fusion with UV-irradiated normal and repair-deficient human fibroblasts. Mutat Res. 1986 Mar;165(2):47–56. doi: 10.1016/0167-8817(86)90059-3. [DOI] [PubMed] [Google Scholar]