Abstract
Oscillatory activity in human electro- or magnetoencephalogram has been related to cortical stimulus representations and their modulation by cognitive processes. Whereas previous work has focused on gamma-band activity (GBA) during attention or maintenance of representations, there is little evidence for GBA reflecting individual stimulus representations. The present study aimed at identifying stimulus-specific GBA components during auditory spatial short-term memory. A total of 28 adults were assigned to 1 of 2 groups who were presented with only right- or left-lateralized sounds, respectively. In each group, 2 sample stimuli were used which differed in their lateralization angles (15° or 45°) with respect to the midsagittal plane. Statistical probability mapping served to identify spectral amplitude differences between 15° versus 45° stimuli. Distinct GBA components were found for each sample stimulus in different sensors over parieto-occipital cortex contralateral to the side of stimulation peaking during the middle 200–300 ms of the delay phase. The differentiation between “preferred” and “nonpreferred” stimuli during the final 100 ms of the delay phase correlated with task performance. These findings suggest that the observed GBA components reflect the activity of distinct networks tuned to spatial sound features which contribute to the maintenance of task-relevant information in short-term memory.
Keywords: auditory spatial processing, gamma-band activity, magnetoencephalography, short-term memory
Introduction
Cortical oscillatory synchronization in the gamma frequency range (∼30–100 Hz) has received increasing interest because of its putative relevance for a variety of cognitive processes (Herrmann et al. 2004; Kaiser and Lutzenberger 2005b; Jensen et al. 2007) and its potential role for brain disorders (Herrmann and Demiralp 2005; Uhlhaas and Singer 2006). Although animal electrophysiology studies have suggested a role of gamma-band activity (GBA) mainly for visual feature binding (Gray et al. 1989; Singer et al. 1997), recordings of fast oscillatory activity with electro- or magnetoencephalography (EEG or MEG) or with intracranial recordings in humans have demonstrated task-dependent GBA modulations during high-level cognitive processes including attention and memory (Jensen et al. 2007). The attentional enhancement of a cortical stimulus representation is thought to involve gamma synchronization of neurons representing an attended stimulus that would increase their impact on downstream target areas. In line with this notion, selective attention has been found to elicit GBA increases to visual (Gruber et al. 1999; Müller and Keil 2004; Vidal et al. 2006), auditory (Tiitinen et al. 1993), audiovisual (Sokolov et al. 2004), and somatosensory stimuli (Brovelli et al. 2005; Bauer et al. 2006).
Similarly, maintenance of an object representation in short-term memory and encoding into long-term memory may rely on the persistent synchronized firing of recurrently connected neurons (Jensen et al. 2007). This hypothesis has been supported by studies showing that gamma power during stimulus encoding predicts recall from long-term memory (Sederberg et al. 2003; Gruber et al. 2004; Osipova et al. 2006). Concerning short-term memory, GBA increases have been found during the maintenance of visual stimuli with EEG (Tallon-Baudry et al. 1998, 1999) and intracranial recordings (Howard et al. 2003; Mainy et al. 2007) as well as for auditory stimuli with MEG (Lutzenberger et al. 2002; Kaiser et al. 2003). GBA in these tasks was found both in sensory and higher nonsensory areas, for example, in MEG sensors over anterior temporal/inferior frontal cortex for sound pattern short-term memory and over posterior temporoparietal cortex during the maintenance of spatial sound features (Kaiser and Lutzenberger 2003).
A common feature of the EEG and MEG studies mentioned above was that they compared oscillatory signals between experimental conditions within which a variety of stimuli were presented. However, as yet there is little evidence for GBA reflecting the cortical representations of individual stimuli. Gamma components with distinct spectral and, possibly, topographical characteristics should characterize the activation of individual networks representing a particular task-relevant stimulus or even a specific attribute of a stimulus only. With MEG we have identified highly local task-specific GBA increases both during auditory (Kaiser and Lutzenberger 2005a; Kaiser et al. 2005; Leiberg et al. 2006) and visual processing (Kaiser et al. 2004). Therefore, MEG may be suitable for the detection of local synchronized networks representing specific stimuli. We have found first evidence for such stimulus-specific GBA components in a recent study that compared oscillatory responses with short sounds of 2 different durations (Kaiser, Leiberg, et al. 2007). During the delay phase of a short-term memory task, maintenance of each sound duration was accompanied by a distinct GBA increase over prefrontal cortex. The aim of the present study was to extend these findings to auditory spatial processing. More precisely, we assessed whether the maintenance of different sound lateralization angles in short-term memory would be characterized by spectrally and/or topographically distinct GBA components.
Networks representing spatial sound features were expected to be localized in areas of the putative auditory dorsal stream including posterior temporal and posterior parietal cortex (Rauschecker 1998). Originally formulated on the basis of single-cell recordings in monkeys (Tian et al. 2001) and anatomical tract tracing (Romanski et al. 1999), evidence from human brain imaging studies has accumulated in support of separate processing streams for spatial versus nonspatial auditory information (Arnott et al. 2004). Areas responsive to auditory spatial processing are posterior parietal and superior frontal cortex (Griffiths et al. 1998; Alain et al. 2001; Maeder et al. 2001; Pavani et al. 2002; Warren et al. 2002; Hart et al. 2004) and posterior temporal cortex (Baumgart et al. 1999; Warren and Griffiths 2003; Krumbholz et al. 2005; Altmann et al. 2007). With MEG, we have found increased GBA over areas of the putative auditory dorsal and ventral streams during auditory spatial versus pattern processing, respectively. These patterns of activations were found both for passive change detection paradigms (Kaiser et al. 2000, 2002), short-term memory tasks (Lutzenberger et al. 2002; Kaiser et al. 2003), and during auditory decision making (Kaiser, Lennert, Lutzenberger 2007).
In the present study, we used an auditory delayed matching-to-sample paradigm akin to previous studies (Lutzenberger et al. 2002; Leiberg et al. 2006). To be able to compute contrasts between presentations of different to-be-memorized sample sounds with sufficient numbers of trials, subjects were subdivided into 2 groups that performed tasks with sample stimuli of only 2 lateralization angles, which were presented either in the right or left hemifield. We hypothesized that stimulus-specific GBA components would be localized in MEG sensors over areas of the putative auditory dorsal stream. In addition, we explored correlations between these GBA components and task performance.
Materials and Methods
Subjects
Twenty-eight adults (10 females, 18 males, mean age 25.4 years, standard deviation [SD] = 2.3 years) gave their informed and written consent to participate in the study. Subjects were randomly assigned to 1 of 2 groups R and L. Group R received only right-lateralized experimental stimuli, whereas group L was presented only left-lateralized stimuli (see section on Procedure and Stimulus Material). Both groups had equal numbers of females (5) and males (9) and did not differ in age (R: 24.8 [SD = 2.1] years, L: 26.0 [SD = 2.5] years, t26 = 1.40). Subjects were paid Euro 10 per hour for participation. The study was approved by the ethics committee of the University of Frankfurt Medical Faculty.
Procedure and Stimulus Material
Subjects were seated upright in a magnetically shielded room (VAC, Hanau, Germany). They were instructed to sit still and keep their eyes open, looking at a fixation cross in the center of their visual field about 2 m in front of them. Auditory stimuli were presented binaurally via air-conducting tubes with ear inserts (E-A-Rtone 3A, Aearo Corporation, IN).
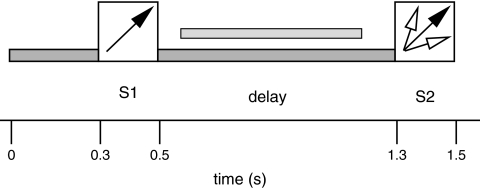
The trial structure of the task is depicted in Figure 1. The onset of the trial was signaled by a soft low-pass filtered midline background noise (at 6 kHz: −24 dB/octave) presented for 300 ms. Then a lateralized noise S1 (sample stimulus) was presented for 200 ms. The intensity of the background sound and the sample stimuli measured with a Reed 120-0014 sound level meter (TechniCal Systems Inc., Hamilton, Canada) amounted to 85 dB(A) and 98 dB(A), respectively. The intensity of the sample sounds was thus in the range that has been shown to elicit pronounced evoked gamma responses to sinusoidal tones in EEG (Schadow et al. 2007). Lateralized sounds were generated by convolution with head-related transfer functions (Gardner and Martin 1995; http://sound.media.mit.edu/KEMAR.html) yielding the impression of lateralized sounds in extrapersonal space. This is achieved by introducing both intrapersonal amplitude and time differences and by simulating the localization-dependent filtering properties of head and outer ears. During the following delay phase the background noise was presented again for 800 ms. This was followed by a second task-relevant lateralized noise S2 (probe stimulus).
Figure 1.
Trial structure of the task. Low-pass filtered noise (pre-S1) and the 200-ms presentation of the sample stimulus (S1) were followed by a delay phase of 800 ms midline noise. Then a probe stimulus (S2) appeared for 200 ms. Subjects had to compare the sound lateralization angle of S1 and S2. Arrows symbolize the lateralization angles of S1 and S2. The light gray horizontal bar above the symbol for the delay phase shows the latency window for spectral analysis (600–1200 ms after trial onset).
The subjects were instructed to compare the lateralization of S1 and S2. Half of the subjects within each group were instructed to respond by triggering a light barrier by raising both index fingers when the lateralization was identical, whereas the other half was to respond when lateralization angles differed between S1 and S2. Responses could be given up to the beginning of the baseline of the subsequent trial. For group R, S1 was presented on the right with a deviation of either 15° or 45° from the midsagittal plane. For group L, S1 was presented on the left at the same lateralization angles. S2 was always presented on the same side as S1. If S1 was presented at 15°, S2 could appear at either 15° (same lateralization), or at 0° or 60° (different lateralizations). If S1 was presented at 45°, S2 appeared at either 45°(same lateralization), or at 5° or 90° (different lateralization). The lateralization angles of S1 were presented in randomized order with equal probabilities for both angles. The lateralization angle of S2 was equal to S1 in half of the trials and different in the other half. The duration of the intertrial interval was randomized between 1700 and 2700 ms.
The task comprised 240 trials, that is, 120 trials with sample sounds S1 lateralized at 15° and 120 trials with S1 lateralized at 45°. Prior to the recordings, subjects performed up to 60 practice trials. In the first half of the practice phase, they received a fixed sequence of example trials with identical versus different S2 lateralization angles. In the second half of the practice phase, subjects had to respond to the stimuli and were given feedback about their performance by the experimenter.
Data Recording
MEG was recorded using a whole-head system (CTF-MEG, VSM MedTech Inc., Port Coquitlam, Canada) comprising 275 magnetic gradiometers with an average distance between sensors of about 2.2 cm. Signals of one defunct channel were discarded. The signals were recorded continuously at a sampling rate of 600 Hz with an antialiasing filter at 150 Hz. The final signal was computed using a synthetic third-order gradiometer configuration to suppress environmental noise and downsampled at 300 Hz. The subject's head position was determined with localization coils fixed at the nasion and the preauricular points at the beginning and at the end of each recording to ensure that head movements did not exceed 0.5 cm. To reduce eye movement and blink artifacts, we rejected trials containing signals exceeding 1.5 pT in frontotemporal sensors. This left an average of ∼95% of trials for analysis.
Data Analysis
Spectral analysis was designed to identify GBA components that distinguished between sample stimuli lateralized at 15° versus 45° in each of the 2 groups. The analyses focused on stimulus maintenance-related activity during the middle 600 ms of the delay phase. All artifact-free trials were included in the analyses. No baseline correction was performed. We followed a procedure that has been applied in a series of previous studies on MEG oscillatory responses (Lutzenberger et al. 2002; Kaiser et al. 2003; Kaiser, Hertrich, et al. 2005). First, spectral analysis was performed to identify the frequency ranges with the most robust differences between both stimuli. Significance of the observed spectral power values for each frequency bin and MEG sensor was tested with a statistical probability mapping including corrections for multiple comparisons. Second, topography (sensors) and time courses of activations were assessed after filtering in the frequency ranges with the most pronounced differences between conditions.
Spectral analysis was conducted for frequencies between 55 and 80 Hz for the time window of 0.6–1.2 s after trial onset, that is, the middle 600 ms of the delay phase starting 100 ms after the offset of S1 and lasting until 100 ms prior to the onset of S2. To reduce the frequency leakage for the different frequency bins, the records were multiplied by Welch windows. The nominal frequency resolution was 1.17 Hz; however, the true frequency resolution was somewhat lower because Welch windowing led to a certain smearing of frequencies across bins. Fast Fourier Transforms were carried out on single-trial basis and square roots of the power values in each frequency bin were computed to obtain more normally distributed spectral amplitude values. These values were averaged across trials to obtain measures of the total spectral activity in response to each of the 2 sample sounds. Spectral activity contrasts were evaluated with a statistical probability mapping procedure that has been used in numerous previous studies (e.g., Kaiser, Hertrich, et al. 2005). It included corrections both for multiple comparisons and for possible correlations between data either from neighboring frequency bins (for spectral analysis) or time points (for time course analysis). Significance criteria (corrected t values tcorr) were determined on the basis of permutation tests (Blair and Karniski 1993). Permutation tests allow to identify the probability to observe a difference of a certain size between 2 experimental conditions on the basis of the distribution obtained by randomly assigning the recorded data to the conditions. In general, the significance criteria obtained from the present procedure correspond to approximately P = 0.003 for 2 neighboring frequency bins.
Starting point was the comparison of group average spectral amplitude values for each of the 2 sample stimuli at each sensor and each frequency bin. This yielded the observed distributions of the t values for all frequency bins i × sensors j. To avoid spurious findings in individual frequency bins, we introduced the requirement that 2 neighboring frequency bins differ significantly between conditions. To ensure that tests for 2 consecutive frequency bins were significant, a new distribution of the minimal t values tm was computed for all pairs of neighboring frequency bins (time points) i and i + 1 at all sensors j:
The next analysis step was designed to take into account possible correlations between neighboring frequency bins. The t value tm and its corresponding P value P0.05 were determined for which 5% of the observed were larger. In the case of highly correlated data, P0.05 would be close to or smaller than 0.05, whereas for highly independent data, P0.05 would be greater than 0.05. The next step was to assess the random distribution of maximal t values in the present data set by exchanging the values for each trial type (or the signs of the differences between the 2 sample stimuli) at a time for all sensors j and frequency bins (time points) i on a subject-by-subject basis. This was done for 214 permutations of the 14 subjects in each group. Each of these permutations now yielded a new maximum t value. The distribution of these maximal t values tmax for each of the nrand = 214 permutations was computed as follows:
The corrected t value tcorr was now defined as the value where P0.05 × nrand of the obtained tmax were greater. This corrected t value tcorr was then applied as significance criterion to the observed data.
To explore the time course and the topographical localization of the observed spectral amplitude differences between conditions, the signals across the recording interval were multiplied with cosine windows at their beginnings and ends and filtered in the frequency ranges in which the statistical probability mapping had yielded significant effects. Noncausal, Gaussian curve-shaped Gabor filters in the frequency domain (width: ±1.5 Hz around center frequency, length in the time domain: 100 ms) were applied to the signals on a single-epoch basis for each of the 2 S1 stimuli. The filtered data were amplitude demodulated by means of a Hilbert transformation (Clochon et al. 1996) and then averaged across epochs for each stimulus. Differences in amplitude between stimuli in the filtered frequency band were assessed with the statistical probability mapping procedure described above.
To depict the topographical localization of the observed differential spectral amplitude enhancements, we assigned the sensor positions with significant spectral amplitude effects of each subject to common spatial coordinates (“common coil system”). Sensor positions with respect to the underlying cortical areas were determined using a volumetric magnetic resonance image of 1 subject. The error that is introduced by not using individual sensor locations was estimated in previous studies by using a single dipole for somatosensory evoked fields and 2 dipoles for the localization of the first auditory evoked component (N1m) (Kaiser et al. 2000). The comparison of individual sensor locations and the common coil system revealed differences ranging below the spatial resolution determined by the sensor spacing.
Results
Behavioral Data
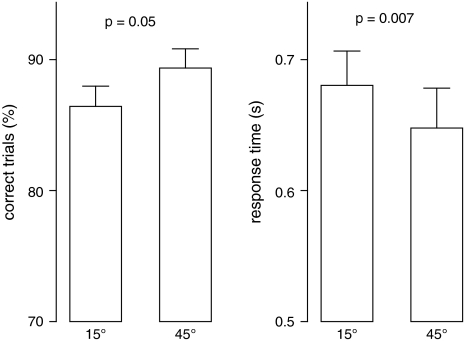
Separate ANOVAs were conducted for correct response rate and reaction time with group (left vs. right stimulation) as between-subjects factor and stimulus (15° vs. 45°) as within-subject factor. Both analyses yielded main effects for stimulus (correct response rate: F1,26 = 4.3, P = 0.048, reaction time: F1,26 = 8.3, P = 0.008). As there were no significant group main effects or group × stimulus interactions, dependent-samples t tests were calculated for both dependent variables across groups (Fig. 2). Correct response rates tended to be lower for sounds lateralized at 15° than 45° (15°: 86.4% [SD = 8.2%], 45°: 89.4% [SD = 7.8%], t27 = 2.05, P = 0.051), and reaction time was longer for 15° than 45° stimuli (15°: 680% [SD = 139%] ms after the onset of S2, 45°: 648% [SD = 161%] ms, t27 = 2.92, P = 0.007). Across all subjects, correct response rate and reaction time were negatively correlated (r = −0.54, P = 0.003).
Figure 2.
Correct response rates and reaction times (means and standard errors) for S1 stimuli presented at 15° and 45° deviation from the midsagittal plane calculated across the entire group of subjects.
Oscillatory Activity
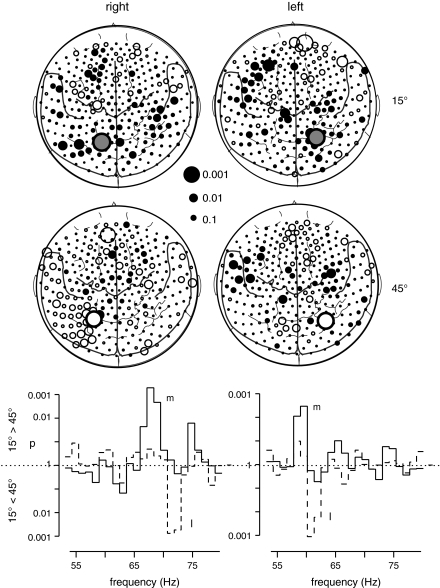
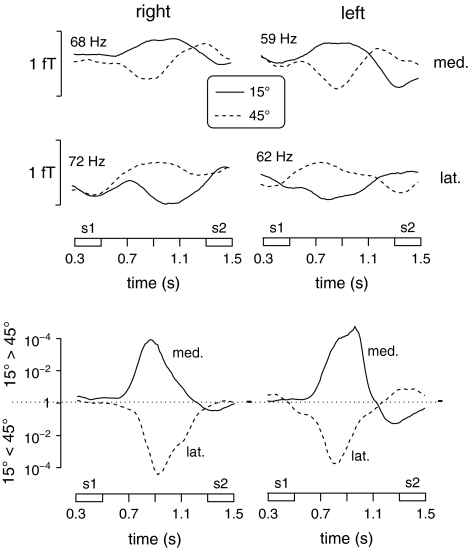
The results of frequency analysis for the comparison of the 2 S1 stimuli during the time window of 0.6–1.2 s after trial onset in each group are depicted in Figure 3. In group R, right-lateralized sample stimuli at 15° deviation from the midsagittal plane were associated with a relative enhancement of GBA at ∼68 Hz at a left parieto-occipital sensor (MLP52). For right-lateralized sample sounds at 45°, higher spectral amplitude was observed at ∼72 Hz at a slightly more lateral parieto-occipital sensor (MLP53). These effects met the criterion of tcorr = 3.41 for 2 consecutive frequency bins in the frequency range of 55–80 Hz. In group L, left-lateralized S1 stimuli at 15° were accompanied by a relative enhancement of GBA at ∼59 Hz at a right parieto-occipital sensor (MRP53). Left-lateralized sample sounds at 45° gave rise to higher spectral amplitude at ∼62 Hz at a more lateral parieto-occipital sensor (MRO13). These effects met the criterion of tcorr = 3.0 for 2 consecutive frequency bins in the frequency range of 58–65 Hz. To explore the time course and topography of these spectral amplitude differences, the data records were Gabor filtered (filter width: ±1.5 Hz around center frequency) in frequency ranges with center frequencies of 68 and 72 Hz for group R, and 59 and 62 Hz for group L, respectively. The time courses of the GBA differences between sample sounds at 15° and 45° in these frequency ranges are depicted as statistical time-frequency plots in Figure 4 and as spectral amplitude and statistical time curves for the filtered signals in Figure 5.
Figure 3.
Comparison of oscillatory responses to S1 stimuli at 15° versus 45° for both groups (left column: group R with stimulus presentation in the right hemifield and right column: group L with stimulus presentation in the left hemifield). The maps depict the topography of GBA differences between both S1 stimuli in the frequency ranges, where the statistical probability mapping had revealed significant effects (top left: 68 ± 1.5 Hz, bottom left: 72 ± 1.5 Hz, top right: 59 ± 1.5 Hz, and bottom right: 62 ± 1.5 Hz). Each circle represents one of the 275 MEG sensors projected onto a 2-dimensional cortical surface map with some major anatomical landmarks (dorsal view, nose up). The size of each circle reflects the statistical strength of the GBA difference between both S1 stimuli. Filled circles symbolize relative spectral amplitude increases in response to 15° stimuli, whereas open circles stand for relative spectral amplitude enhancements for 45° stimuli. The circles with the bold borders represent the sensors with the most robust GBA differences between stimuli, that is, where the statistical criterion was fulfilled for 2 neighboring frequency bands. The more medially located sensors showed a preference for 15°, the more lateral sensors for 45° stimuli.
The graphs at the bottom show the results (p values) of t-tests comparing spectral amplitudes between both S1 stimuli at the 2 sensors showing the most pronounced effects. The solid line gives p values for the comparison of S1 at 15° versus 45° at the more medial sensor (m) responding more strongly to S1 at 15°, whereas the dotted line represents p values for the opposite contrast (plotted downwards) at the more lateral sensor (l) responding more strongly to S1 at 45°.
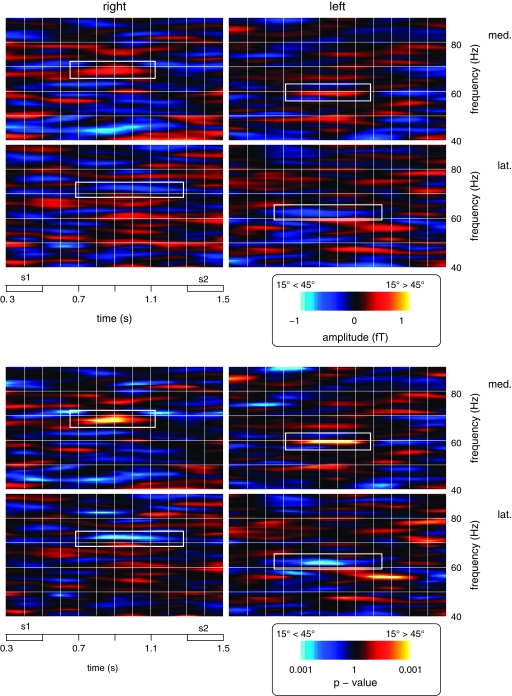
Figure 4.
Time-frequency plots depicting the spectral amplitude values and statistical strength (top and bottom panels, respectively) of differences between 15° and 45° sample stimuli (warm colors: relative increases for S1 at 15°, cold colors: relative increases for S1 at 45°) for both groups. Data are shown for the interval from the onset of S1 to the offset of S2 and for frequencies between 40 and 90 Hz. The top left graphs in each panel depict activity differences at the more medial posterior sensor for group R (med., symbolized by the largest circle in the top left map of Fig. 3), the bottom left graphs show activity differences for the more lateral parieto-occipital sensor for group R (lat., symbolized by the largest circle in the bottom left map of Fig. 3). The plots in the left half of the figure show the corresponding sensors for group L. Effects that met the statistical significance criteria described in the Materials and Methods are marked with white rectangles.
Figure 5.
Time courses between the onset of S1 and the offset of S2 of filtered signals for the frequency ranges with the most pronounced differences between sample stimuli at 15° versus 45° for group R and L (left and right columns, respectively). The graphs in the top 2 rows show spectral amplitude time courses, the graphs in the bottom row depict the time course of the statistical difference between 15° and 45° S1 stimuli. The top left graph depicts spectral amplitude (68 ± 1.5 Hz) time courses at the more medial posterior sensor (med., symbolized by the largest circle in the top left map of Fig. 3) for sample sounds at 15° and 45° (symbolized by the solid and dotted lines, respectively). The middle left graph depicts spectral amplitude (72 ± 1.5 Hz) time courses at the more lateral posterior sensor (lat., symbolized by the largest circle in the bottom left map of Fig. 3) for both sample sounds. The top and middle graphs on the right depict amplitude time courses at 59 and 62 ± 1.5 Hz at the more medial and lateral sensors shown in the right maps of Figure 3, respectively. Time courses of P values for the statistical difference between 15° and 45° stimuli at each sensor (solid lines: medial sensors, hatched lines: lateral sensors) are depicted in the bottom part of the figure.
In group R, right-lateralized sample stimuli at 15° deviation from the midsagittal plane gave rise to a spectral amplitude enhancement at 68 ± 1.5 Hz at a left parieto-occipital sensor (Fig. 3, top left map) that was maximal at 0.8–1.0 s after trial onset. The difference amplitude for this sensor during this time window amounted to 0.55 fT (SD = 0.11 fT), t13 = 4.82, P < 0.001. Right-lateralized sample sounds at 45° were accompanied by a relative GBA enhancement at 72 ± 1.5 Hz at a more lateral left parieto-occipital sensor (Fig. 3, bottom left map). Here, the mean difference amplitude during the same time window of 0.8–1.0 s after trial onset amounted to 0.51 (SD = 0.09) fT, t13 = 5.90, P < 0.001.
In group L, left-lateralized sample stimuli at 15° deviation from the midsagittal plane were associated with a spectral amplitude enhancement at 59 ± 1.5 Hz at a right parieto-occipital sensor (Fig. 3, top right map) that was maximal at 0.7–0.9 s after trial onset. The difference amplitude for this sensor during this time window amounted to 0.58 fT (SD = 0.12 fT), t13 = 4.99, P < 0.001. Left-lateralized sample sounds at 45° induced a relative GBA enhancement at 62 ± 1.5 Hz at a slightly more lateral right parieto-occipital sensor (Fig. 3, bottom right map). Here, the mean difference amplitude during the same time window of 0.7–0.9 s after trial onset amounted to 0.52 (SD = 0.11) fT, t13 = 4.85, P < 0.001.
Based on previous findings (Lutzenberger et al. 2002; Leiberg et al. 2006; Kaiser, Leiberg, et al. 2007), the present analyses focused on activity in the higher gamma range. In addition, we also explored differences in oscillatory activity between the 2 sample sounds in the lower frequency ranges including theta, alpha, beta, and the lower gamma range up to 55 Hz. Here, no significant effects were found.
Correlations between Oscillatory Activity and Task Performance
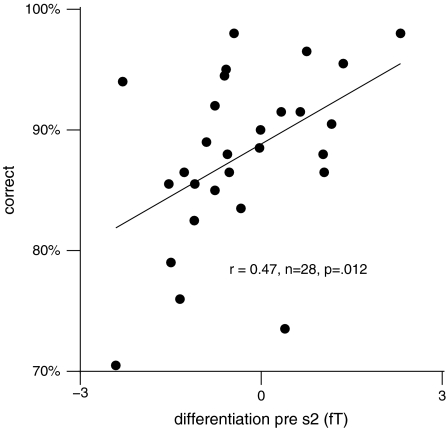
To explore a possible relationship between the stimulus-specific GBA components and task performance, we calculated an index of strength of representation of the 2 S1 stimuli across groups. First, for each subject the spectral amplitude differences in response to the 15° minus the 45° sample stimulus were calculated at the more medial and the more lateral parieto-occipital sensors, respectively. Second, the difference was computed between these amplitude difference values at the medial minus the lateral sensor. The resulting score thus reflected the degree to which oscillatory signals differentiated between the 2 stimuli. Positive values indicated a “consistent” differentiation with larger amplitudes to the preferred stimulus (in the sense of the initial statistical parametric mapping), whereas negative values stood for an “inconsistent” differentiation with larger amplitudes to the nonpreferred sound. This score was then correlated with correct response rate, that is, the combined proportion of hits and correct rejections. As subjects had to respond to 1 type of S1–S2 comparison only (either to matches or nonmatches), a distinction between both types of responses was not possible. Across groups, a significant positive correlation of r = 0.47 (P = 0.012) was observed between correct response rate and the averaged differentiation score for the final 100 ms of the delay phase only (Fig. 6), that is, a more pronounced differentiation was associated with better performance. In contrast, there was no significant correlation between GBA amplitude and reaction time during this time window (r = 0.07).
Figure 6.
Correlations between correct response rate (ordinate) and a spectral amplitude measure reflecting the strength of differentiation between the 2 sample stimuli (abscissa) for the entire subject sample across both groups (N = 28). The differentiation measure was computed as the difference between the stimulus-specific GBA spectral amplitude changes at the 2 sensors where these effects were localized during the final 100 ms of the delay phase.
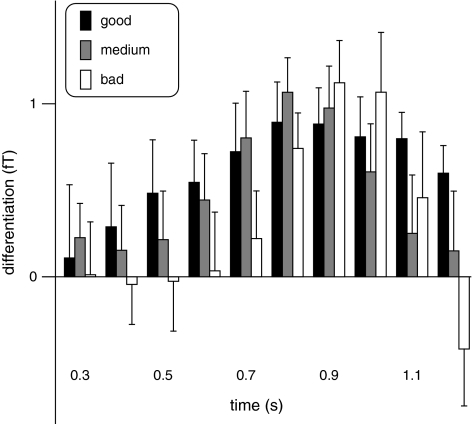
As the correlation between performance and differentiation score was observed for a time window when in the group average, there was no differentiation between the 2 sample stimuli; for exploratory purposes, we split the subject group into 3 groups of 10 good, 8 medium, and 10 poor performers. The mean amplitudes and standard errors of the differentiation index in these 3 groups are plotted in Figure 7 for ten 100-ms time windows between 0.3 s after trial onset (onset of S1) and 1.2 s (end of the delay phase). Good performers upheld the consistent differentiation for longer than average or poor performers whose differentiation score decreased or even changed its sign prior to the onset of S2. The figure further suggests that there were no substantial differences in amplitude variability between groups.
Figure 7.
Amplitudes and standard errors of the differentiation index for ten 100-ms time windows between 0.3 and 1.2 s after trial onset for groups of 10 good, 8 medium, and 10 bad task performers.
Discussion
The present study investigated induced GBA during the delay phase of an auditory spatial delayed matching-to-sample task requiring the maintenance of the lateralization angle of a sample noise sound in short-term memory and to compare it with a subsequent probe stimulus. In contrast to previous work using a similar paradigm (Lutzenberger et al. 2002; Leiberg et al. 2006), here we did not contrast this task with a nonmemory control condition but we compared oscillatory responses between 2 different sample sounds lateralized at 15° or 45° deviation from the midsagittal plane, respectively. Oscillatory responses to these stimuli were investigated in 2 nonoverlapping groups of subjects who were either presented with stimuli lateralized in the left or right hemifield only. Statistical probability mapping revealed distinct GBA components to each of the sample sounds. These components had an intermediate amplitude during the presentation of S1 and showed subsequently either an amplitude increase in response to their “preferred” stimulus or a decrease to the “nonpreferred” stimulus (Fig. 5). The maximum differentiation between preferred and nonpreferred stimuli was reached during the middle of the delay phase approximately 0.2–0.5 s after the offset of S1. The average differentiation returned to 0 immediately prior to the onset of S2. GBA components distinguishing between the 2 lateralization angles were observed at parieto-occipital sensors contralateral to the side of stimulation (Fig. 3). These sensors were localized over homologous areas for the 2 groups. The present study thus demonstrates that distinct GBA components for each stimulus lateralization angle can be identified in MEG. Effects were replicated in a similar frequency range and with a highly comparable topography for 2 independent groups, arguing for the robustness of the findings.
Increased GBA in EEG in response to attentively perceived familiar sounds compared with unfamiliar acoustic stimuli has been interpreted as reflecting matches with representations in long-term memory (Lenz et al. 2007). In contrast, the present findings were obtained with meaningless noise stimuli, suggesting that GBA represents the activation of networks processing task-relevant information also for abstract stimuli that do not have a meaningful long-term memory representation (Başar 2005). The finding of distinct oscillatory components in response to each sample stimulus is in keeping with our hypothesis that GBA reflects the cortical representations of individual stimuli. These components could only be identified by directly contrasting 2 stimuli. As they showed amplitude increases for their preferred stimulus but decreases for the nonpreferred one, they would not be visible if data were averaged across stimuli. In earlier studies where we compared oscillatory activity during a memory task with a control condition (Lutzenberger et al. 2002; Kaiser et al. 2003; Leiberg et al. 2006), GBA during the delay phase reflected memory-specific activations that were common to the different sample stimuli maintained during this phase. The present results show that direct contrasts between 2 stimuli reveal spectrally narrow and topographically local GBA components in MEG, possibly reflecting networks tuned to a task-relevant stimulus feature-like sound lateralization angle. In both groups, the 15° sample stimuli elicited GBA components at lower central frequencies than the sounds lateralized at 45°. As lower frequencies have been related to increased cortical activation (Herculano-Houzel et al. 1999), this finding could be attributed tentatively to the fact that the 15° stimuli were more difficult to process in short-term memory as indicated by lower correct response rates and longer reaction times.
The topography of stimulus-specific components seems to depend on the particular feature that is to be attended or maintained in short-term memory. During our previous sound duration matching-to-sample task, stimulus-specific GBA components were found over prefrontal cortex (Kaiser, Leiberg, et al. 2007), whereas here the maintenance of spatial sounds elicited GBA over posterior cortical regions. The topography of the present GBA components is consistent with the notion of a putative auditory dorsal stream involved in the processing of auditory spatial information (Rauschecker 1998). Previous studies of spatial sound processing have found activations in or over posterior parietal areas with functional magnetic resonance imaging (Alain et al. 2001; Arnott et al. 2004) and MEG (Kaiser et al. 2000, 2005; Lutzenberger et al. 2002; Kaiser, Lennert, Lutzenberger 2007). However, the existence of an auditory dorsal spatial processing stream is debated (Belin and Zatorre 2000); activations in posterior parietal areas could also reflect supramodal spatial attention or visual imagery (Bidet-Caulet and Bertrand 2005). The oscillatory activations in the present study were localized in slightly more posterior sensors than in our previous MEG studies. Their topography is akin to the one reported by Siegel et al. (2007) for magnetoencephalographic high-frequency gamma activity in relation to visual motion strength where sources were localized in occipitoparietal and lateral occipitotemporal regions attributed to human area MT+/V5. GBA peaks at similar sensor positions over motion-relevant areas possibly including V3A, the kinetic occipital region and the dorsal intraparietal sulcus have also been found in a previous unpublished visual motion processing study from our laboratory. Recently, it has been suggested that human area V5 may be involved in auditory motion processing (Poirier et al. 2005). However, it is quite likely that in the present study representations of the sound lateralization angles were coded by visual or supramodal space processing networks in posterior parietal/occipitoparietal areas (Macaluso and Driver 2005) and that visuospatial imagery processes might have been involved in stimulus maintenance during the delay phase. This interpretation is supported by a postexperimental interview in which 14 out of 19 available participants indicated having used a visual (12) or an audiovisual (2) strategy. The fact that the present stimulus-specific GBA components were localized in sensors contralateral to the side of stimulation and that stimuli lateralized at 15° were consistently accompanied by more medial GBA than stimuli lateralized at 45° could reflect the existence of spatial maps in posterior parietal cortex (Sereno et al. 2001).
Similar to our previous studies, we have chosen a conservative statistical procedure to identify the most robust differences between the 2 acoustic stimuli. This procedure included the determination of a statistical threshold on the basis of nonparametric permutation tests and required that t tests comparing conditions reach a certain critical t value in 2 neighboring frequency bins. In previous investigations where, for example, memory tasks were compared with control tasks (Lutzenberger et al. 2002; Kaiser et al. 2003), this analysis procedure has typically yielded effects for small numbers of sensors only. Although this approach may include a certain risk to overlook more transient effects, the effects that we have reported previously could usually be replicated in independent studies (Kaiser and Lutzenberger 2003, 2005a), arguing in favor of such a conservative approach. In the present study where we assessed the differential representation of sound lateralization angles, effects at single sensors were expected because it seemed plausible that such a subtle difference would be processed by highly local networks.
In general, the topography of the current effects has to be interpreted with caution because the relationship between surface data and the underlying generators is not straight forward. The present surface GBA patterns do not suggest simple dipolar sources which would produce 2 patches with strong magnetic fields. In contrast, the single patches typically found both in the present study and in our previous work could possibly be attributed to a more complex structure of local sources that might generate a relatively weak field which is maximal over the area between the dipoles (see Kaiser et al. 2000, for a detailed discussion of the possible source structure). According to this model, the cortical generators would thus have to be localized in the vicinity of the sensors showing the strongest activations. Moreover, differential effects were found in sensors separated only by short distances. This topography may reflect the activities of partly overlapping sources.
The relative strength of the present stimulus-specific GBA components correlated moderately with task performance, that is, the more pronounced the relative GBA increase to the preferred and the relative decrease to the nonpreferred stimulus was, the higher the correct response rate (Fig. 6). This supports the notion that the stimulus-specific oscillatory activity reflected processes relevant to the short-term memory maintenance of acoustic information. Interestingly, the correlation was only found for relative GBA differences during the final 100 ms of the delay phase, when the mean differentiation between the 2 sample stimuli had already returned to 0. In contrast, there was no correlation between the peak amplitude of S1-related gamma components and correct response rate or reaction time. Apparently, good performance relied more on the maintenance of the consistent representation at the end of the delay phase than on the strength of the differentiation earlier during the delay period. Good performers seemed to be able to maintain a representation of S1 until the end of the delay period even if it may have been a weak one. Their differentiation score showed a broader temporal distribution than in average or poor performers who both showed a clearer differentiation peak and a more pronounced subsequent decrease (Fig. 7). At the end of the delay phase, poor performers even showed an inverse differentiation with higher spectral amplitudes to the incorrect stimuli. However, good and poor performers did not differ in the variability of their differentiation amplitudes. The larger variance between subjects during the final part of the delay phase may have helped to find a significant correlation.
Towards the end of the delay phase, the time course of the average stimulus-related oscillatory activity returned to the intermediate level found during S1 presentation (Fig. 5). This is a phenomenon already observed in earlier studies on visual short-term memory. For example, Tallon-Baudry et al. (1998) argued that with a fixed 800-ms delay phase (as the one used in the present study), it was difficult to distinguish whether the gamma response during the delay was transient or sustained. They also speculated that GBA decreased because S2 could be anticipated and it may not have been necessary to maintain the full strength of this activity until the end of the delay period. In a subsequent study using variable delay durations, sustained posterior gamma components were described which, however, also showed a constant power decrease over time (Tallon-Baudry et al. 1999). Also, this might suggest that GBA amplitude increases do not represent the only relevant mechanism underlying stimulus maintenance in short-term memory. Previous studies have suggested that corticocortical gamma-band synchronization between higher sensory areas and frontal regions may play an important role in short-term memory maintenance (Kaiser, Leiberg, Lutzenberger 2005). Alternatively, a temporal modulation of GBA would be in keeping with the proposed correlation of this activity with the cycle of power in the theta band (Canolty et al. 2006). A future study may employ delay periods of different lengths to assess the effects of delay duration on the temporal dynamics of the GBA components.
In summary, spectrally and topographically distinct oscillatory components in the higher gamma range were associated with the maintenance of different sound lateralization angles during the delay phase of a short-term memory task. These components were localized at MEG sensors over parieto-occipital cortex contralateral to the side of stimulation, suggesting an involvement of this region in the representation of sound lateralization angles. The present findings add to the growing number of studies demonstrating that GBA not only plays a role in sensory feature binding but may reflect representations of task-relevant stimulus attributes that are modulated by attention or memory processes (Jensen et al. 2007). Moreover, GBA may index the specific contents of short-term memory, that is, the stimulus representation itself.
Funding
German Federal Ministry of Education and Research, Brain Imaging Center, Frankfurt (DLR 01GO0507).
Acknowledgments
We are grateful to Benjamin Rahm for helpful comments. Conflict of Interest: None declared.
References
- Alain C, Arnott SR, Hevenor S, Graham S, Grady CL. “What” and “where” in the human auditory system. Proc Natl Acad Sci USA. 2001;98:12301–12306. doi: 10.1073/pnas.211209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann CF, Bledowski C, Wibral M, Kaiser J. Processing of location and pattern changes of natural sounds in the human auditory cortex. Neuroimage. 2007;35:1192–1200. doi: 10.1016/j.neuroimage.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Arnott SR, Binns MA, Grady CL, Alain C. Assessing the auditory dual-pathway model in humans. Neuroimage. 2004;22:401–408. doi: 10.1016/j.neuroimage.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Başar E. Memory as the “whole brain work”: a large-scale model based on “oscillations in super-synergy.”. Int J Psychophysiol. 2005;58:199–226. doi: 10.1016/j.ijpsycho.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Bauer M, Oostenveld R, Peeters M, Fries P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci. 2006;26:490–501. doi: 10.1523/JNEUROSCI.5228-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgart F, Gaschler-Markefski B, Woldorff MG, Heinze HJ, Scheich H. A movement-sensitive area in auditory cortex. Nature. 1999;400:724–726. doi: 10.1038/23390. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ. ‘What’, ‘where’ and ‘how’ in auditory cortex. Nat Neurosci. 2000;3:965–966. doi: 10.1038/79890. [DOI] [PubMed] [Google Scholar]
- Bidet-Caulet A, Bertrand O. Dynamics of a temporo-fronto-parietal network during sustained spatial or spectral auditory processing. J Cogn Neurosci. 2005;17:1691–1703. doi: 10.1162/089892905774589244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RC, Karniski W. An alternative method for significance testing of waveform difference potentials. Psychophysiol. 1993;30:518–524. doi: 10.1111/j.1469-8986.1993.tb02075.x. [DOI] [PubMed] [Google Scholar]
- Brovelli A, Lachaux JP, Kahane P, Boussaoud D. High gamma frequency oscillatory activity dissociates attention from intention in the human premotor cortex. Neuroimage. 2005;28:154–164. doi: 10.1016/j.neuroimage.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313:1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clochon P, Fontbonne J, Lebrun N, Etevenon P. A new method for quantifying EEG event-related desynchronization: amplitude envelope analysis. Electroencephalogr Clin Neurophysiol. 1996;98:126–129. doi: 10.1016/0013-4694(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Gardner WG, Martin KD. HRTF measurements of a KEMAR. J Acoust Soc Am. 1995;97:3907–3908. [Google Scholar]
- Gray CM, König P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Rees G, Rees A, Green GG, Witton C, Rowe D, Büchel C, Turner R, Frackowiak RS. Right parietal cortex is involved in the perception of sound movement in humans. Nat Neurosci. 1998;1:74–79. doi: 10.1038/276. [DOI] [PubMed] [Google Scholar]
- Gruber T, Müller MM, Keil A, Elbert T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin Neurophysiol. 1999;110:2074–2085. doi: 10.1016/s1388-2457(99)00176-5. [DOI] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Montaldi D, Müller MM. Induced gamma band responses: an early marker of memory encoding and retrieval. Neuroreport. 2004;15:1837–1841. doi: 10.1097/01.wnr.0000137077.26010.12. [DOI] [PubMed] [Google Scholar]
- Hart HC, Palmer AR, Hall DA. Different areas of human non-primary auditory cortex are activated by sounds with spatial and nonspatial properties. Hum Brain Mapp. 2004;21:178–190. doi: 10.1002/hbm.10156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Munk MH, Neuenschwander S, Singer W. Precisely synchronized oscillatory firing patterns require electroencephalographic activation. J Neurosci. 1999;19:3992–4010. doi: 10.1523/JNEUROSCI.19-10-03992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8:347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30:317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Bühler M, Lutzenberger W. Magnetoencephalographic gamma-band responses to illusory triangles in humans. Neuroimage. 2004;23:551–560. doi: 10.1016/j.neuroimage.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Hertrich I, Ackermann H, Mathiak K, Lutzenberger W. Hearing lips: gamma-band activity during audiovisual speech perception. Cereb Cortex. 2005;15:646–653. doi: 10.1093/cercor/bhh166. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Leiberg S, Lutzenberger W. Let's talk together: memory traces revealed by cooperative activation in the cerebral cortex. Int Rev Neurobiol. 2005;68:51–78. doi: 10.1016/S0074-7742(05)68003-8. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Leiberg S, Rust H, Lutzenberger W. Prefrontal gamma-band activity distinguishes between sound durations. Brain Res. 2007;1139:153–162. doi: 10.1016/j.brainres.2006.12.085. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lennert T, Lutzenberger W. Dynamics of oscillatory activity during auditory decision making. Cereb Cortex. 2007;17:2258–2267. doi: 10.1093/cercor/bhl134. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Induced gamma-band activity and human brain function. Neuroscientist. 2003;9:475–484. doi: 10.1177/1073858403259137. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Cortical oscillatory activity and the dynamics of auditory memory processing. Rev Neurosci. 2005a;16:239–254. doi: 10.1515/revneuro.2005.16.3.239. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Human gamma-band activity: a window to cognitive processing. Neuroreport. 2005b;16:207–211. doi: 10.1097/00001756-200502280-00001. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W, Ackermann H, Birbaumer N. Dynamics of gamma-band activity induced by auditory pattern changes in humans. Cereb Cortex. 2002;12:212–221. doi: 10.1093/cercor/12.2.212. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W, Preissl H, Ackermann H, Birbaumer N. Right-hemisphere dominance for the processing of sound-source lateralization. J Neurosci. 2000;20:6631–6639. doi: 10.1523/JNEUROSCI.20-17-06631.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Ripper B, Birbaumer N, Lutzenberger W. Dynamics of gamma-band activity in human magnetoencephalogram during auditory pattern working memory. Neuroimage. 2003;20:816–827. doi: 10.1016/S1053-8119(03)00350-1. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Walker F, Leiberg S, Lutzenberger W. Cortical oscillatory activity during spatial echoic memory. Eur J Neurosci. 2005;21:587–590. doi: 10.1111/j.1460-9568.2005.03867.x. [DOI] [PubMed] [Google Scholar]
- Krumbholz K, Schönwiesner M, von Cramon DY, Rübsamen R, Shah NJ, Zilles K, Fink GR. Representation of interaural temporal information from left and right auditory space in the human planum temporale and inferior parietal lobe. Cereb Cortex. 2005;15:317–324. doi: 10.1093/cercor/bhh133. [DOI] [PubMed] [Google Scholar]
- Leiberg S, Kaiser J, Lutzenberger W. Gamma-band activity dissociates between matching and nonmatching stimulus pairs in an auditory delayed matching-to-sample task. Neuroimage. 2006;30:1357–1364. doi: 10.1016/j.neuroimage.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Lenz D, Schadow J, Thaerig S, Busch NA, Herrmann CS. What's that sound? Matches with auditory long-term memory induce gamma activity in human EEG. Int J Psychophysiol. 2007;64:31–38. doi: 10.1016/j.ijpsycho.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Lutzenberger W, Ripper B, Busse L, Birbaumer N, Kaiser J. Dynamics of gamma-band activity during an audiospatial working memory task in humans. J Neurosci. 2002;22:5630–5638. doi: 10.1523/JNEUROSCI.22-13-05630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macaluso E, Driver J. Multisensory spatial interactions: a window onto functional integration in the human brain. Trends Neurosci. 2005;28:264–271. doi: 10.1016/j.tins.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Maeder PP, Meuli RA, Adriani M, Bellmann A, Fornari E, Thiran JP, Pittet A, Clarke S. Distinct pathways involved in sound recognition and localization: a human fMRI study. Neuroimage. 2001;14:802–816. doi: 10.1006/nimg.2001.0888. [DOI] [PubMed] [Google Scholar]
- Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Neural correlates of consolidation in working memory. Hum Brain Mapp. 2007;28:183–193. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Keil A. Neuronal synchronization and selective color processing in the human brain. J Cogn Neurosci. 2004;16:503–522. doi: 10.1162/089892904322926827. [DOI] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernandez G, Maris E, Jensen O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci. 2006;26:7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavani F, Macaluso E, Warren JD, Driver J, Griffiths TD. A common cortical substrate activated by horizontal and vertical sound movement in the human brain. Curr Biol. 2002;12:1584–1590. doi: 10.1016/s0960-9822(02)01143-0. [DOI] [PubMed] [Google Scholar]
- Poirier C, Collignon O, Devolder AG, Renier L, Vanlierde A, Tranduy D, Scheiber C. Specific activation of the V5 brain area by auditory motion processing: an fMRI study. Brain Res Cogn Brain Res. 2005;25:650–658. doi: 10.1016/j.cogbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Curr Opin Neurobiol. 1998;8:516–521. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nat Neurosci. 1999;2:1131–1136. doi: 10.1038/16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadow J, Lenz D, Thaerig S, Busch NA, Fründ I, Herrmann CS. Stimulus intensity affects early sensory processing: sound intensity modulates auditory evoked gamma-band activity in human EEG. Int J Psychophysiol. 2007;65:152–161. doi: 10.1016/j.ijpsycho.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. J Neurosci. 2003;23:10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. High-frequency activity in human visual cortex is modulated by visual motion strength. Cereb Cortex. 2007;17:732–741. doi: 10.1093/cercor/bhk025. [DOI] [PubMed] [Google Scholar]
- Singer W, Engel AK, Kreiter A, Munk MHJ, Neuenschwander S, Roelfsema PR. Neuronal assemblies: necessity, signature and detectability. Trends Cogn Sci. 1997;1:252–261. doi: 10.1016/S1364-6613(97)01079-6. [DOI] [PubMed] [Google Scholar]
- Sokolov A, Pavlova M, Lutzenberger W, Birbaumer N. Reciprocal modulation of neuromagnetic induced gamma activity by attention in the human visual and auditory cortex. Neuroimage. 2004;22:521–529. doi: 10.1016/j.neuroimage.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci. 1998;18:4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C, Kreiter A, Bertrand O. Sustained and transient oscillatory responses in the gamma and beta bands in a visual short-term memory task in humans. Vis Neurosci. 1999;16:449–459. doi: 10.1017/s0952523899163065. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Näätänen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Vidal JR, Chaumon M, O'Regan JK, Tallon-Baudry C. Visual grouping and the focusing of attention induce gamma-band oscillations at different frequencies in human magnetoencephalogram signals. J Cogn Neurosci. 2006;18:1850–1862. doi: 10.1162/jocn.2006.18.11.1850. [DOI] [PubMed] [Google Scholar]
- Warren JD, Griffiths TD. Distinct mechanisms for processing spatial sequences and pitch sequences in the human auditory brain. J Neurosci. 2003;23:5799–5804. doi: 10.1523/JNEUROSCI.23-13-05799.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JD, Zielinski BA, Green GG, Rauschecker JP, Griffiths TD. Perception of sound-source motion by the human brain. Neuron. 2002;34:139–148. doi: 10.1016/s0896-6273(02)00637-2. [DOI] [PubMed] [Google Scholar]