Sir,
We reported the in vitro activity of tigecycline against 148 strains of Acinetobacter spp. from the infectious diseases research laboratory at Siriraj Hospital, Bangkok, Thailand, which showed that only 3.4% of the Acinetobacter spp. strains were considered resistant to tigecycline due to their inhibition zone diameters (<13 mm) and MICs determined by broth microdilution (>2 mg/L).1 The service microbiology laboratory of our hospital has reported tigecycline disc diffusion susceptibility results for Acinetobacter spp. based on the interpretative breakpoints proposed by Jones et al.,2 which indicated that most clinical isolates of Acinetobacter spp. were not susceptible to tigecycline. There were several observations on the effect of the susceptibility test media on the MICs of tigecycline. Hope et al.3 found that the MIC of tigecycline was raised in aged media. Fernandez-Mazarasa et al.4 reported that high concentrations of manganese in Mueller–Hinton agar (MHA) increased the MIC90 of tigecycline for A. baumannii determined by Etest from 1 to 4 mg/L. The service microbiology laboratory at Siriraj Hospital used MHA (Oxoid), whereas the infectious diseases research laboratory used MHA [Becton–Dickinson (BD)] for disc diffusion susceptibility for Acinetobacter spp. Therefore, a discrepancy in tigecycline susceptibility for Acinetobacter spp. might be due to a difference in the type of MHA.
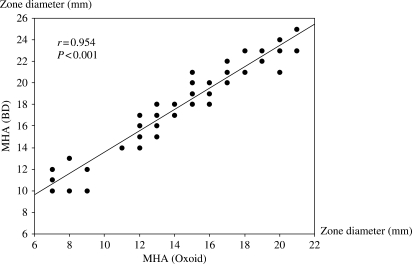
We performed tigecycline disc diffusion susceptibility for 102 strains of Acinetobacter spp. isolated from different patients. These isolates were resistant to all β-lactams, aminoglycosides and fluoroquinolones. In vitro susceptibility of Acinetobacter spp. to tigecycline was determined by the Kirby–Bauer disc diffusion method using a paper disc containing 15 µg of tigecycline per disc (BD, USA). The MHAs were purchased from BD (Thailand) and Oxoid Company (Thailand), and they were freshly prepared. The methodology for susceptibility testing was performed by direct colony suspension according to the guidelines suggested by the CLSI.5 Quality control was performed by testing the susceptibility of Escherichia coli ATCC 25922. The susceptibility tests using both types of MHAs were performed concurrently under identical conditions. The comparison of tigecycline inhibition zone diameters on MHA (BD) and MHA (Oxoid) for 102 isolates of Acinetobacter spp. is shown in Figure 1. The inhibition zone diameters observed on MHA (Oxoid) were consistently smaller than those on MHA (BD), with a mean difference of 3.5 mm and a range from 1 to 6 mm. The inhibition zone diameters on MHA (Oxoid) and MHA (BD) were significantly correlated (r = 0.95, P < 0.001). The distribution of tigecycline disc diffusion susceptibility for Acinetobacter spp. performed on MHA (BD) and MHA (Oxoid) is shown in Table 1. The susceptibility of Acinetobacter spp. to tigecycline using MHA (BD) was 86.2%, whereas that using MHA (Oxoid) was only 28.5%. We also measured the content of manganese in both types of MHAs by atomic absorption and found that the content of manganese in MHA (Oxoid) was three times more than that in MHA (BD). Therefore, the discrepancy in inhibition zone diameters between MHA (Oxoid) and MHA (BD) might be due to a difference in the manganese content in MHAs. However, there could be other associated factors for such a discrepancy, and more studies are required. The aforementioned observations warrant a clinical study to determine the efficacy of tigecycline for therapy of Acinetobacter spp. infections in order to consider which type of MHA is more appropriate for tigecycline disc diffusion susceptibility for Acinetobacter spp. Meanwhile, the results of tigecycline disc diffusion susceptibility for Acinetobacter spp. in MHA (Oxoid) should be cautiously reported and interpreted.
Figure 1.
Comparison of tigecycline inhibition zone diameters on MHA (BD) and MHA (Oxoid) for 102 isolates of Acinetobacter spp.
Table 1.
Distribution of tigecycline disc diffusion susceptibility for Acinetobacter spp. performed on MHA (BD) and MHA (Oxoid)
| Inhibition zone diameter (mm) | MHA (Oxoid) | MHA (BD) |
|---|---|---|
| ≤12 (resistant) | 14 (13.7%) | 7 (6.9%) |
| 13–15 | 59 (57.8%) | 7 (6.9%) |
| ≥16 (susceptible) | 29 (28.5%) | 88 (86.2%) |
Funding
None.
Transparency declarations
None to declare.
References
- 1.Thamlikitkul V, Tiengrim S, Tribuddharat C. Comment on: High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2007;60:177–8. doi: 10.1093/jac/dkm142. [DOI] [PubMed] [Google Scholar]
- 2.Jones RN, Ferraro MR, Reller LB, et al. Multicenter studies of tigecycline disk diffusion susceptibility results for Acinetobacter spp. J Clin Microbiol. 2007;35:227–30. doi: 10.1128/JCM.01588-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hope R, Warner M, Mushtaq S, et al. Effect of medium type, age and aeration on the MICs of tigecycline and classical tetracyclines. J Antimicrob Chemother. 2005;56:1042–6. doi: 10.1093/jac/dki386. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Mazarasa C, Mazarrasa O, Calvo J, et al. High concentrations of manganese in Mueller–Hinton agar increase MICs of tigecycline determined by Etest. Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007; Chicago, IL, USA. Washington, DC, USA: American Society for Microbiology; Abstract D-227, p. 154. [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Fifteenth Informational Supplement M100-S15. Wayne, PA, USA: CLSI; 2005. [Google Scholar]