Abstract
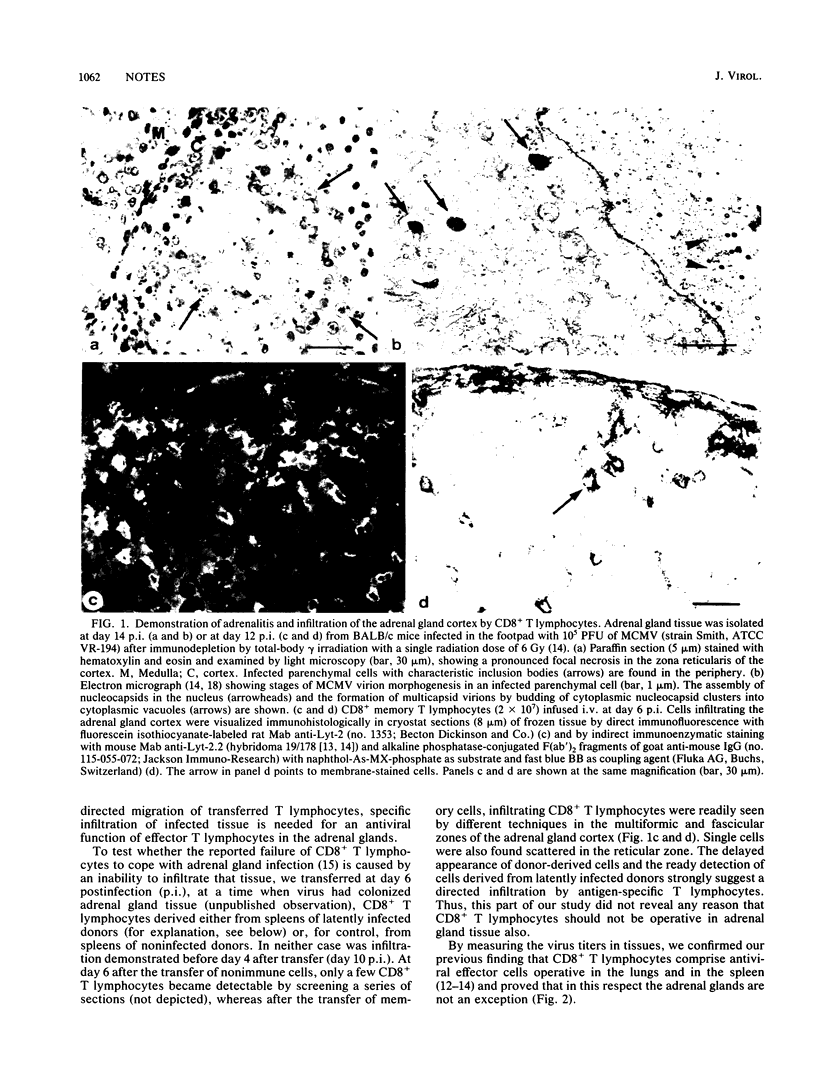
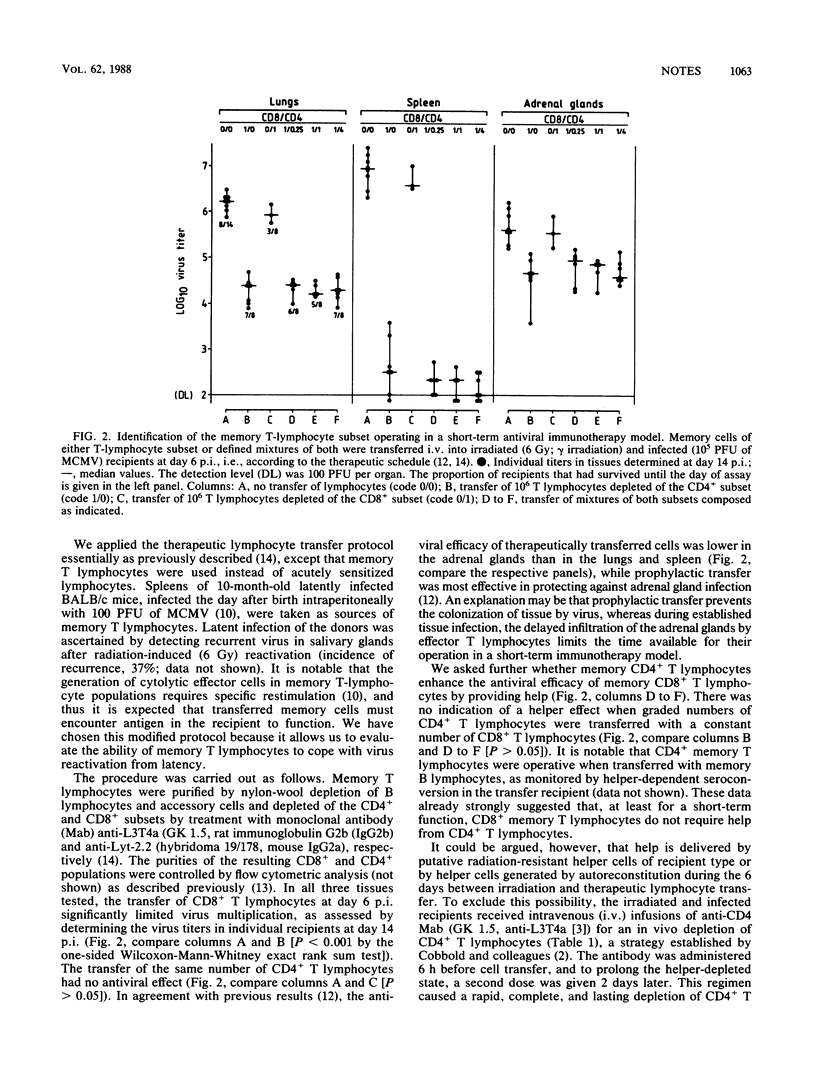
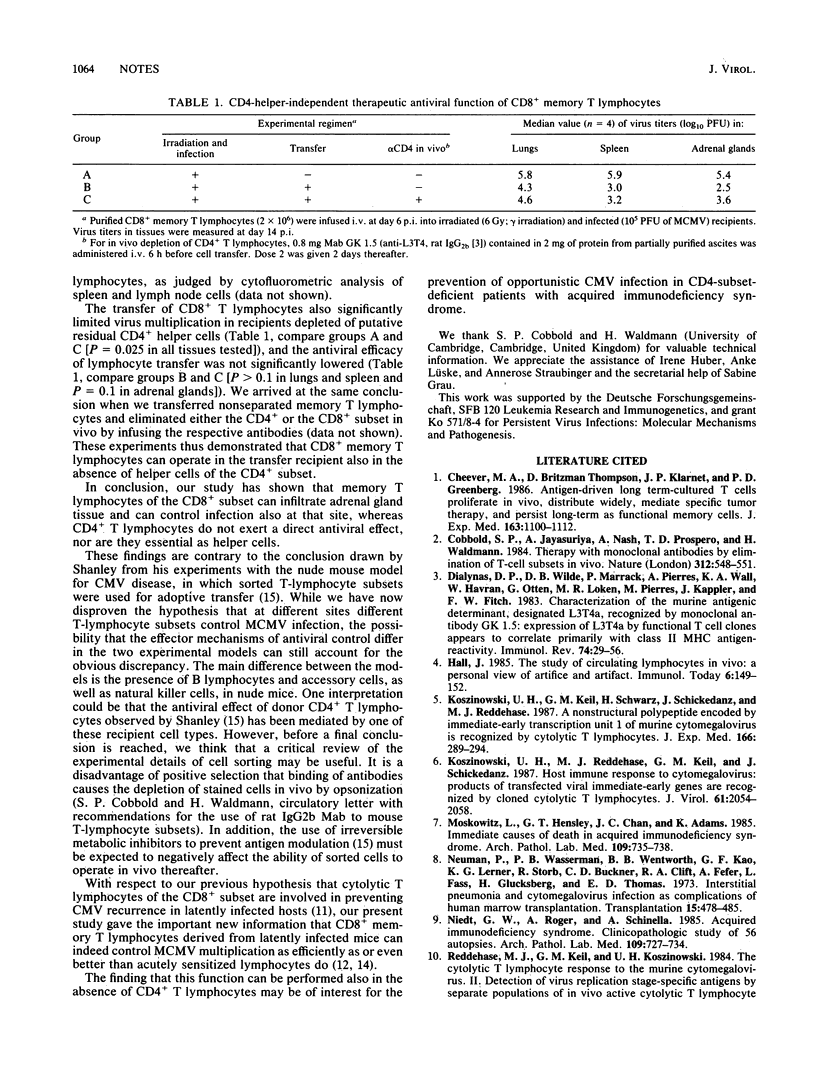
The ability of memory T lymphocytes derived from latently infected mice to control murine cytomegalovirus disease in the immunocompromised host was studied by adoptive transfer experiments. At a stage of pathogenesis when virus had already colonized target tissues, a therapeutic antiviral function could be ascribed to the CD8+ subset. This in vivo function was not restricted to sites in which intravenously infused lymphocytes usually are trapped or home in, such as the lungs or the spleen, respectively, but was also evident in the adrenal glands, a site to which antiviral effector cells have to specifically migrate. Specific infiltration of adrenal gland cortical tissue by donor-derived CD8+ memory T lymphocytes was demonstrated. CD4+ memory T lymphocytes had no antiviral effect by themselves and also were not required for the function of the CD8+ effector cells in this short-term immunotherapy model. These findings should help settle the debate about which subset of T lymphocytes comprises the effector cells that can directly control cytomegalovirus infection in the murine model system.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheever M. A., Thompson D. B., Klarnet J. P., Greenberg P. D. Antigen-driven long term-cultured T cells proliferate in vivo, distribute widely, mediate specific tumor therapy, and persist long-term as functional memory T cells. J Exp Med. 1986 May 1;163(5):1100–1112. doi: 10.1084/jem.163.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Koszinowski U. H., Keil G. M., Schwarz H., Schickedanz J., Reddehase M. J. A nonstructural polypeptide encoded by immediate-early transcription unit 1 of murine cytomegalovirus is recognized by cytolytic T lymphocytes. J Exp Med. 1987 Jul 1;166(1):289–294. doi: 10.1084/jem.166.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U. H., Reddehase M. J., Keil G. M., Schickedanz J. Host immune response to cytomegalovirus: products of transfected viral immediate-early genes are recognized by cloned cytolytic T lymphocytes. J Virol. 1987 Jun;61(6):2054–2058. doi: 10.1128/jvi.61.6.2054-2058.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz L., Hensley G. T., Chan J. C., Adams K. Immediate causes of death in acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1985 Aug;109(8):735–738. [PubMed] [Google Scholar]
- Neiman P., Wasserman P. B., Wentworth B. B., Kao G. F., Lerner K. G., Storb R., Buckner C. D., Clift R. A., Fefer A., Fass L. Interstitial pneumonia and cytomegalovirus infection as complications of human marrow transplantation. Transplantation. 1973 May;15(5):478–485. [PubMed] [Google Scholar]
- Niedt G. W., Schinella R. A. Acquired immunodeficiency syndrome. Clinicopathologic study of 56 autopsies. Arch Pathol Lab Med. 1985 Aug;109(8):727–734. [PubMed] [Google Scholar]
- Reddehase M. J., Keil G. M., Koszinowski U. H. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984 Jan;14(1):56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Koszinowski U. H. In vivo application of recombinant interleukin 2 in the immunotherapy of established cytomegalovirus infection. J Exp Med. 1987 Mar 1;165(3):650–656. doi: 10.1084/jem.165.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Mutter W., Münch K., Bühring H. J., Koszinowski U. H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987 Oct;61(10):3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddehase M. J., Weiland F., Münch K., Jonjic S., Lüske A., Koszinowski U. H. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985 Aug;55(2):264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley J. D. Modification of acute murine cytomegalovirus adrenal gland infection by adoptive spleen cell transfer. J Virol. 1987 Jan;61(1):23–28. doi: 10.1128/jvi.61.1.23-28.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley J. D., Pesanti E. L. Murine cytomegalovirus adrenalitis in athymic nude mice. Arch Virol. 1986;88(1-2):27–35. doi: 10.1007/BF01310887. [DOI] [PubMed] [Google Scholar]
- Tapper M. L., Rotterdam H. Z., Lerner C. W., Al'Khafaji K., Seitzman P. A. Adrenal necrosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Feb;100(2):239–241. doi: 10.7326/0003-4819-100-2-239. [DOI] [PubMed] [Google Scholar]
- Weiland F., Keil G. M., Reddehase M. J., Koszinowski U. H. Studies on the morphogenesis of murine cytomegalovirus. Intervirology. 1986;26(4):192–201. doi: 10.1159/000149701. [DOI] [PubMed] [Google Scholar]



