Abstract
Objectives
For smoking cessation, physical activity (PA) may help manage withdrawal symptoms, mood, stress, and weight; yet studies of PA as an aid for smoking cessation have been mixed. This study examined: (1) the impact of an extended relapse prevention program on increasing moderate to vigorous PA (MVPA) in adults enrolled in a tobacco cessation treatment trial; (2) whether changes in MVPA were associated with sustained abstinence from smoking; and (3) mechanisms by which MVPA may support sustained abstinence from smoking.
Method
In a randomized controlled trial conducted from 2003-2006 in San Francisco, California, 407 adult smokers received a 12 week group-based smoking cessation treatment with bupropion and nicotine patch with the quit date set at week 3. At week 12, participants were randomized to no further treatment or to 40 weeks of bupropion or placebo with or without an 11-session relapse prevention intervention of which 2 sessions (held at weeks 16 and 20) focused on PA. Participants receiving the PA intervention (n=163) received a pedometer, counseling to increase steps 10% biweekly towards a 10,000 steps/day goal, and personalized reports graphing progress with individualized goals. The International Physical Activity Questionnaire assessed weekly minutes of MVPA at baseline and weeks 12 and 24. Sustained abstinence from tobacco at week 24 was validated with expired carbon monoxide.
Results
In a repeated mixed model analysis, intervention participants significantly increased their MVPA relative to control participants, F(1,475)=3.95, p=.047. Pedometer step counts also increased significantly, t(23)=2.36, p=.027, though only 15% of intervention participants provided 6 weeks of pedometer monitoring. Controlling for treatment condition, increased MVPA predicted sustained smoking abstinence at week 24, odds ratio=1.84 (95% CI: 1.07, 3.05). Among participants with sustained abstinence, increased MVPA was associated with increased vigor (r=0.23, p=.025) and decreased perceived difficulty with staying smoke-free (r=-0.21, p=.038).
Conclusion
PA promotion as an adjunct to tobacco treatment increases MVPA levels; changes in MVPA predict sustained abstinence, perhaps by improving mood and self-efficacy.
Précis
In a randomized controlled trial (N=407), physical activity promotion as an adjunct to a smoking cessation intervention was associated with increased physical activity levels and maintenance of nonsmoking.
Keywords: smoking cessation, physical activity, randomized trial, relapse prevention, pedometer
Tobacco use is the leading preventable cause of death in the United States (CDC, 2005). Only 3% to 5% of smokers who try to quit unaided achieve prolonged abstinence at 6 to 12 months (Hughes, et al., 2004). Even with evidence-based smoking cessation treatments, relapse is common (Piasecki, 2006), often in response to mood changes (Shiffman, et al., 2004), withdrawal symptoms (Piasecki, et al., 2000), weight gain (Borrelli, et al., 2001), and cravings (Killen, et al., 1997).
Physical activity (PA) may be useful in addressing the physiological and psychological causes of relapse to smoking (Bock, et al., 1999, Ussher, et al., 2001). A systematic review of 12 studies comparing a bout of exercise with a passive condition reported a positive effect for reducing cigarette cravings, negative affect, withdrawal symptoms, and smoking behavior (Taylor, et al., 2007). Two additional studies concluded the effect of PA was irrespective of the intensity level (moderate vs. vigorous). Further, the mechanism by which PA reduces desire to smoke and nicotine withdrawal symptoms is distinct from simple cognitive distraction (Daniel, et al., 2006).
Though most people who quit smoking will gain less than 10 pounds (Williamson, et al., 1991), weight gain concerns are predictive of smoking initiation, reluctance to quit smoking, and smoking relapse (Gritz, et al., 1991; Klesges, et al., 1989; Perkins, et al., 1995). For managing weight gain associated with quitting smoking, clinical practice guidelines recommend PA, rather than dieting (Fiore, et al., 2000). In general practice, PA is an effective strategy for weight gain prevention (USDHHS, 1996).
Adults who smoke tend to be less physically active than nonsmokers (Kaczynski, et al., 2008). Unger (1996) observed that adults preparing to quit smoking exercised more than smokers in the earlier stages of change. Further, a significant positive association has been found between one’s self-efficacy for quitting smoking and self-efficacy for maintaining PA (Boudreaux, et al., 2003).
The evidence for PA as a strategy for supporting smoking cessation, however, is not strong. A Cochrane review of 11 randomized controlled trials examining PA as a smoking cessation strategy concluded that while exercise promotion did not appear to harm smoking cessation efforts, there was limited evidence that it helped (Ussher, 2005). Only one of the 11 trials found evidence for PA aiding smoking cessation at long term follow-up (Marcus, et al., 1999). The program was highly structured, supervised, and promoted PA of vigorous intensity. A follow up study that promoted engagement in moderate PA and used more of a home-based approach failed to find an effect for long-term cessation relative to a standard cognitive behavioral cessation program (Marcus, et al., 2005). In the Cochrane review, only two studies reported changes in PA, limiting our understanding of the feasibility of smokers making changes in their tobacco use and PA patterns concurrently (Ussher, 2005). A follow up study reported no long-term effect of a PA intervention on tobacco abstinence, but also reported no difference in PA levels between the intervention and control conditions (Ussher, et al., 2007).
When quitting smoking, the timing of the PA program and the intensity of PA promoted (vigorous vs. moderate) may be important. Moderate or “lifestyle” exercise programs may offer greater efficacy and practicality for dissemination (Dunn, et al., 1998, King, 1998), greater participant adherence (King, et al., 1995), and greater appeal to sedentary smokers (Ussher, et al., 2001). The current study examined: (1) the impact of an extended relapse prevention program on increasing moderate to vigorous PA (MVPA) in adults enrolled in a tobacco cessation treatment trial; (2) whether changes in MVPA were associated with sustained abstinence from smoking; and (3) mechanisms by which MVPA may support sustained abstinence from smoking. Hypothesized mechanisms included enhanced psychological states, reduced withdrawal symptoms, improved health functioning, reduced weight gain, and greater motivation and self-efficacy to stay tobacco-free. Baseline correlates of PA also were examined with sample demographic and tobacco use characteristics.
Methods
Participants
Participants were 407 adults who smoked at least 10 cigarettes daily for 5 or more years and smoked within 30 minutes of awakening. The study, conducted in the San Francisco Bay Area, recruited smokers interested in quitting via direct mail and media advertisements. Recruitment spanned February 2003 - December 2005 with the 24 week assessments completed in June 2006. The study was limited to English speakers. Exclusion criteria included contraindications to bupropion use (e.g., elevated seizure risk), nicotine patch (e.g., recent myocardial infarction, dermatitis), current pregnancy or lactation, and physical limitations to participation in moderate PA as assessed with the Physical Activity Readiness-Questionnaire (PAR-Q) (Thomas, et al., 1992). Additionally, smokers with current substance use problems, active psychotropic use, or current depression were excluded. The University of California, San Francisco Institutional Review Board approved the study, and participants provided informed consent.
Study Design
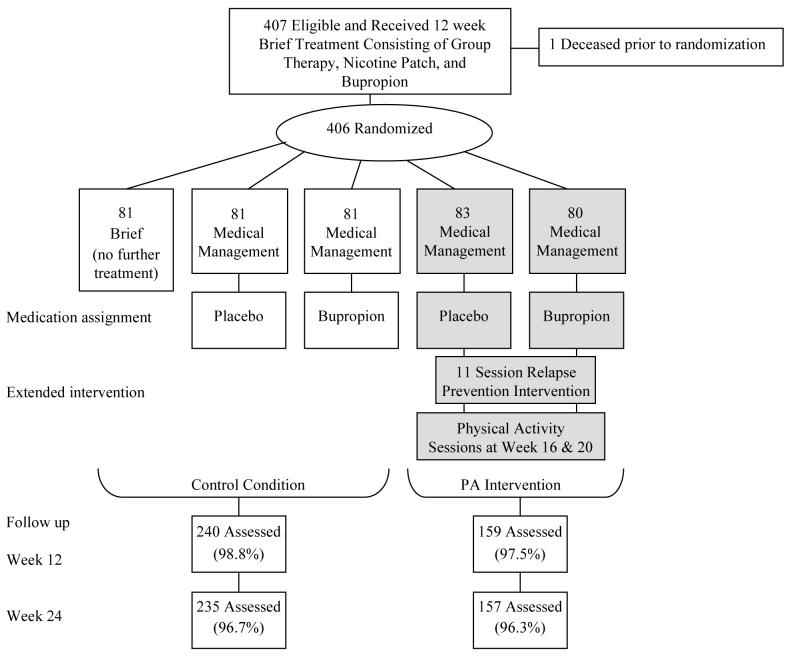
All participants received a 12 week smoking cessation treatment consisting of nicotine patch therapy, sustained-release bupropion (150 mg twice a day), and five group sessions with the quit date set at week 3. Following the 12 week treatment, participants were randomized to no further treatment or one of four extended 40-week intervention conditions (see Figure 1). Randomization was stratified by gender and smoking status at week 11. The extended conditions provided 40 more weeks of bupropion (52 weeks in total), or placebo, crossed with medication management, alone or with an extended relapse prevention program.
Figure 1.
Study Randomization and Follow-Up through Week 24. Medication management consisted of brief (10-15 minute) sessions with a nurse practitioner to review medication use and any side effects. The extended 11-session relapse prevention intervention included 2 sessions, at weeks 16 and 20, dedicated to physical activity promotion. Shaded boxes in the figure indicate conditions receiving the physical activity intervention. The study was conducted in San Francisco, CA with recruitment started in February 2003 and the week 24 assessments completed in June 2006.
The two extended relapse prevention conditions, with placebo or active bupropion, were combined for the current analyses to examine the impact of the PA intervention (shaded boxes in Figure 1). The relapse prevention program focused on optimizing health while living smoke-free; consisted of 11 individual sessions staggered over 40 weeks time; and covered ongoing motivation, social support, mood management, nicotine dependence and withdrawal, and weight gain prevention through promotion of moderate-to-vigorous PA (Fiore, et al., 2000). The current paper focuses on the impact of the two dedicated PA sessions of the relapse prevention intervention, delivered at weeks 16 and 20, and described below.
PA Intervention
At week 14, participants receiving the relapse prevention program (n=163) were provided a Yamax pedometer and instructed to track their steps for 2 weeks to provide a baseline indication of their PA level. Participants were to wear the pedometer every day, all day, except when sleeping, swimming, or showering; record their steps at the end of each day; and reset the pedometer each morning. A form was provided to record time spent in activity not assessed by the pedometer (e.g., swimming, biking) for study counselors to convert into step estimates. An inexpensive and unobtrusive tool that captures varied levels and kinds of PA, pedometers have established validity and reliability (Felton, et al., 2006, Tudor-Locke, et al., 2001, Tudor-Locke, et al., 2002) and are increasingly being used to promote PA (Merom, et al., 2007, Rooney, et al., 2003, Stovitz, et al., 2005).
At week 16, counselors entered participants’ recorded steps into an Excel-based program graphing the counts relative to the 10,000 steps/day goal (Tudor-Locke, et al., 2004). The program calculated participants’ mean steps/day and recommended a 10% increase in daily steps for the next 2 weeks. Counselors reviewed the printed report with participants and provided encouragement and support with increasing PA to meet their individual goals. At week 20, counselors entered participants’ step recording for the previous 4 weeks, reviewed progress in meeting the step goals, and printed a second report with updated goals reflecting a further 10% increase. Over the remaining 20 weeks of the extended relapse prevention program, ongoing step monitoring was encouraged with bi-weekly 10% increases in steps towards meeting the 10,000 steps/day goal. The PA program was designed to be individually tailored, encourage reasonable increases in PA over time, and brief and easy for master’s level counselors to implement.
Measures
The following measures were administered to all participants at baseline and weeks 12 and 24, unless otherwise indicated.
Tobacco Measures
Tobacco Use was assessed as the number of cigarettes smoked in the past 7 days verified with an expired air carbon monoxide (CO) level using a Bedfont Smokerlyzer. At follow up assessments, nonsmoking status was defined as no use of tobacco in the past 7 days and a CO<11 (SRNT Subcommittee on Biochemical Verification, 2002). Sustained abstinence was defined as being smoke-free at both weeks 12 and 24. Fagerström Test of Nicotine Dependence assessed level of dependence to nicotine at baseline (Heatherton, et al., 1991). Thoughts about Abstinence scale assessed desire, expectancy of success, and anticipated difficulty with quitting smoking rated on 10-point scales and abstinence goal (Hall, et al., 1990). Minnesota Nicotine Withdrawal Scale (MNWS) assessed withdrawal symptoms over the past 24 hours (Hughes, et al., 1986).
PA Measure
International Physical Activity Questionnaire (IPAQ), short form (7-items) assessed time spent sitting, walking, and engagement in other moderate and vigorous PA over the past 7-days. The measure specifies broad PA domains including transportation and household chores. The outcome variable for our analyses was total minutes of MVPA. The IPAQ, used widely in the literature, has demonstrated adequate reliability and criterion validity against accelerometers (Craig, et al., 2003, Fogelholm, et al., 2006, Hagstromer, et al., 2006), yet has shown problems with skew and is often analyzed using a log transformation (De Bourdeaudhuij, et al., 2003, Rzewnicki, et al., 2003). In the current sample, a log10 transformation of participants’ MVPA minutes at week 12 approximated a normal distribution and correlated significantly (r=.33, p=.048) with intervention participants’ objective pedometer counts during the initial 2-week baseline period (weeks 14 to 16).
Mood and Health Measures
Profile of Mood States (POMS) provides six subscales (Depression, Tension, Anger, Confusion, Fatigue, and Vigor) and a Total Mood Disturbance score. POMS scores are predictive of smoking relapse (McNair, et al., 1971) and used widely in the sport and exercise psychology field (LeUnes, 2000). Perceived Stress Scale, 10-items, assessed frequency of experienced stressors in the past month (Cohen, et al., 1983). Medical Outcome Studies 36-item Short-Form Health Survey (SF-36) provides a global self-rating of health and composite scales of general physical and mental health functioning (Ware, et al., 1997). Body Mass Index (BMI) was assessed in stocking feet with a balance beam scale and calculated as height in inches divided by weight in kilograms squared.
Demographic Measure
Participants reported their gender, age, ethnicity or race, education, employment, marital status, and income.
Analyses
Analyses were performed using SAS 9.1.3. Pearson correlations examined associations among baseline PA levels and participant demographic and smoking characteristics. Analyses compared outcomes for participants in the two extended relapse prevention conditions (referred to as the PA intervention) to the three other conditions (referred to as the control condition). For aim 1, we ran a repeated mixed model analysis to compare changes in MVPA from baseline to weeks 12 and 24 based on randomization to the PA intervention. The comparison groups’ sample sizes provided 80% power to detect a difference in MVPA levels of moderate magnitude (Cohen’s d=.28) at an alpha level of .05. For aim 2, we ran a logistic regression to examine changes in MVPA as a function of continued abstinence, controlling for the 5-group treatment condition assignment, thereby controlling for effects of the medication assignment (bupropion or placebo). Lastly, for aim 3, we ran Pearson correlations to test associations between increased MVPA and hypothesized constructs, among participants with sustained abstinence at week 24. In all analyses, IPAQ data were analyzed as log10 MVPA minutes.
Results
Participant Baseline Characteristics
The sample was 61% male with a mean age of 40.7 years (SD=9.8). Race or ethnic identification was Caucasian (71%), African American (8%), Asian/Pacific Islander (6%), Hispanic (3%), multiracial (10%), and other (2%). Most participants (74%) had attended some college; 45% held at least a bachelor’s degree; 72% were employed. The measured BMI of the sample averaged 26 (SD=5) for men and 26 (SD=6) for women at baseline; 54% of the men and 47% of the women were classified as overweight or obese. Participants smoked M=18.6 cigarettes per day (SD=7.9), had an FTND score of M=4.9 (SD=2.1), and a CO reading of M=21.6 ppm (SD=11.3) at baseline.
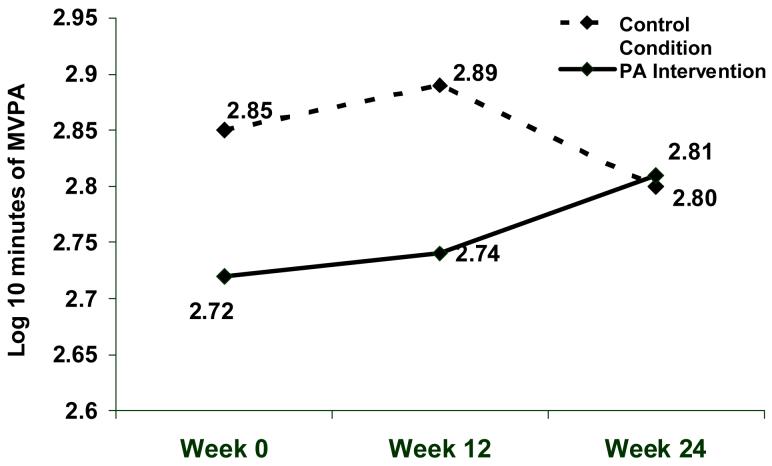
The two groups did not differ on any demographic or tobacco-use characteristic; however, log10 MVPA minutes were significantly lower at both baseline, F(1,354)=4.98, p=0.026, and week 12, F(1,266)=5.61, p=0.019, for participants in the PA intervention relative to the control condition (see Figure 2). Based on pedometer step recording during weeks 14 to 16, 76% of participants in the PA intervention averaged less than the recommended 10,000 steps/ day goal.
Figure 2.
Change in log10 minutes of moderate-to-vigorous physical activity (MVPA) on the IPAQ overtime by treatment condition. The study was conducted in San Francisco, CA with recruitment started in February 2003 and the week 24 assessments completed in June 2006.
Baseline Correlates of PA
Participants’ MVPA at baseline did not differ significantly by gender, age, education, or employment status or for any of the tobacco use variables (cigarettes per day, FTND, Thoughts about Abstinence, or CO level). Baseline MVPA correlated significantly with the POMS subscales of Tension (r=-0.12), Confusion (r=-0.13), Depression (r=-0.16), Fatigue (r=-0.16), and Vigor (r=0.23), and the Total Mood Disturbance score (r= -0.17), all p-values<.05; the Perceived Stress Scale (r= -0.12, p=.03); and the SF-36 global rating of health (r=-0.20, p=.001) and the general physical functioning composite score (r=0.11, p=.04). MVPA correlated significantly with BMI for women (r=-0.22, p<.01), but not men, r=0.03.
Change in PA overtime by Condition
In a repeated mixed model analysis, participants randomized to the PA intervention significantly increased their MVPA over time relative to control participants, F(1,475)=3.95, p=.047. Figure 2 shows the change in log10 minutes of MVPA over time by condition. Assessments at baseline and week 12 allowed for an extended baseline assessment of PA since the PA sessions occurred at weeks 16 and 20. The significant change in MVPA over time by condition reflected both an increase in MVPA among participants in the PA intervention group coupled with a decline in MVPA among control participants.
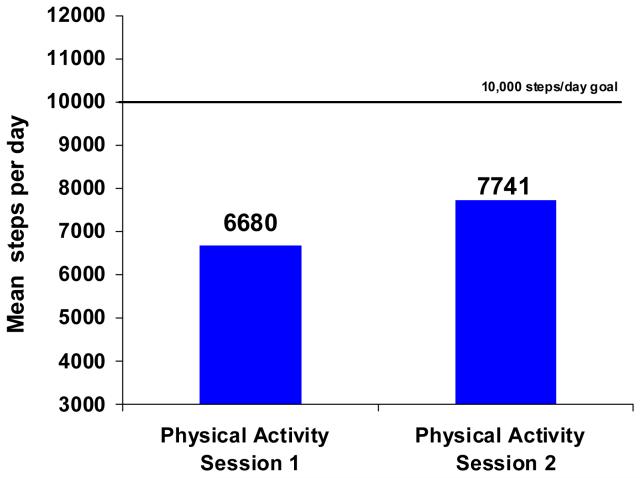
Pedometer data were available at weeks 16 and 20 for 24 of the 163 participants in the relapse prevention condition. Pedometer data for these participants indicated a significant increase in average daily steps from the first to the second PA session, paired samples t(23)=2.36, p=.027. The change in steps averaged an increase of 16% (see Figure 3). Comparison of the 24 PA intervention participants with consistent step recording to the other PA intervention participants indicated no significant difference on any of the measured demographic, tobacco use, health, or PA measures.
Figure 3.
Average daily step counts among PA intervention participants with pedometer recording at both weeks 12 to 14 and weeks 16 to 20 (n=24). The study was conducted in San Francisco, CA with recruitment started in February 2003 and the week 24 assessments completed in June 2006.
Change in PA & Sustained Abstinence
Abstinence rates by condition will be reported in the main outcomes paper for the study. For the current analyses, we examined whether changes in log10 minutes MVPA were associated with sustained smoking abstinence at week 24. Controlling for treatment condition, increased MVPA predicted sustained smoking abstinence at week 24, odds ratio=1.84, p=.028 (95% CI: 1.07, 3.05).
Changes in log10 minutes MVPA were examined for intervention and control participants by sustained abstinence status at week 24. Intervention participants who sustained abstinence at week 24 increased their MVPA (M=.17, SD=.48), control participants who were abstinent (M=.01, SD=.50) and intervention participants who relapsed (M=.00, SD=.52) had no change, and the MVPA of control participants who returned to smoking at week 24 declined (M=-0.15, SD=.55).
Correlates of Change in MVPA among Participants with Sustained Abstinence
Among subjects with sustained abstinence (n=153), increased MVPA was associated with increased vigor (r=0.23, p=.025) and decreased perceived difficulty with staying smoke-free (r=-0.21, p=.038) from baseline to week 24. Change in MVPA was not significantly correlated with change in other psychological states as measured by the POMS nor change in withdrawal symptoms, perceived stress, weight gain, or health functioning.
Discussion
The current study examined promotion of PA as part of an extended relapse prevention program for smoking cessation. The PA component was brief (2 sessions), individualized, encouraged self-monitoring with a pedometer, and promoted gradual increases in lifestyle MVPA. As hypothesized, we found significant increases in MVPA from baseline to week 24 among participants receiving the PA intervention, whereas MVPA among control participants declined. Among intervention participants who did their pedometer recording, daily steps increased an average of 16% or 1061 steps between the two PA sessions. In a recent review of pedometer use, increases averaged 2491 steps/day in randomized controlled intervention trials and 2183 in observational studies, reflecting a 27% increase over baseline levels (Bravata, et al., 2007). The pedometer interventions reviewed tended to be of longer duration than that employed in the current study, M=18 weeks (range 3 to 104 weeks).
Change in MVPA significantly predicted sustained abstinence from smoking at week 24. Increases in PA were associated with a greater likelihood of sustained abstinence from smoking, while relapse to smoking was associated with decline in activity. The findings are consistent with a recent prospective 7-year observational study with 750 Japanese men in which increased habitual exercise was associated with smoking cessation, while smoking relapse was associated with reduced habitual exercise (Nagaya, et al., 2007).
Examining MVPA changes by abstinence status and condition, MVPA increased among PA intervention participants who sustained abstinence, remained unchanged among intervention participants who relapsed or control participants with sustained abstinence, and declined among control participants who relapsed. The PA intervention appeared to increase PA among participants who quit smoking and mitigate declines in PA among those who relapsed.
Among participants with sustained abstinence at week 24, increased MVPA was associated with decreased perceived difficulty with remaining smoke-free and an increased state of vigor. Engagement in PA may have reinforced participants’ commitment to a healthy lifestyle, which did not include smoking. In the literature, a significant cross-sectional association has been found between self-efficacy for smoking cessation and exercise adoption (King, et al., 1996). Individuals working on increasing their PA seem confident about decreasing their smoking and vice versa. Previous studies also have reported higher ratings of vigor among adults who are more physically active (LeUnes, 2000). Engagement in PA may help to offset the fatigue and sleep problems characteristic of nicotine withdrawal (American Psychiatric Association [APA], 1994).
The lack of significant correlations between changes in MVPA and other nicotine withdrawal symptoms and BMI may reflect the timing of the intervention and assessment schedule. The PA sessions came 13 weeks after the scheduled quit date, likely too late to impact most nicotine withdrawal symptoms, which typically last only 2 to 4 weeks (APA, 1994). For weight gain prevention, the week 24 assessment (only 4 weeks after the second PA session) may have been too soon to detect an effect.
Strengths of the current study include a large sample size and use of a validated PA self-report measure. Though used largely as a surveillance tool in the literature, the IPAQ demonstrated sensitivity to detecting changes overtime associated with an intervention. A limitation of the IPAQ, however, was the high degree of skew making use of the raw data for examining baseline PA levels misleading. Despite this limitation, brief measures, such as the IPAQ, are particularly useful in studies targeting and assessing multiple health behaviors given concerns with respondent burden. In the current sample, self-reported MVPA correlated significantly with objective pedometer counts.
The study is limited in that it focused on short-term effects of a PA intervention on smoking abstinence. Despite a randomized design, participants in the relapse prevention group reported lower levels of MVPA at baseline relative to control participants. The difference was stable with the repeated baseline assessment design, apparent at both the baseline and week 12 assessments. The low compliance with pedometer monitoring also limits the study findings. Only 15% of participants in the PA intervention provided 6 weeks of pedometer monitoring. The PA intervention was part of an extended relapse prevention intervention with multiple objectives: nicotine withdrawal, mood management, social support, ongoing motivation, and weight gain prevention through PA promotion. Given the multiple foci, participants may have opted to focus on the issues of greatest concern or interest to them. A study of smokers with severe mental illness reported that 63% were interested in assistance to increase their PA levels while quitting smoking (Faulkner, et al., 2007). Given the high rates of tobacco use and inactivity among persons with mental illness, this group presents a unique population worthy of study with perhaps even greater potential synergism for PA’s effects on enhancing mood and reducing nicotine withdrawal symptoms.
The leading causes of morbidity and mortality in the US -- heart disease, stroke, diabetes, and cancer -- are influenced by tobacco use and sedentary lifestyles. Risk behaviors have been shown to cluster and tobacco users, in particular, tend to have poor behavioral profiles, with about 92% of smokers having at least one additional risk behavior (Fine, et al., 2004, Klesges, et al., 1990, Pronk, et al., 2004). In the 2001 National Health Interview Study, 70% of current smokers were classified as physically inactive (Fine, et al., 2004). Interventions that address multiple behaviors, such as tobacco use and PA, have the potential to offer greater health benefits, more adequately address participants’ behavioral profiles, maximize health promotion opportunities, and reduce health care costs.
The current study demonstrated that the addition of a low cost, two session PA program to a smoking cessation intervention served to increase participants’ MVPA with changes predictive of sustained abstinence at 24 weeks. The timing of the PA sessions (13 weeks post quit date), promotion of lifestyle activity of moderate intensity, and the tailoring of step goals to participants’ baseline activity levels are factors that likely contributed to the significant changes observed.
Acknowledgements
This work was supported by the National Institute on Drug Abuse (#R01 DA015732, #K05 DA016752, #K23 DA018691 and #P50 DA09253) and the State of California Tobacco-Related Disease Research Program (#13KT-0152). The authors have no financial interests related to the material in the manuscript. The study was presented orally at the Annual Meeting of the Society of Behavioral Medicine in San Diego, CA on March 28, 2008. We thank Kevin Ahern for his assistance with data management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict Behav. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. J Consult Clin Psychol. 2001;69:511–515. doi: 10.1037//0022-006x.69.3.511. [DOI] [PubMed] [Google Scholar]
- Boudreaux ED, Francis JL, Carmack Taylor CL, Scarinci IC, Brantley PJ. Changing multiple health behaviors: Smoking and exercise. Prev Med. 2003;36:471–478. doi: 10.1016/s0091-7435(02)00048-8. [DOI] [PubMed] [Google Scholar]
- Bravata DM, Smith-Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. Jama. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Annual smoking-attributable mortality, years of potential life lost, and productivity losses --- United States, 1997--2001. MMWR. 2005;54:625–628. [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Daniel JZ, Cropley M, Fife-Schaw C. The effect of exercise in reducing desire to smoke and cigarette withdrawal symptoms is not caused by distraction. Addiction. 2006;101:1187–1192. doi: 10.1111/j.1360-0443.2006.01457.x. [DOI] [PubMed] [Google Scholar]
- De Bourdeaudhuij I, Sallis JF, Saelens BE. Environmental correlates of physical activity in a sample of Belgian adults. Am J Health Promot. 2003;18:83–92. doi: 10.4278/0890-1171-18.1.83. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Andersen RE, Jakicic JM. Lifestyle physical activity interventions. History, short-and long-term effects, and recommendations. Am J Prev Med. 1998;15:398–412. doi: 10.1016/s0749-3797(98)00084-1. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Taylor A, Munro S, Selby P, Gee C. The acceptability of physical activity programming within a smoking cessation service for individuals with severe mental illness. Patient Educ Couns. 2007;66:123–126. doi: 10.1016/j.pec.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Felton GM, Tudor-Locke C, Burkett L. Reliability of pedometer-determined free-living physical activity data in college women. Res Q Exerc Sport. 2006;77:304–308. doi: 10.1080/02701367.2006.10599364. [DOI] [PubMed] [Google Scholar]
- Fine LJ, Philogene GS, Gramling R, Coups EJ, Sinha S. Prevalence of multiple chronic disease risk factors; 2001 National Health Interview Survey. American Journal of Preventive Medicine. 2004;27:18–24. doi: 10.1016/j.amepre.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Rockville MD. Clinical Practice Guideline. U.S. Department of Health and Human Services, Public Health Service; 2000. Treating Tobacco Use and Dependence. al. e. [Google Scholar]
- Fogelholm M, Malmberg J, Suni J, Santtila M, Kyrolainen H, Mantysaari M, Oja P. International Physical Activity Questionnaire: Validity against fitness. Med Sci Sports Exerc. 2006;38:753–760. doi: 10.1249/01.mss.0000194075.16960.20. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Crane LA. Use of diet pills and amphetamines to lose weight among smoking and nonsmoking high school seniors. Health Psychol. 1991;10:330–335. doi: 10.1037//0278-6133.10.5.330. [DOI] [PubMed] [Google Scholar]
- Hagstromer M, Oja P, Sjostrom M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Commitment to abstinence and acute stress in relapse to alcohol, opiates, and nicotine. J Consult Clin Psychol. 1990;58:175–181. doi: 10.1037//0022-006x.58.2.175. [DOI] [PubMed] [Google Scholar]
- Hall SM, Havassy BE, Wasserman DA. Effects of commitment to abstinence, positive moods, stress, and coping on relapse to cocaine use. J Consult Clin Psychol. 1991;59:526–532. doi: 10.1037//0022-006x.59.4.526. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Kaczynski AT, Manske SR, Mannell RC, Grewal K. Smoking and physical activity: a systematic review. Am J Health Behav. 2008;32:93–110. doi: 10.5555/ajhb.2008.32.1.93. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- King AC, Haskell WL, Young DR, Oka RK, Stefanick ML. Long-term effects of varying intensities and formats of physical activity on participation rates, fitness, and lipoproteins in men and women aged 50 to 65 years. Circulation. 1995;91:2596–2604. doi: 10.1161/01.cir.91.10.2596. [DOI] [PubMed] [Google Scholar]
- King AC. How to promote physical activity in a community: research experiences from the US highlighting different community approaches. Patient Educ Couns. 1998;33:S3–12. doi: 10.1016/s0738-3991(98)00004-4. [DOI] [PubMed] [Google Scholar]
- King TK, Marcus BH, Pinto BM, Emmons KM, Abrams DB. Cognitive-behavioral mediators of changing multiple behaviors: smoking and a sedentary lifestyle. Preventive Medicine. 1996;25:684–691. doi: 10.1006/pmed.1996.0107. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Eck LH, Isbell TR, Fulliton W, Hanson CL. Smoking status: effects on the dietary intake, physical activity, and body fat of adult men. American Journal of Clinical Nutrition. 1990;51:784–789. doi: 10.1093/ajcn/51.5.784. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- LeUnes A. Updated bibliography on the Profile of Mood States in sport and exercise psychology research. Journal of Applied Sport Psychology. 2000;12:110–113. [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, Niaura RS, Abrams DB. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med. 1999;159:1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, Parisi AF, Niaura R, Abrams DB. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine Tob Res. 2005;7:871–880. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Manual for the Profile of Mood States. San Diego, CA: 1971. [Google Scholar]
- Merom D, Rissel C, Phongsavan P, Smith BJ, Van Kemenade C, Brown WJ, Bauman AE. Promoting walking with pedometers in the community: the step-by-step trial. Am J Prev Med. 2007;32:290–297. doi: 10.1016/j.amepre.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Yoshida H, Takahashi H, Kawai M. Cigarette smoking weakens exercise habits in healthy men. Nicotine Tob Res. 2007;9:1027–1032. doi: 10.1080/14622200701591575. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Fonte C, Mitchell SL, Grobe JE. Gender, dietary restraint, and smoking’s influence on hunger and the reinforcing value of food. Physiol Behav. 1995;57:675–680. doi: 10.1016/0031-9384(94)00320-3. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Niaura R, Shadel WG, Abrams D, Goldstein M, Fiore MC, Baker TB. Smoking withdrawal dynamics in unaided quitters. J Abnorm Psychol. 2000;109:74–86. doi: 10.1037//0021-843x.109.1.74. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Pronk NP, Anderson LH, Crain AL, Martinson BC, O’Connor PJ, Sherwood NE, Whitebird RR. Meeting recommendations for multiple healthy lifestyle factors; Prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. American Journal of Preventive Medicine. 2004;27:25–33. doi: 10.1016/j.amepre.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Rooney B, Smalley K, Larson J, Havens S. Is knowing enough? Increasing physical activity by wearing a pedometer. Wmj. 2003;102:31–36. [PubMed] [Google Scholar]
- Rzewnicki R, Vanden Auweele Y, De Bourdeaudhuij I. Addressing overreporting on the International Physical Activity Questionnaire (IPAQ) telephone survey with a population sample. Public Health Nutr. 2003;6:299–305. doi: 10.1079/PHN2002427. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Stovitz SD, VanWormer JJ, Center BA, Bremer KL. Pedometers as a means to increase ambulatory activity for patients seen at a family medicine clinic. J Am Board Fam Pract. 2005;18:335–343. doi: 10.3122/jabfm.18.5.335. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q) Can J Sport Sci. 1992;17:338–345. [PubMed] [Google Scholar]
- Tudor-Locke C, Ainsworth BE, Whitt MC, Thompson RW, Addy CL, Jones DA. The relationship between pedometer-determined ambulatory activity and body composition variables. Int J Obes Relat Metab Disord. 2001;25:1571–1578. doi: 10.1038/sj.ijo.0801783. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Williams JE, Reis JP, Pluto D. Utility of pedometers for assessing physical activity: convergent validity. Sports Med. 2002;32:795–808. doi: 10.2165/00007256-200232120-00004. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Bassett DR., Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- Unger JB. Stages of change of smoking cessation: relationships with other health behaviors. Am J Prev Med. 1996;12:134–138. [PubMed] [Google Scholar]
- US Department of Health and Human Services . Physical activity and health: a report of the Surgeon General. US Department of Health and Human Services, Public Health Service, CDC, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, Georgia: 1996. [Google Scholar]
- Ussher M, Nunziata P, Cropley M, West R. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology (Berl) 2001;158:66–72. doi: 10.1007/s002130100846. [DOI] [PubMed] [Google Scholar]
- Ussher M. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2005:CD002295. doi: 10.1002/14651858.CD002295.pub2. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, McEwen A, Taylor A, Steptoe A. Randomized controlled trial of physical activity counseling as an aid to smoking cessation: 12 month follow-up. Addict Behav. 2007;32:3060–3064. doi: 10.1016/j.addbeh.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. The Health Institute, New England Medical Center; Boston, MA: 1997. [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. New England J Med. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]