Abstract
The premotor theory of attention suggests that target processing and generation of a saccade to the target are interdependent. Temporally precise transcranial magnetic stimulation (TMS) was delivered over the human frontal eye fields, the area most frequently associated with the premotor theory in association with eye movements, while subjects performed a visually instructed pro-/antisaccade task. Visual analysis and saccade preparation were clearly separated in time, as indicated by 2 distinct time points of TMS delivery that resulted in elevated saccade latencies. These results show that visual analysis and saccade preparation, although frequently enacted together, are dissociable processes.
Keywords: eye movements, transcranial magnetic stimulation, vision
The premotor theory of attention (Rizzolatti, 1983; Sheliga et al. 1994; Sheliga et al. 1995; cf., Klein. 1980) in its strongest form states that the same mechanisms are responsible for controlling action and spatial attention. Whereas a number of behavioral studies support such a link (Shepherd et al. 1986; Deubel and Schneider, 1996), Hunt and Kingstone (2003a, 2003b) have elegantly demonstrated that attention and eye movements are independent processes in both volitional and reflexive experimental tasks. At a neurophysiological level, the dissociation of spatial attention and saccade preparation has also been found in nonhuman primate microstimulation experiments (Juan et al. 2004a, cf. Kustov and Robinson, 1996).
This dissociation is a contentious issue in human neuroimaging studies. Some imaging studies support the proposal that there is a single locus of activity corresponding to a unitary function of visual selection and saccade generation (Corbetta, 1998) whereas others indicate less complete overlap of the areas involved (Gitelman et al. 2002). Another approach to the problem in the human brain is to ask whether the functions can be dissociated temporally. Imaging studies cannot parse the temporal dynamics of vision and eye movements, and it remains possible that visual analysis and saccade preparation can be dissociated temporally in the human brain, notwithstanding any potential overlap of the anatomical areas involved.
A visual stimulus in a peripheral location can be analyzed in the absence of saccades to the stimulus location. In the monkey frontal eye fields (FEF), the visuomotor area responsible for saccade generation and that most frequently associated with the premotor theory, neurons respond selectively to stimuli in the absence of a saccade (Schall, 2004). Although it is possible that a saccade is prepared but not executed, thus playing a part in visual analysis, evidence from microstimulation studies in macaques suggests that this is not the case. Microstimulation of FEF neurons evokes involuntary saccades with a fixed vector. If a saccade is being programed, these microstimulation-induced saccades are integrated with the ongoing voluntary saccade program and this is reflected in a deviation of the direction of the evoked saccades. Given that the strongest form of the premotor theory assumes that the deployment of covert attention is equivalent to the preparation of a saccade to that location, it would predict that the microstimulation-induced saccade would deviate toward the location of covert attention. However, the direction of the covert attention and the direction in which saccades are made are usually coupled together. Using a task in which the orientation of a colored singleton instructed monkeys whether to make a pro- or antisaccade, Juan et al. (2004a) temporally dissociated the 2 processes. In the antisaccade condition, covert attention was first deployed to the location of the singleton and the monkey was then required to saccade to the location opposite to this singleton. Consequently, the directions of the covert attention and the saccade direction were dissociated momentarily. Microstimulation applied over the FEF with various timings during this task was used to test whether the saccadic deviation during the transition between covert attention and saccade generation deviated to the instruction singleton first then redirected to the opposite location, as the premotor theory would predict. They found that microstimulation of FEF evoked saccades that deviated toward the singleton on prosaccade trials only. For antisaccades, the end point of evoked saccades only ever deviated toward a correct saccade end point and never the singleton. The dissociation of spatial attention and saccade preparation has thus been found at a neurophysiological level in monkeys (Juan et al. 2004; cf. Kustov and Robinson, 1996). Klein (2004) has comprehensively reviewed behavioral and neuroscientific evidence for a link between covert and overt orienting, and one of his conclusions is that “Endogenous covert orienting of attention is not mediated by endogenously generated saccadic programming.” Klein (2004) has also pointed out that the demonstration of Kustov and Robinson that covert orienting is closely coupled with saccade programing could be the result of confounding covert attention and motor preparation in their experimental design (see also Klein and Shore, 2000).
The causal role of human FEF in visual selection has been demonstrated in several studies (Grosbras and Paus, 2002; Muggleton et al. 2003; O'Shea et al. 2004; Smith et al. 2005). However, whereas effects on saccade latencies have been found with TMS delivered relative to the expected saccade onset (Thickbroom et al. 1996), the temporal dynamics of covert attention and overt eye movements responses in human FEF have not been tested directly. We used transcranial magnetic stimulation (TMS) to investigate the role of human FEF in performance of a task similar to that employed by Juan et al. (2004a) in the studies of nonhuman primate electrophysiology. This task can dissociate the processes of covert attention and overt saccade execution in time, the neural mechanisms of which have been addressed in monkey FEF. The aim of this study is to clarify the issues raised by neuroimaging studies regarding the overlapping neural mechanisms between covert attention and saccade programming in the human brain. We tested the hypothesis that if, as has been demonstrated in the macaque, visual and saccade-related processes can be separated, then there should be 2 time points during which TMS over FEF would alter task performance, with an early visual processing–related effect being present in addition to the previously reported effects of TMS related to expected saccade latency. If the 2 processes are one and the same then this would not be expected to be the case and there would be a single period susceptible to disruption. It has been shown that TMS over the primary visual cortex can modulate the performance of visual tasks in discrete time windows, and this line of evidence indicates that a brain region can serve different functions at different time points (Juan and Walsh, 2003; Pascual-Leone and Walsh, 2001; for review, see Juan et al. 2004b). Following the same logic, the dynamic interplay between visual selection and motor preparation in FEF can be probed with TMS applied in different time windows. Neurophysiological evidence from awake, behaving monkeys has demonstrated that the interchange between these processes does happen at different time points in FEF (e.g., Sato and Schall, 2003). However, no such similar evidence exists in human FEF research. It is therefore essential to establish the temporal dynamics of visual selection and saccade preparation in human FEFs, as neuroimaging studies cannot parse these processes in the temporal domain.
Materials and Methods
Subjects
Six subjects, aged 23–35 years (mean 30.2), all male, took part. All these took part in the first part of the experiment and 5 completed both parts. All gave informed consent prior to participating, and the study was approved by the local ethics committee of University College London. All subjects had prior experience of TMS and, with the exception of one of the authors who took part (NM), were naive to the purpose and hypotheses underlying the experiment. Exclusion criteria conforming to current guidelines for rTMS research were applied (Wasserman, 1998).
TMS
TMS was delivered by a Magstim 200 Super Rapid Stimulator via a 50-mm figure-of-eight coil, with the coil anterior to the handle, with the handle parallel to the midline. Stimulation level was fixed at 65% of the machine output because 1) this has previously been shown to be effective when delivered over FEF (Muggleton et al. 2003; O'Shea et al. 2004) and 2) it has been shown that motor cortex excitability, which can be readily measured with TMS, does not necessarily correlate with excitability in other brain areas (Stewart et al. 2001).
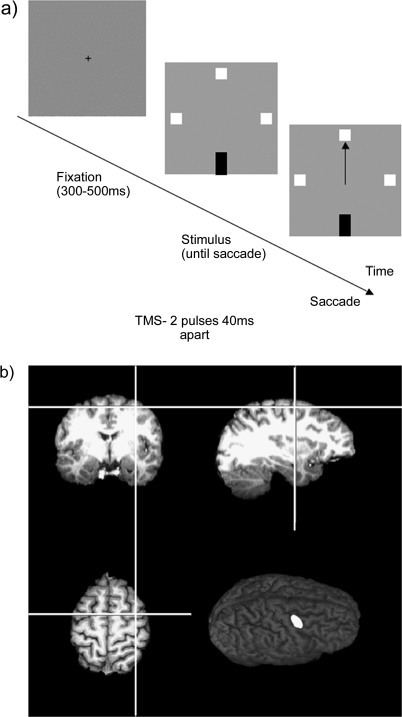
The site for FEF stimulation was located using a simple antisaccade task during which TMS was delivered for 500 ms at 10 Hz over candidate sites anterior to the hand motor area in the right hemisphere using a grid of points separated from each other by 1 cm. The site that resulted in the longest saccade latencies was marked, and the anatomical position was then located by coregistering the head of each subject with their individual high-resolution magnetic resonance imaging scans using the Brainsight system (Rogue Research, Montreal, Canada). TMS locations were consistent with FEF locations in previous studies (Muggleton et al. 2003; O'Shea et al. 2004) (see Fig. 1a) and were a mean of 4.7 cm [standard error of mean (SEM = 0.21)] lateral and 3.3 cm (SEM = 0.21) anterior to the vertex.
Figure 1.
(a) Site of TMS stimulation shown on coronal, parasaggital, and axial sections through the highlighted region on the surface of the brain. (b) The saccade task. The orientation of the oddball element indicated whether a pro- or antisaccade was the correct response. Blocks could be all prosaccades, all antisaccades, or interleaved at random. All 3 types were presented without TMS whereas TMS blocks consisted of only interleaved trials. In one session, pairs of TMS pulses, with 40 ms between pulses, were delivered with the first pulse at 0, 40, 80, 120, or 160 ms following array onset. In a second session, TMS was delivered with the first pulse 200 ms prior to the median saccade latency for the subject or at the median latency. Blocks where no TMS was delivered were included as controls in both sessions.
Task Details
The task was presented using an SMI Eyelink I system, modified for use with TMS, which recorded eye position information at 250 Hz throughout experimental trials. Each trial began with a correction of any eye drift and then followed the time course detailed in the main text. Stimulus arrays subtended 10° of visual angle such that targets and distractors were 5° from fixation. Colors in the array were isoluminant red and green (20 cd m-2) presented on a uniform gray background.
Subjects were required, upon presentation of the stimulus array (see Fig. 1b), to make an eye movement either toward or away from a colored singleton on the basis of its orientation. For each session, the orientations indicating the saccade types were assigned at random (i.e., in one session the horizontal element could require a prosaccade response whereas it could require an antisaccade to be made in the next session).
Subjects took part in 3 sessions of testing. The first of these (Experiment 1) involved no TMS and consisted of blocks of just prosaccade trials, just antisaccade trials, or interleaved trials where half of the trials required prosaccades and half required antisaccades to be made. The order of trial types was randomized in the interleaved blocks. Each block contained 40 trials (as was also the case for subsequent sessions) with 5 blocks each of the prosaccade and antisaccade conditions and 10 blocks of the interleaved condition. The second session (Experiment 2) involved presentation of the interleaved trials with TMS, consisting of pairs of pulses separated by 40 ms, delivered at different times with respect to stimulus onset. TMS could be delivered with the first pulse at 0, 40, 80, 120, or 160 ms following onset of the visual stimulus. In each block of 40 trials, only one TMS onset time was used and 2 blocks were presented for each stimulation time. Additionally, 2 blocks were presented with no TMS. In this study, we did not employ a sham condition. Sham is one of the ways of controlling for any nonspecific auditory effects of TMS. In the current paper, however, nonspecific effects are controlled for by the presentation of real TMS at different times and in different tasks. For example, the difference between the pro- and antisaccade tasks shown in Experiment 2 could not be explained as an auditory or tactile artifact because this would be the same in both conditions. With the exception of one subject (author NGM), subjects were not informed that different TMS timings were employed in the study and when questioned afterwards all indicated that they had not noticed any difference between TMS blocks. In the final session (Experiment 3), pairs of pulses (for 2 blocks of 40 trials per condition) were delivered at times relative to individual subjects’ saccade latencies. Two TMS times were used. The first was the median saccade latency (425 ± 15.4 ms). Inspection of the distribution of latencies showed that the latest time with no saccades made was 200 ms prior to the median latency, and this was used as the second TMS time, with the first of the pair of pulses delivered at this time point. Again, 2 no-TMS blocks were also presented and pro- and antisaccades were interleaved in each block. In all sessions the order of blocks was randomized.
Saccade Analysis
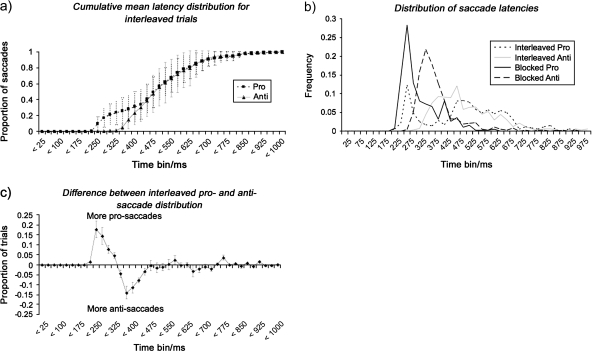
Only correct responses were analyzed. These were defined as saccades with an amplitude of more than 1° and an angle such that it was in the quadrant containing the element toward which a saccade was required. Saccades were automatically identified by the Eyelink system according to acceleration and amplitude criteria (minimum speed 30°/s, minimum acceleration 8000°/s2), meaning that eye drift or small eye movements were not erroneously scored as saccades. Additionally, distributions of saccade latencies for pro- and antisaccades were plotted (see Fig. 2a,b) for all conditions. Inspection of these suggested that there was a subpopulation of responses in the interleaved prosaccade trials compared with the interleaved antisaccade trials and so differences between the 2 distributions were calculated (see Fig. 2c). This showed that there were 2 ranges in which the distributions differed. The latency ranges for these differences agreed with the latencies seen for the same type of trial presented in blocks (i.e., noninterleaved blocks). This suggests that in the interleaved condition, some responses were made which were correct but with latencies suggesting that these were performed in the same way as blocked trials, that is, were correct because a pro- or antisaccade had been made but not following analysis of the singleton with these sometimes being correct for the particular trial presented (see Fig. 2b) and with latencies in the range for pro- or antisaccades, respectively, when presented in blocks. Consistent with this, the number of these types of responses were similar irrespective of whether they were pro- or antisaccades, again suggesting they were made without analysis of the singleton in the array. In summary, the responses on a subpopulation of the interleaved trials had short latencies within the range of those observed when pro- and antisaccades trials were presented blocks. The important point here is that in the blocked condition they would either saccade to or away from the stimulus—in other words, no visual analysis was required. This created a distribution of the prosaccade trials in the blocked condition with a distribution around a shorter mean than in the interleaved condition. In the interleaved condition, there was a bimodal distribution of prosaccade latencies, one distributed around a mean similar to the latencies in the blocked condition and one distributed around a longer mean latency. When we investigated these distributions at the individual subject level, we found that the rapid prosaccades in the interleaved condition and the prosaccades in the blocked condition did not overlap with the longer prosaccades in the interleaved condition. This was not the case for antisaccade trials, where the 2 populations overlapped. We therefore think that the short latencies in the interleaved prosaccade condition are likely to be due to anticipatory response executed without visual analysis. We therefore excluded these saccades in the case of prosaccades in subsequent analysis. Our grounds for this exclusion are 2-fold: the short latency subpopulation in the interleaved prosaccades was statistically significantly different from the longer latency subpopulation in the same condition (t(5) = 10.592, P < 0.001); the short latency subpopulation in the interleaved prosaccades was not statistically significantly different from the population of blocked prosaccades (t(5) = 1.091, P = 0.325). In the case of the antisaccades, there was no statistically significant distribution difference between any ranges of latencies, and all were therefore included in subsequent analysis.
Figure 2.
Distribution of saccade latencies on behavioral trials when the trial types were interleaved. (a) Cumulative distributions suggest a subpopulation of responses in the prosaccades. (b) Distributions of latencies for different saccade and block types. The prosaccade trials can be seen to be made up of 2 distributions with the early one showing the same latencies as blocked prosaccade trials. (c) Differences between the pro- and antisaccade distribution plots. This clearly shows both an early population in the prosaccade trials (positive region) as well as a later population in the antisaccade trials (negative region).
Results
Experiment 1: Behavioural Data
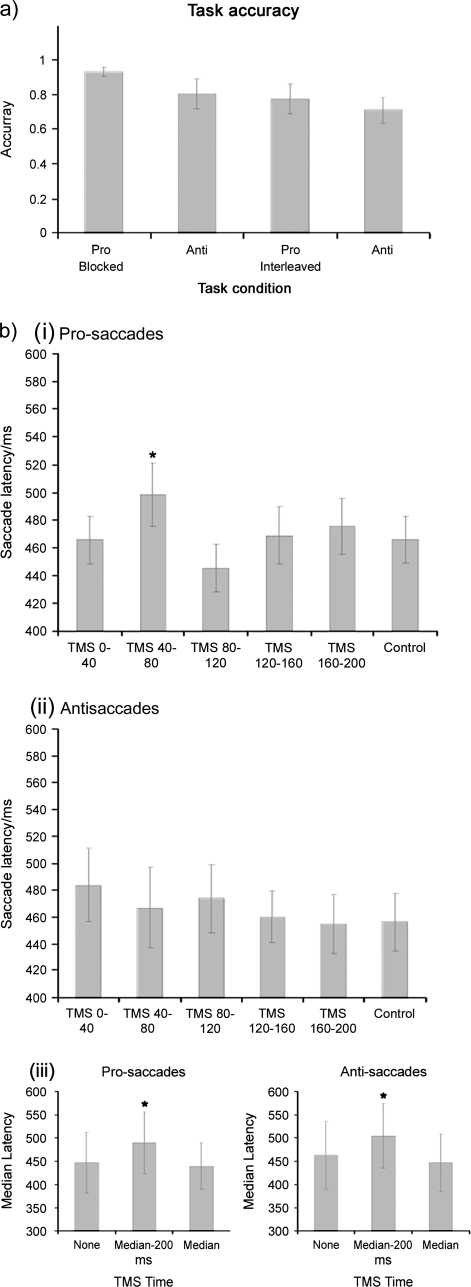
Data are illustrated in Figure 3a. Repeated measures analysis of variance (ANOVA) was used with a factor of blocking (blocked or unblocked trials) and of saccade type (pro- or antisaccade) to evaluate saccade reaction times. Significant main effects of blocking (F(1,5) = 35.38, P = 0.002) and of saccade type (F(1,5) = 10.12, P = 0.025) as well as a significant interaction (F(1,5) = 7.55, P = 0.04) were seen. Post hoc t-tests showed that prosaccades latencies were significantly lower than antisaccades when presented as blocks (P < 0.001) but not when interleaved (P = 0.90). Additionally, both pro- and antisaccades were slower when interleaved than when blocked (P = 0.012 and P = 0.006, respectively). No effects were seen on accuracy.
Figure 3.
Results. (a) Data from behavioral blocks (Experiment 1). (i) There was no difference between pro- and antisaccade latency when these trials were interleaved. Latency distribution curves for each trial type showed that there were 2 times at which these trials did differ. The timing of these differences was consistent with latencies for the respective response types seen when presented as blocks. (b) TMS data. Experiment 2: (i) There was a significant effect of TMS timing on prosaccade latency. Post hoc comparisons showed that this was due to increased latencies when TMS was delivered starting at 40 ms following array onset. (ii) Elevated latencies were not significant for antisaccade trials (possibly due to containing 2 populations of responses). Experiment 3: (iii) For later TMS delivery times, both pro- and antisaccade latencies were significantly increased by TMS prior to but not during saccade execution.
TMS Data
Experiment 2: Early TMS Effects: (Visual Processing)
There were no effects of TMS on prosaccades when the subpopulation of lower latency responses was included in the data analysis (repeated measures ANOVA with a factor of TMS × time: F(5,25) = 1.037, P = 0.418) Subsequent analysis was carried out with the early population of saccades excluded from the prosaccade data (see above).
Data from TMS trials are shown in Figure 3b. Pro- and antisaccade trial latencies and accuracies were each analyzed using repeated measures ANOVA with a factor of TMS time and of saccade type. This showed a significant interaction of time × type (F(5,25) = 2.968, P = 0.031). This was investigated with a repeated measures ANOVA on pro- and antisaccade trials separately with a factor of TMS time. This showed that, for prosaccades, there was a significant effect of TMS time (F(5,25) = 2.942, P = 0.032). Post hoc comparisons showed that this was due to elevated latencies for the 40/80 ms TMS time that were significantly higher than no TMS (P = 0.028) as well as the 80/120 ms and 120/160 ms TMS (P = 0.004 and P = 0.035, respectively). No differences were seen for the antisaccade trials. No effects were seen on accuracy measures.
Experiment 3: Late TMS Effects: (Saccade Processing)
Latencies and accuracies were again analyzed using repeated measures ANOVA with factors of TMS time and of saccade type. This showed a significant main effect of time (F(2,8) = 4.442, P = 0.05) on saccade latencies but no interaction. Post hoc t-tests showed this to be due to elevated latencies when TMS was delivered 200 ms prior to the expected saccade latency (P = 0.025). There were no significant effects on accuracy.
Discussion
Our findings showed a clear temporal dissociation of saccade preparation and attention and were therefore contra to the premotor theory of attention that views saccade generation and spatial attention as interdependent, and in its strongest form, as being controlled by the same process. A prediction of this theory is that prosaccades made as a consequence of the outcome of the processing of the identity of a visual stimulus should be faster than antisaccades that depend on similar processing. If processing the visual stimulus and preparing a saccade were dissociable, then such a benefit should not be present. We investigated the claims of this form of the premotor theory using a task where pro- and antisaccade trials were interleaved, with the required saccade type instructed by a visual stimulus. As such, identification of the orientation of a singleton, defined by color, was required to indicate the type of saccade required on each trial. When blocks consisted of only one type of trial, the pattern of saccade latencies seen were as expected from previous studies, that is, prosaccade blocks showed lower latencies than antisaccade blocks. In contrast, performance when the pro- and antisaccade trials were interleaved thus requiring visual analysis of the singleton for correct performance, there was no significant difference between latencies for the 2 trial types (see Fig. 1a for task details and Fig. 3a for results).
When the trial types are blocked, the shorter latency may be due to the appropriate motor response being known in advance. In the case of prosaccade trials, locating the odd element in the display is all that is needed for the correct response to be made, as locating the target also means the saccade end point is known. In the case of antisaccades, the saccade end point must be derived from this location (i.e., a transformation must be applied), so the saccade latencies may be expected to be higher because of this extra step. Also, it has been shown that the processes that inhibit the tendency to make saccades to a singleton may contribute to the antisaccade cost (Olk and Kingstone, 2003, see also Godijn and Kramer, 2006). In Experiment 2, the interleaving of the pro- and antisaccades within blocks meant that the direction of the saccade to be made is unknown until the singleton in the array has been analyzed. Therefore, the programming of a saccade has to be withheld until this point, after which the saccade end point can be determined. This means that until the end of the visual processing stage, there is no difference between the pro- and antisaccade trials. The absence of a difference in the saccade latencies suggests that there is also no difference between the pro- and antisaccades in terms of how long it takes to determine the appropriate saccade end point and execute the saccade. This inhibitory process may be involved in every trial because subjects could not determine the saccade type to be made until each visual search array was presented. However, in the block design, subjects can predict the saccade direction once they have seen the singleton without any need to analyze it. The saccade RTs in Experiment 1 when the required saccade types were blocked are therefore quicker than those in the Experiment 2 when they were interleaved (for both pro- and antisaccades), as there are always more steps required for successful performance. Furthermore, counter to the premotor predictions, there was no benefit, in terms of speed of responding, when an eye movement was required to the location, which had to be processed for successful saccade selection.
TMS delivered over FEF was used to probe the time course of the involvement of this area in performance of the pro-/antisaccade task. Prosaccades showed increased latencies due to paired pulses of TMS at 2 time points; when the pulses occurred at 40 and 80 ms following presentation of the visual stimulus (at time point consistent with previously reported effects of TMS over FEF on a visual task which did not involve eye movements, O'Shea et al. 2004) and when pulses were delivered at a later time point, prior to saccade execution. Antisaccades were only disrupted when TMS was delivered later, prior to saccade execution (see Fig. 3). The lack of the early effect on antisaccades at the time point when disruption occurred for prosaccades seems to reflect the presence of a subpopulation of saccades seen in the responses made. These had latencies in the range seen on the blocks of trials where all of the responses required were antisaccades (in other words, blocks where the visual analysis of the stimulus was unnecessary). Unlike the equivalent subpopulation for prosaccades, these overlapped with the main population of saccade latencies and so could not be excluded from analysis of these trials (see Fig. 2). This seems to offer a reasonable explanation for the absence of an early effect on antisaccade trials, although manipulating the relative populations of the saccade latencies would be beneficial to confirm this hypothesis (possibly through altering the difficulty of the singleton analysis). Consistent with this, the TMS effects on prosaccade latencies were not present when the faster population of prosaccades was included in the analysis.
Disruption of task performance by TMS delivered over FEF at 2 distinct time windows is incompatible with the view that processing of a target and preparation of a saccade are the same process. The earlier time point of disruption indicates that TMS disrupts the visual selection of the stimulus and that this is distinct from the later disruption of saccade preparation. The early disruption is consistent with the timing of TMS disruption of performance of a visual search tasks that does not require eye movements. When small search arrays were presented for brief durations where eye movements were not necessary for performance (nor were any made), TMS delivered over FEF was found to disrupt performance where the target was defined by a conjunction of features but not when it was defined by a single attribute (Muggleton et al. 2003). Furthermore, the timing of involvement of this area was found to be early, with TMS pulses delivered at 40 and 80 ms following array onset disrupting performance (O'Shea et al. 2004). The early disruption seen in the present experiment is therefore consistent with the effect being due to disruption of processing of the visual stimulus. The second time point of disruption was when TMS was delivered immediately prior to saccade execution, that is, during a period likely to be when saccade preparation is taking place. This is consistent with previous findings that TMS delivered prior to saccade execution increases saccade latencies (Priori et al. 1993; Ro et al. 2002).
The pattern of effects seen in the data reported here strongly support the argument that visual analysis of the target must be completed before the outcome can be used to prepare the appropriate saccade. Thus, although a saccade may be the outcome of visual processing, this link between the 2 processes is neither obligatory nor necessary and, although they may share anatomical circuits, they can be separated temporally and thus constitute 2 processes.
Funding
National Science Council, Taiwan (94-2413-H-008-001; 94-2752-H-010-003-PAE) and Academia Sinica, Taiwan (AS-94-TP-C06) to CHJ, OJLT, and DLH; Wellcome Trust, UK, to VW and NGM.
Acknowledgments
Conflict of Interest: None declared.
References
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci USA. 1998;95:831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Parrish TB, Friston KJ, Mesulam MM. Functional anatomy of visual search: regional segregations within the frontal eye fields and effective connectivity of the superior colliculus. Neuroimage. 2002;15:970–982. doi: 10.1006/nimg.2001.1006. [DOI] [PubMed] [Google Scholar]
- Godijn R, Kramer AF. Prosaccades and antisaccades to onsets and color singletons: evidence that erroneous prosaccades are not reflexive. Exp Brain Res. 2006;172:439–448. doi: 10.1007/s00221-006-0351-8. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. J Cogn Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Kingstone A. Covert and overt voluntary attention: linked or independent? Brain Res Cogn Brain Res. 2003a;18:102–105. doi: 10.1016/j.cogbrainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Hunt AR, Kingstone A. Inhibition of return: dissociating attentional and oculomotor components. J Exp Psychol Hum Percept Perform. 2003a;29:1068–1074. doi: 10.1037/0096-1523.29.5.1068. [DOI] [PubMed] [Google Scholar]
- Juan C-H, Walsh V. Feedback to V1: a reverse hierarchy in vision. Exp Brain Res. 2003;150:259–263. doi: 10.1007/s00221-003-1478-5. [DOI] [PubMed] [Google Scholar]
- Juan C-H, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci USA. 2004a;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan C-H, Campana G, Walsh V. Prog Brain Res. Vol. 144. 2004b. Cortical interactions in vision and awareness: hierarchy in reverse; pp. 117–130. [DOI] [PubMed] [Google Scholar]
- Klein R. Does oculomotor readiness mediate cognitive control of visual attention? In: Nickerson R, editor. Attention and performance. Vol. 8. New York: Academic Press; 1980. pp. 259–2760. [Google Scholar]
- Klein RM. On the control of visual orienting. In: Posner M, editor. Cognitive neuroscience of attention. New York: Guilford Press; 2004. pp. 29–44. [Google Scholar]
- Klein RM, Shore DI. Relations among modes of visual orienting. In: Monsell S, Driver J, editors. Attention and performance XVIII: control of cognitive processes. Cambridge, MA: MIT Press; 2000. pp. 195–208. [Google Scholar]
- Kustov AA, Robinson DL. Nature. Vol. 384. Shared neural control of attentional shifts and eye movements; pp. 74–77. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Juan C-H, Cowey A, Walsh V. Human frontal eye fields and visual search. J Neurophysiol. 2003;89:3340–3343. doi: 10.1152/jn.01086.2002. [DOI] [PubMed] [Google Scholar]
- Olk B, Kingstone A. Why are antisaccades slower than prosaccades? A novel finding using a new paradigm. Neuroreport. 2003;14:151–155. doi: 10.1097/00001756-200301200-00028. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye fields. J Cogn Neurosci. 2004;16:1060–1067. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Science. Vol. 292. 2001. Fast backprojections from the motion to the primary visual area necessary for visual awareness; pp. 510–512. [DOI] [PubMed] [Google Scholar]
- Priori A, Bertolasi L, Rothwell JC, Day BL, Marsden CD. Some saccadic eye movements can be delayed by transcranial magnetic stimulation of the cerebral cortex in man. Brain. 1993;116:355–367. doi: 10.1093/brain/116.2.355. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G. Mechanisms of selective attention in mammals. In: Ewert J, Capranica RR, Ingle DJ, editors. Advances in vertebrate neuroethology. New York: Plenum Press; 1983. pp. 261–297. [Google Scholar]
- Ro T, Farnè A, Chang E. Locating the human frontal eye fields with transcranial magnetic stimulation. J Clin Exp Neuropsychol. 2002;24:930–940. doi: 10.1076/jcen.24.7.930.8385. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38:637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Orienting of attention and eye movements. Exp Brain Res. 1994;98:507–522. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Spatial attention and eye movements. Exp Brain Res. 1995;105:261–275. doi: 10.1007/BF00240962. [DOI] [PubMed] [Google Scholar]
- Shepherd M, Findlay JM, Hockey RJ. The relationship between eye movements and spatial attention. Q J Exp Physiol. 1986;38:475–491. doi: 10.1080/14640748608401609. [DOI] [PubMed] [Google Scholar]
- Smith DT, Jackson SR, Rorden C. Transcranial magnetic stimulation of the left human frontal eye fields eliminates the cost of invalid endogenous cues. Neuropsychologia. 2005;43:1288–1296. doi: 10.1016/j.neuropsychologia.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Stewart LM, Walsh V, Rothwell JC. Motor and phosphene thresholds: a transcranial magnetic stimulation correlation study. Neuropsychologia. 2001;39:415–419. doi: 10.1016/s0028-3932(00)00130-5. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Stell R, Mastaglia FL. Transcranial magnetic stimulation of the human frontal eye field. J Neurol Sci. 1996;144:114–118. doi: 10.1016/s0022-510x(96)00194-3. [DOI] [PubMed] [Google Scholar]
- Wasserman EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]