Abstract
Regeneration of bone is driven by the action of numerous biomolecules. However, most osteobiologic devices mainly depend on delivery of a single molecule. The present studies were directed at investigating a polymeric system that enables localized, alternating delivery of two or more biomolecules. The osteotropic biomolecules studied were simvastatin hydroxyacid (Sim) and parathyroid hormone (1-34) (PTH(1-34)), and the antimicrobial peptide cecropin B (CB) was also incorporated. Loaded microspheres were made using the complexation polymer system of cellulose acetate phthalate and Pluronic F-127 (blend ratio, 7:3). By alternating layers of the different types of microspheres, 10-layer devices were made to release CB and Sim, CB and PTH, or Sim and PTH. In vitro experiments showed five discrete peaks for each molecule over a release period of approximately two weeks. MC3T3-E1 osteoblastic cells alternately exposed to the osteotropic biomolecules showed enhanced proliferation and early osteoblastic activity. Alternating delivery of 10 nM Sim and either 500 pg/ml or 5 ng/ml PTH showed additive effects compared to the CB/Sim or CB/PTH devices. These implantable formulations may be useful for alternating delivery of different biomolecules to stimulate concurrent biological effects in focal tissue regeneration applications.
Keywords: Controlled release, Alternating release, Simvastatin, Parathyroid hormone (1-34), Cecropin B
INTRODUCTION
Controlled release technology enables delivery of a variety of biomolecules at the proper time, in the appropriate amount, and to the desired site. Most commonly, investigators seek to release one drug at constant concentrations for extended periods. However, such zero-order kinetics can be undesirable. For example, parathyroid hormone (1-34) (PTH(1-34)) can have either anabolic or catabolic effects on bone, depending on the mode of delivery. Daily, intermittent administration of low doses of PTH stimulates bone formation and increases bone mass in animals and humans in vivo [1–3], whereas continuous exposure has a catabolic effect [4–6].
Simvastatin (Sim), a member of the statin family of drugs known for lowering serum cholesterol, also has been reported to stimulate bone formation. Sim induces expression of bone morphogenetic protein 2 (BMP-2) [7,8], and daily subcutaneous injections of Sim stimulated bone formation on the calvaria of mice [7]. Other studies showed that oral dosing [9,10] as well as localized delivery [11–13] can enhance osteogenesis in different animal models.
Regeneration of bone in focal defects, such as fracture gaps and periodontal lesions, is of significant clinical and commercial interest, with an estimated 500,000 bone grafting procedures per year in the U.S. alone [14]. Healing in such sites can be complex and involves sequential cascades of events [15]. Furthermore, they face the threat of microbial infection. Infections in bony sites can be difficult to treat, often requiring multiple surgeries, prolonged antibiotic treatment, and causing long-term complications [16]. Therefore, every effort is required to prevent this problem with early treatment. Cecropin B (CB) is a naturally occurring cationic, amphipathic peptide derived from moths that has broad spectrum activity against not only certain gram-positive bacteria but also gram-negative bacteria, fungi, and parasites [17–19].
Currently, therapies for tissue regeneration in the body mainly rely on the delivery of single biomolecules. Microspheres, nanospheres, and porous scaffolds comprising numerous polymers, such as polylactides, polyglycolides, poly(lactide-co-glycolides), polyanhydrides, and polyorthoesters, have been developed for delivery of one biomolecule at a time [20–26], but comparatively few studies have investigated delivery of multiple biomolecules [27–30]. However, multiple signals are required to drive the regeneration process to completion in a timely and efficient manner, and consequently the success of current efforts releasing a single biomolecule to the defect site may be limited [29]. An approach to overcome such limitations is release of multiple molecules with distinct kinetics to trigger the desired cell and tissue responses.
In previous studies, we further developed a controlled release system based on blends of cellulose acetate phthalate (CAP) and Pluronic F-127 (PF-127) for intermittent release of small and large biomolecules [13,31,32]. The CAP/PF-127 polymer system can be used for programmed drug delivery, with the rate, duration, and amount of released drug controlled by blending ratio, number of layers, and thickness [31–33].
The objective of this research was to investigate the CAP/PF-127 system for alternating delivery of different biomolecules and to determine their effects on osteoblastic cells.
MATERIALS AND METHODS
CAP/PF-127 Microspheres
Cecropin B (Anaspec, San Jose, CA) and PTH(1-34) (Bachem Torrance, CA) were labeled with Alexa Fluor 350 carboxylic acid, succinimidyl ester (Molecular Probes, Eugene, OR) and Alexa Fluor 488 carboxylic acid, succinimidyl ester (Molecular Probes), respectively, to enable measurement of the released amounts. Simvastatin (Aldrich, Milwaukee, WI) was hydrolyzed to its active hydroxyacid form, which also increases water solubility [31]. CAP (Fluka, Buchs, Switzerland) and PF-127 (Sigma, St Louis, MO) microspheres were made by a water-acetone-oil-water (W/A/O/W) triple emulsion process as reported previously [31,32]. In brief, CAP and PF-127 polymer blend (7:3 ratio fraction of CAP to PF-127) was dissolved in acetone. Phosphate-buffered saline (PBS), pH 7.4, containing CB (250 or 500 µg/ml), PTH(1-34) (50 or 100 µg/ml), or Sim (420 or 840 µg/ml) was added to the polymer solution, homogenized into corn oil, and then sonicated for 20 seconds. The CAP/PF-127 suspension and 5% Triton X-100 solution were added to deionized water and then stirred for 5 minutes to harden the microspheres. Following collection, microspheres were washed, filtered, and lyophilized overnight. Blank (drug-free) microspheres served as controls.
Release Devices
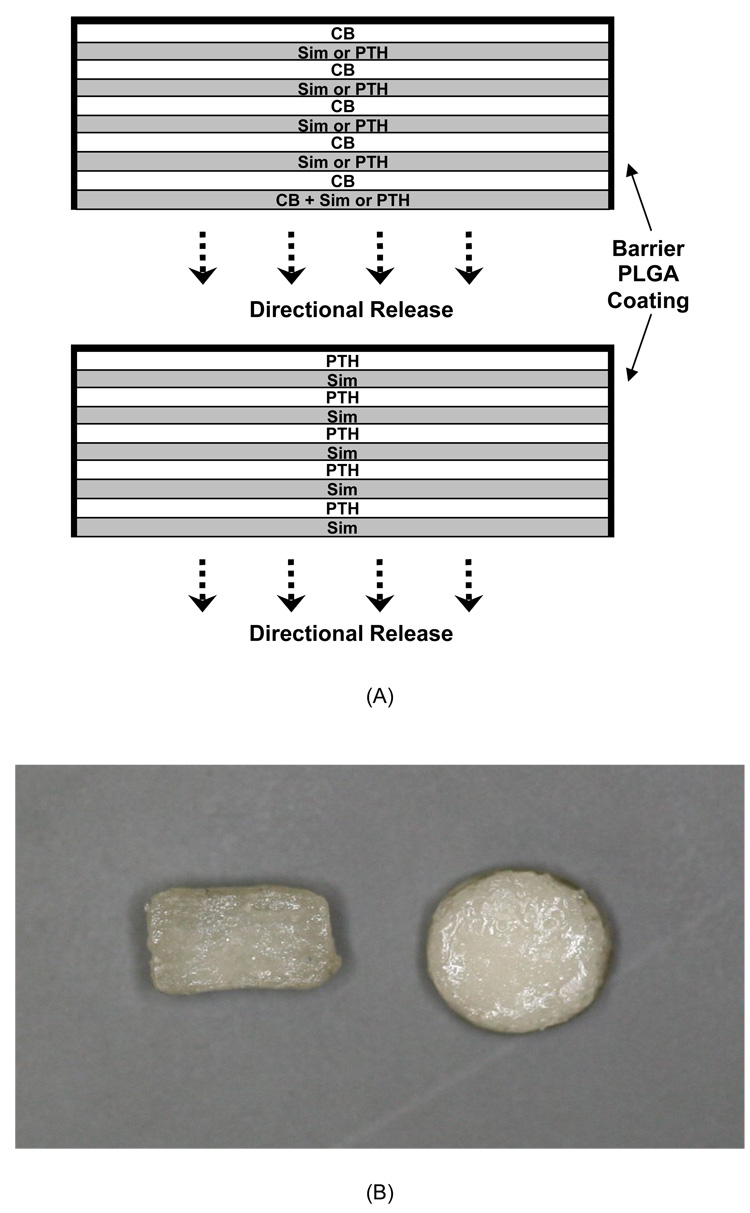
Release devices were fabricated using a pressure-sintering process and consolidated in acetone vapor. The first layer of CB/Sim and CB/PTH devices was made with 20 mg of mixed UV-sterilized microspheres (10 mg of each type). They were placed in the wells of a Delrin mold and consolidated by applying 20 MPa pressure for 5 sec. Next, microspheres containing only CB were added on top of the first layer, and pressure was reapplied. The third layer consisted of only Sim-containing microspheres for CB/Sim release devices or PTH-loaded microspheres for CB/PTH devices. Ten-layer devices (diameter, 6.2 mm; thickness, ~3 mm) were made by repeatedly alternating CB and Sim or PTH(1-34). Sim/PTH release devices were made by alternating layers of PTH- and Sim-containing microspheres. Blank microspheres were used throughout to make control devices. To provide directional control of drug release, the bottom and sides of the devices were coated three times with 10% poly(lactic-co-glycolic acid) (75:25, Mw~75 kDa; Alkermes, Cincinnati, OH) solution in methylene chloride. Figure 1 depicts the design of the three devices and shows images of the finished devices.
Figure 1.
A) Schematic representation of CB/Sim, CB/PTH, and Sim/PTH devices. B) Cross-sectional and top view images of the finished devices.
Characterization of Microspheres
Drug content was determined by dissolving 100 mg of microspheres in 5 ml of PBS followed by precipitation of CAP in the solution with 50 µl of 0.1 M hydrochloric acid. After centrifugation at 190g for 5 minutes, supernatants were placed into 96-well assay plates, along with standard solutions of Sim, fluorophore-labeled CB, and fluorophore-labeled PTH. Each standard was made by serial diluting from a known stock solution. Concentrations of released Sim, CB, and PTH(1-34) were determined fluorometrically using a SpectraMAX Gemini XS (PTH(1-34): λex=346 nm, λem=442 nm; CB: λex=495 nm, λem=519 nm; and Sim: λex=390 nm, λem=413 nm). Mean diameter of microspheres was measured using a Partica LA-950 Laser Diffraction Particle Size Analyzer (Horiba Instruments, Irvine, CA). For the size measurements, the following refractive indices were used: D.I. water, 1.333; CAP, 1.475.
In Vitro Release
For release experiments, samples were immersed in 5 ml of 150 mM PBS, pH 7.4, and incubated at 37°C with gentle shaking. All of the supernatant was collected and replaced daily to maintain sink conditions and a constant volume. To allow quantification of released biomolecules, CAP was precipitated from the supernatant by treatment with 50 µl of 0.1 M hydrochloric acid, and fluorescence was measured as described in the previous section. In order to investigate pH changes, devices were immersed in 15 ml of 150 mM PBS, pH 7.4, at 37°C, and the pH was measured each day.
Cell culture
MC3T3-E1 preosteoblastic cells (CRL-2593; ATCC, Manasas, VA) were seeded at a density of 15,000/cm2 into 24-well tissue culture plates in α-Minimum Essential Medium (MEM) containing 10% fetal bovine serum (GIBCO/Invitrogen, Carlsbad, CA), 50 µg/ml ascorbic acid (Sigma), and 5 mM ß-glycerophosphate (Sigma). In CB/Sim, CB/PTH, and Sim/PTH release cultures, cells were alternatingly exposed to CB and Sim, CB and PTH, and Sim and PTH(1-34), respectively, according to the schedule of the release studies described above. Concentrations were diluted to appropriate in vitro levels of 1 µg/ml CB, 100 pM-1 µM Sim, and 500 pg/ml to 50 ng/ml PTH(1-34). In control cultures, medium was changed on the same schedule, but it did not contain biomolecules. Previous studies examined cell responses to sustained levels of Sim [31] and PTH(1-34) [34].
DNA and AP Assays
After 3, 7, 10, and 14 days of culture, cells were harvested and then lysed by sonication in high salt solution (0.05M NaH2PO4, 2M NaCl, and 2mM EDTA). DNA standards were prepared by serial diluting calf thymus DNA in the high salt solution. Hoechst 33258 (final concentration, 0.5 µg/ml; Sigma) was added to DNA standards and samples and allowed to react in the dark for 10 minutes [35]. DNA content of the lysates was quantified by measuring fluorescence (λex=356 nm, λem=458 nm).
Alkaline phosphatase (AP) activity was determined by a colorimetric assay. Lysates were incubated with substrate solution prepared by dissolving 10 mM of Sigma 104 phosphatase substrate in Sigma 221 alkaline buffer solution. After 30 minutes, 0.25 N NaOH was added to each well to immediately stop enzyme activity. Absorbance at 410 nm was measured with an MR5000 microplate reader (Dynatech Laboratories, Chantilly, VA), and the amount of substrate cleaved was determined using ε=1.7×104 M−1cm−1. Activity was expressed as nM of substrate cleaved per minute and then normalized by DNA content.
Statistical Analysis
All data were analyzed by analysis of variance (ANOVA) using InStat (Graphpad Software, San Diego, CA). Post-hoc comparisons were made using the Tukey-Kramer Multiple Comparisons Test when the p-value was significant (p<0.05).
RESULTS
Characterization of Microspheres
Characteristics of the three types of biomolecule-containing and blank microspheres are shown in Table 1. Microsphere yields were similar for all types. Drug content per g polymer and encapsulation efficiencies of CB, Sim, and PTH(1-34) microspheres were 11.2, 85.1, and 111 µg and 53.1, 47.2, and 39%, respectively. The mean size of blank, CB-, Sim-, and PTH(1-34)-loaded microspheres was 104, 161, 141, and 159 µm, respectively. Compared to blank microspheres, loaded particles were 37–56% larger in mean size (p<0.05).
Table 1.
Characteristics of microspheres used to make the release devices.
| Initial CAP/PF- 127 (g) |
Drug Added (µg) |
M.S. Harvested (g) [Yield (%)] |
Total Drug Content (µg) [E.E.* (%)] |
Drug Content per g Polymer (µg) |
M.S. Size (µm) | |
|---|---|---|---|---|---|---|
| PTH | 1.4/0.6 = 2 | 50 | 1.65±0.12 [82.7±7.5] |
24.6±9.1 [39±6.9] |
11.2 | 159.1±90.2 |
| Cecropin B | 1.4/0.6 = 2 | 250 | 1.56 ±0.22 [78.2±13.7] |
132.8±17.5 [53.1±13.1] |
85.12 | 161.2±113.3 |
| Simvastatin | 1.4/0.6 = 2 | 420 | 1.79 ±0.30 [89.4±16.7] |
198.6±18.7 [47.2±9.4] |
111.1 | 141.2±89.8 |
| Blank | 1.4/0.6 = 2 | -- | 1.67±0.22 [88.5±13.7] |
-- | -- | 103.9±67.9 |
Encapsulation efficiency
Data are the mean of replicates from four batches of microspheres.
Sample pH and Release Profiles
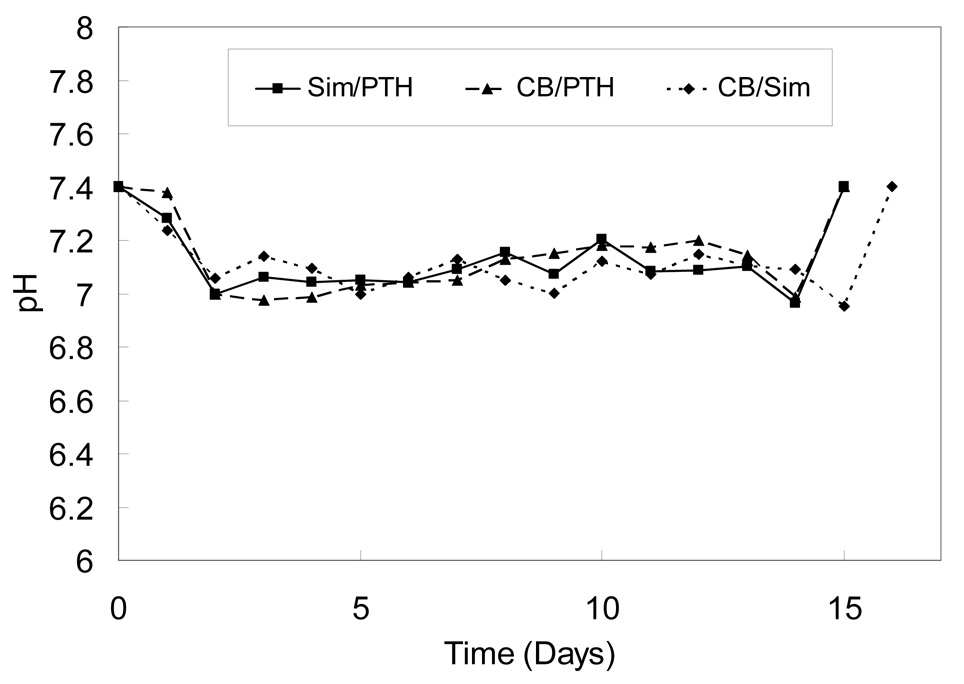
CB/PTH and Sim/PTH devices degraded over a period of 14 days, and CB/Sim devices degraded over 15 days. Figure 2 shows the pH at each day for the three different devices developed. Solution pH decreased from 7.4 to 6.96–7.04 over the first 1–2 days and then was stable until the last time points, when the devices were fully eroded as demonstrated by the absence of CAP/PF-127 polymer (data not shown). The ranges of pH for CB/Sim, CB/PTH, and Sim/PTH devices were statistically similar at 6.95–7.23, 6.97–7.37 and 6.96–7.28, respectively.
Figure 2.
Supernatant pH during degradation of blank 10-layer devices. Data are the mean of six replicates. Error bars, which ranged from 11–28% of the mean, are not shown to prevent obscuring the curves.
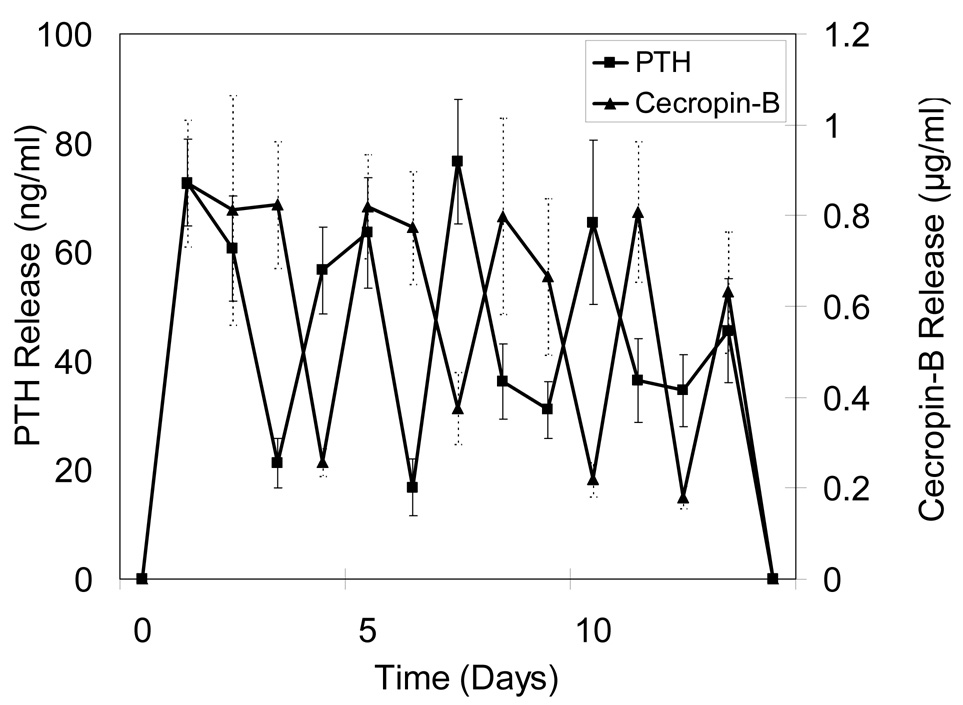
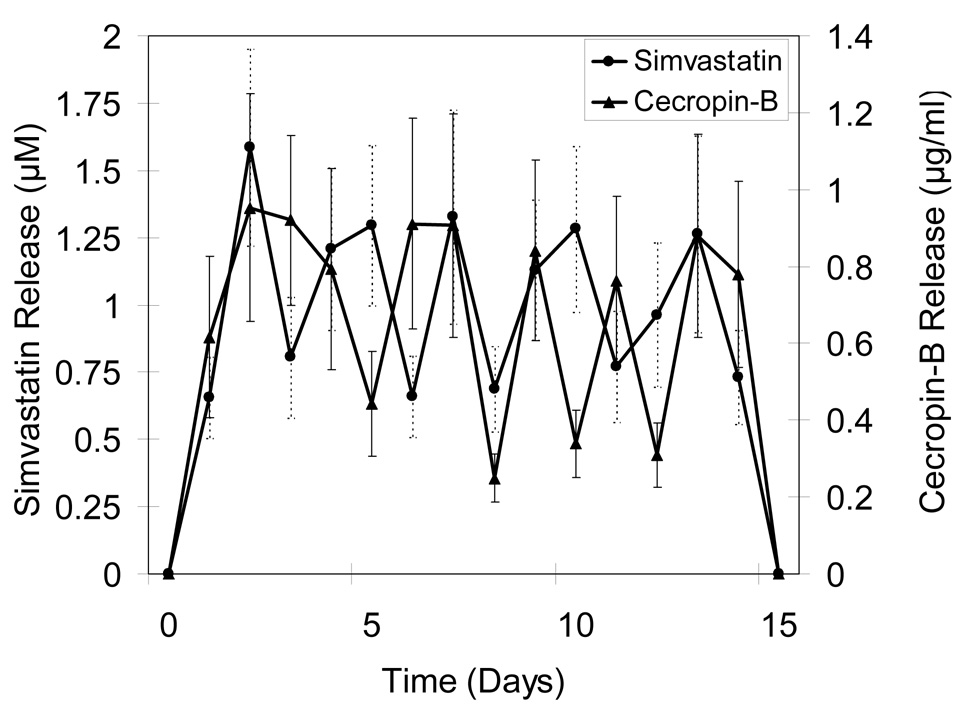
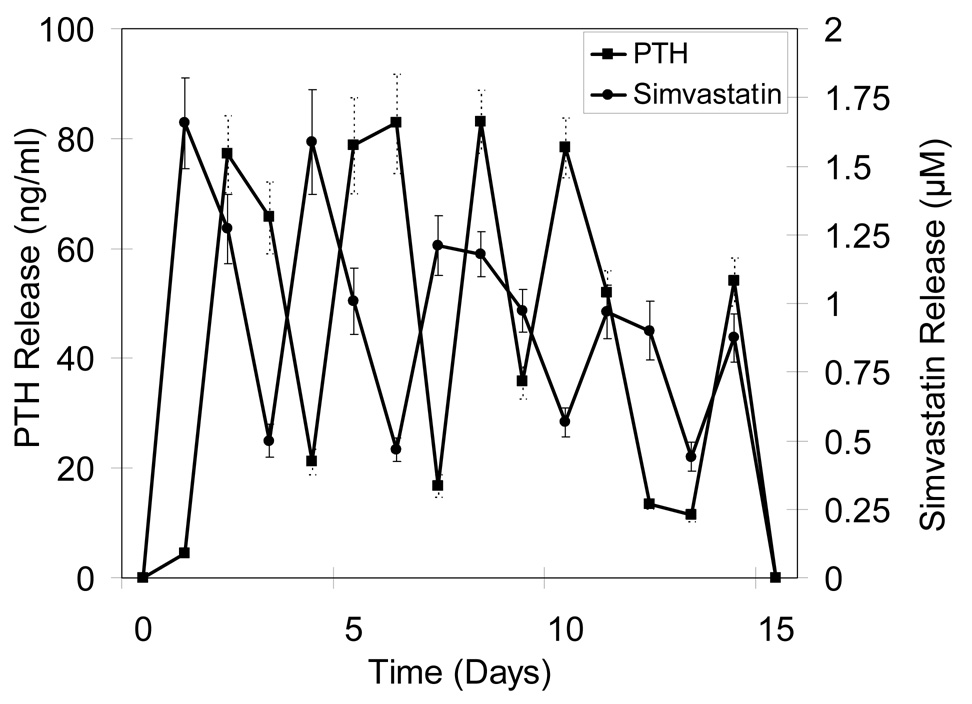
Alternating release profiles were achieved by sequentially layering microspheres loaded with different biomolecules. Figure 3a shows release profiles for devices designed to alternately release CB and PTH(1-34). Overall, five discrete release peaks were observed each for CB and PTH(1-34). To prevent early stage infection, CB release was maintained for three days at the beginning. During the release period, CB showed uniform peak concentrations (0.68–0.94 µg/ml), and PTH(1-34) also had relatively even maximum release (59–78 ng/ml), except for the last peak when integrity of the devices was lost. Figure 3b shows release characteristics of devices alternately fabricated with CB and Sim. Again, prolonged CB release along with a shorter delivery of Sim was observed over the first three days and was followed by four subsequent release peaks for each component over a 15 day period. The range of concentrations of CB and Sim were 0.76–0.95 µg/ml and 1.26 –1.56 µM, respectively.
Figure 3.
Release profiles for 10-layered devices containing A) CB/PTH, B) CB/Sim, and C) Sim/PTH. Data are mean ± standard deviation (n=6).
Figure 3c shows results for devices alternately loaded with Sim and PTH(1-34). Each profile had five distinguishable peaks. For Sim release, the first two peaks were sharp and high (1.58–1.65 µM), but the next two were relatively broad and gave lower concentrations (0.97–1.12 µM) than first two. PTH(1-34) peaks showed relatively even release for the first four (77.2–83.16 ng/ml), but a lower concentration was observed for the last (54.1 ng/ml).
Effect of Combined Delivery of Biomolecules on Osteoblastic Cells
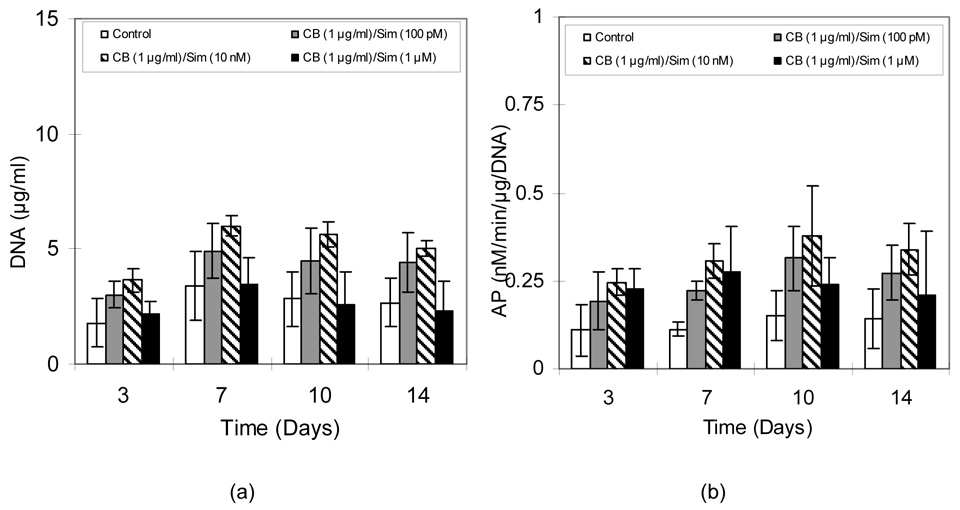
Figure 4a shows that DNA contents in all MC3T3-E1 cultures treated with CB/Sim increased until the seventh day and then showed relatively unchanging levels. This observation reflects that after being seeded at moderately high density, the cells proliferated to confluence, after which only slight multilayering was seen (confirmed by microscopic inspection; not shown). Cells exposed to 1 µg/ml CB with 10 nM Sim showed higher DNA contents (5.64 µg/ml) than any other group (p<0.05). AP activity in all groups increased through the tenth day and then decreased, except for the 1 µM Sim group, which was flat (Figure 4b). As was observed for DNA content, the 10 nM Sim group had the highest AP activity (p<0.05). Unlike DNA content, however, AP activity was higher for the 1 µM Sim group compared to controls (p<0.05).
Figure 4.
A) DNA content and B) AP activity in MC3T3-E1 cultures exposed to alternating CB and Sim. Data are mean ± standard deviation (n=6).
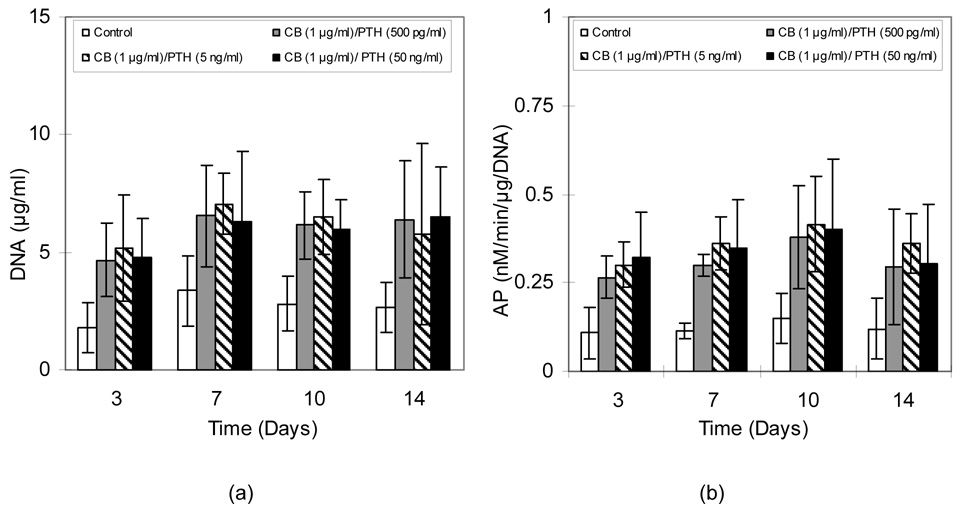
As shown in Figure 5a, all CB and PTH(1-34) treatment groups had higher amounts of DNA (2.99-7.06 µg/ml) than did the control cultures (1.78– 3.37 µg/ml) (p<0.05). Although not significantly different, cultures treated with 1 µg/ml CB and 5 ng/ml PTH(1-34) showed slightly higher DNA content as the cells continued to proliferate and multilayer until day 10. As seen for DNA contents, all CB and PTH(1-34) treatment groups had higher AP activity (0.26-0.4 nM/min/µg/DNA) than did the controls (0.11–0.15 nM/min/µg/DNA) (p<0.05) (Figure 5b). Activity gradually increased through the tenth day and then decreased slightly for all groups.
Figure 5.
A) DNA content and B) AP activity in MC3T3-E1 cultures exposed to alternating CB and PTH. Data are mean ± standard deviation (n=6).
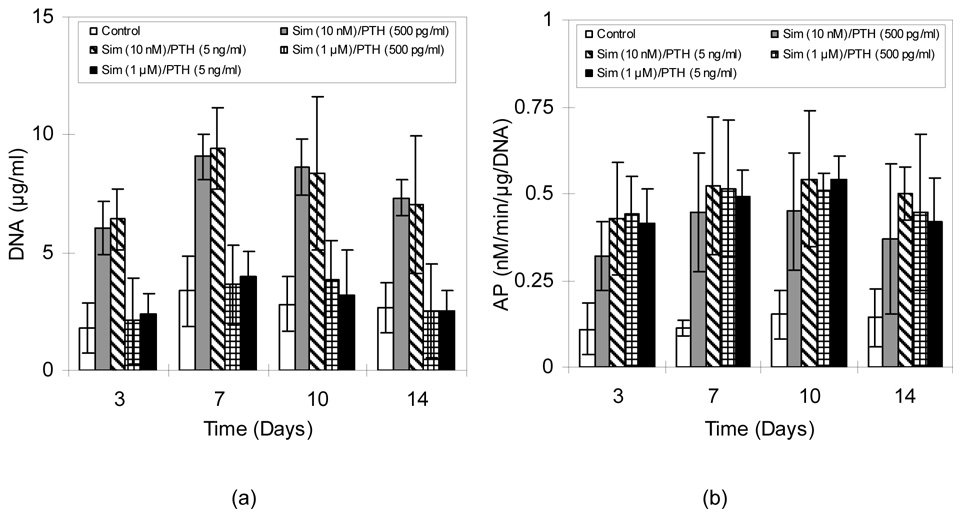
In cultures exposed to alternating Sim and PTH(1-34), DNA contents mainly depended on Sim concentration (Figure 6a). DNA contents for cells treated with 1 µM Sim were only marginally higher than controls at the early time points, whereas treatment with 10 nM Sim resulted in 163–259% greater amounts of DNA than the controls (p<0.01) as the cells quickly grew to confluence and began to form multilayers. Furthermore, these two groups (10 nM Sim plus either 500 pg/ml or 5 ng/ml PTH(1-34)) showed 39.8–101% and 14.6–40.3% higher DNA content than CB/Sim and CB/PTH groups, respectively (p<0.05). Alternating Sim and PTH cultures showed higher AP activity than did the control groups (p<0.05) (Figure 6b). Furthermore, even 1 µM Sim treatment stimulated higher AP activity than in control groups throughout the two week experiments (p<0.05).
Figure 6.
A) DNA content and B) AP activity in MC3T3-E1 cultures exposed to alternating Sim and PTH. Data are mean ± standard deviation (n=6).
DISCUSSION
The present study demonstrates alternating delivery of different biomolecules and shows that such exposure increases cell proliferation and early osteoblastic activity, which are important first steps to enhancing osteogenesis. The profiles were based on reports of intermittent delivery of Sim and PTH(1-34) both promoting osteoblastic responses and bone formation [5,7,12,36–39]. These erodible polymeric devices, developed to allow intermittent release of a single molecule, alternating release of different molecules, or sequential release of multiple molecules, may offer a powerful approach in bone regeneration as well as in other medical therapies that involve multiple biologically active agents. Rather than being intended for subcutaneous implantation and subsequent systemic distribution of the encapsulated molecules, the formulations would be for use in treating focal defects, such as fracture nonunions or periodontal lesions.
The present formulations comprise CAP, which is a well known enteric coating material in the pharmaceutical industry (e.g., [40]), and the nonionic surfactant, PF-127, which is used for stabilizing proteins and peptides (e.g., [41]). Whereas effects following intravenous and intramuscular delivery of Pluronics have been studied [42–44], parenteral use of CAP is comparatively untested. Studies in which 10-layer CAP/PF-127 devices were implanted in rats, however, demonstrated an absence of adverse effects [13]. Regardless, the objective of this work was to illustrate alternating release of bioactive molecules for potential use in local tissue regeneration applications.
Drug loading and encapsulation efficiency are affected by volume of organic solvent used in dissolving polymer and the initial drug concentration [45,46]. A smaller volume of organic solvent in the system promotes precipitation of polymer. Drug loading increases with decreasing organic solvent volume and with increasing drug concentration. The present drug loadings and encapsulation efficiencies differed for the three types of biomolecules used because the amounts of CB, Sim, and PTH(1-34) incorporated were selected to achieve biologically relevant release concentrations. The size of CAP/PF-127 microspheres used for the release devices can be controlled by modifying the emulsification process, such as by altering agitation velocity, time, temperature, and the parameters that influence the kinematic viscosity, including concentration of polymer and emulsifier [47]. In these experiments, microsphere sizes ranged from 104 to 161 µm. It is thought that because other processing parameters were kept the same, the size and nature of the molecules encapsulated were the main reasons for the differences. Because microspheres are subsequently consolidated into a thin, solid layer of polymer, initial variations in particle size are not expected to affect release behavior of the devices, which is primarily dependent on exposed surface area and ratio of CAP to PF-127.
Blends of CAP and PF-127 form a complexation polymer that undergoes surface erosion and shows zero-order release [31,32]. A number of ether sites in PF-127 and carboxylic acid groups in CAP form hydrogen bonds. When exposed to an aqueous environment, carboxyl groups on CAP become deprotonated, and hydrogen bonds with ether groups in PF-127 are consequently lost, resulting in surface erosion of the material. Deprotonation also results in locally decreased pH. Measurement of pH showed only slight acidification below PBS’s initial pH of 7.4. Supernatants remained close to neutral, with the lowest pH of just 6.95 occurring at the last time point sampled when the material was finally completely eroded. Disthabanchong et al. reported that a slightly acidic condition (pH 7.1) up-regulated PTH/PTHrP receptors and increased PTH binding to osteoblast-like cells compared to pH 7.4 [48]. The slight acidification measured in the present experiments would not be expected to adversely affect differentiation of mesenchymal cells, which is reported to occur down to pH 6.8 [49], and may even up-regulate expression of PTH receptors by cells. Implantation of CAP/PF-127 devices on the calvaria of rats verified the absence of detrimental effects [13].
The CAP/PF-127 system is a flexible platform for controlled release. The type and number of molecules that can be used are not restricted. Small molecule drugs, peptides, and proteins have been released [31,32,34]. The duration of release from each layer can be controlled via the amount of microspheres used and the ratio of CAP:PF-127 [31,32]. Furthermore, size of the formulations is scalable, however the ultimate thickness of the device resulting from additional layers could be a constraint. The maximal allowable size would depend on the particular tissue defect requiring treatment.
In the present study, two of the biomolecules were used because of their osteotropic effects and one because of its antimicrobial activity. Many in vitro and in vivo studies have been conducted to explain the anabolic effect of intermittent administration of PTH on bone formation. PTH enhances proliferation of primary osteoblastic cells from humans and animals [50,51]. In addition, PTH exerts diverse effects on osteoblast differentiation depending on differentiation stage [36,52,53]. Simvastatin promotes bone formation associated with increasing BMP-2 transcription both in vitro and in animal models [7,8,11,12]. To provide the ability to prevent infection, which can have devastating consequences when present in bone, the antimicrobial peptide CB was used instead of traditional antibiotics. In addition to their broad-spectrum activity [17,19], as naturally occurring agents in host defenses, antimicrobial peptides are considered less likely for microorganisms to develop mechanisms for resistance.
Devices used in this study were fabricated to fluctuate between maximum and minimum release of drug at approximately daily intervals, and it was expected that these changes would occur in a regular way. Ideally, the minimum concentrations in each profile would decrease to zero, because the adjacent layers do not contain the same biomolecules. However, low but measurable concentrations of drug were detected. It is thought that during fabrication of the multilayered devices, some microspheres in adjacent layers became intermingled under pressure, resulting in “contamination” of neighboring layers with the other drugs. Interlayer diffusion during incubation at 37°C also may have contributed to lowering and broadening of release peaks at later times. If absolutely zero concentration between peaks was needed, thicker layers could be made, although at the expense of increased overall thickness of the device. In some release profiles, the last one or two peaks were lower than the first peaks because the drug may have penetrated the PLGA coating before the device finished degrading. A less permeable coating could be applied, but it also should be degradable, with a time course that avoids chronic inflammation. To maintain a stricter range of concentrations, drug loading of microspheres used for the last layers could be increased, if necessary. It is also possible that the true minimum concentrations were missed by daily analyses, and more frequent sample collection would give more precise information about release profiles.
Cultures treated with CB/Sim and CB/PTH showed increased DNA content and AP activity, except those exposed to 1 µM of simvastatin had an adverse effect on DNA content compared to other concentrations. This result corresponds to previous findings, in which too much (sustained) inhibition of the HMG-CoA reductase pathway was cytotoxic or less effective than pulsatile exposure to simvastatin [31]. Similarly, pulsatile delivery of PTH(1-34) has been shown to be more effective in stimulating osteoblastic responses than does continuous exposure [34]. Sim/PTH delivery devices stimulated greater DNA content and AP activity compared to CB/Sim and CB/PTH devices. The alternating combination of Sim and PTH(1-34) had more of an additive than a synergistic effect, however. Additional combinations of concentrations could be investigated to maximize biological effects. The mechanism of Sim action on bone formation is different from that of PTH. PTH stimulates multiple intracellular signal pathways, mediated by cAMP, and activates both protein kinase A (PKA) and C (PKC) [51]. Sim induces BMP-2 transcription presumably through inhibition of HMG-CoA reductase activity and thus protein prenylation, resulting in the depletion of mevalonate in osteoblasts [54]. However, the roles of each signal transduction system in osteoblast proliferation and differentiation resulting from the CAP/PF-127 release devices are unknown and beyond the scope of the present work. Because of the significantly different nature of cells needed for testing bioactivity of CB, detailed results are not presented here. Preliminary studies have confirmed the antibacterial activity of CAP/PF-127 release supernatants in cultures of E. coli and A. actinomycetemcomitans, and more comprehensive testing against a panel of microorganisms is ongoing.
Compared to the vast amount of literature on drug delivery, relatively few reports describe controlled release of multiple molecules. The simplest way to deliver two or more drugs is to co-dissolve them in polymer [27,55]. Release kinetics then depend on solubility and diffusivity of the molecules in the matrix. A more sophisticated approach involved encapsulating platelet-derived growth factor (PDGF) in PLGA microspheres and then mixing them with vascular endothelial growth factor (VEGF) [29]. VEGF tended to be segregated to the surface and therefore was released more quickly, whereas PDGF was delivered as the polymer degraded. Alternatively, scaffolds have been successively coated with different proteins to modulate release profiles [56]. Dual growth factor release was also obtained from gelatin hydrogels [30]. Release profiles for transforming growth factor β and insulin-like growth factor I were altered by changing crosslinking and charges on the gelatin. The present CAP/PF-127 devices differed in that they provide short-term, alternating delivery of molecules rather than simultaneous release and/or sustained release. The materials released their encapsulated biomolecules and were completely eroded in approximately two weeks, after which the biological events initiated by the molecules continued.
CONCLUSION
By alternating layers of CAP/PF-127 microspheres loaded with CB and Sim, CB and PTH(1-34), or Sim and PTH(1-34), intermittent release with five distinguishable peaks for each of the two molecules was achieved. In vitro experiments showed that alternating delivery enhanced bioactivity of osteoblastic cells. Furthermore, alternating treatment with Sim and PTH(1-34) had an additive effect on cell responses. Overall, CAP/PF-127 polymer is a useful system for alternating, intermittent, or sequential delivery of multiple molecules, and as such it could be further developed for treating focal bone defects.
ACKNOWLEDGMENTS
This work was supported by the Kentucky Science and Education Foundation (KSEF-148-502-03-67) and the National Institutes of Health (AR048700).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;110:506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- 2.Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993;14:690–709. doi: 10.1210/edrv-14-6-690. [DOI] [PubMed] [Google Scholar]
- 3.Lane NE, Kimmel DB, Nilsson MH, Cohen FE, Newton S, Nissenson RA, et al. Bone-selective analogs of human PTH(1-34) increase bone formation in an ovariectomized rat model. J Bone Miner Res. 1996;11:614–625. doi: 10.1002/jbmr.5650110509. [DOI] [PubMed] [Google Scholar]
- 4.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J Bone Miner Res. 1992;7:65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- 5.Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1-34) on rat bone. Bone. 1995;16:477–484. doi: 10.1016/8756-3282(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 6.Rubin MR, Cosman F, Lindsay R, Bilezikian JP. The anabolic effects of parathyroid hormone. Osteoporos. Int. 2002;13:267–277. doi: 10.1007/s001980200026. [DOI] [PubMed] [Google Scholar]
- 7.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 8.Sugiyama M, Kodama T, Konishi K, Abe K, Asami S, Oikawa S. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem. Biophys. Res. Commun. 2000;271:688–692. doi: 10.1006/bbrc.2000.2697. [DOI] [PubMed] [Google Scholar]
- 9.Oxlund H, Dalstra M, Andreassen TT. Statin given perorally to adult rats increases cancellous bone mass and compressive strength. Calcif. Tissue Int. 2001;69:299–304. doi: 10.1007/s00223-001-2027-5. [DOI] [PubMed] [Google Scholar]
- 10.Skoglund B, Forslund C, Aspenberg P. Simvastatin improves fracture healing in mice. J. Bone Miner. Res. 2002;17:2004–2008. doi: 10.1359/jbmr.2002.17.11.2004. [DOI] [PubMed] [Google Scholar]
- 11.Thylin MR, McConnell JC, Schmid MJ, Reckling RR, Ojha J, Bhattacharyya I, et al. Effects of simvastatin gels on murine calvarial bone. J. Periodontol. 2002;73:1141–1148. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 12.Stein D, Lee Y, Schmid MJ, Killpack B, Genrich MA, Narayana N, et al. Local simvastatin effects on mandibular bone growth and inflammation. J. Periodontol. 2005;76:1861–1870. doi: 10.1902/jop.2005.76.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon JH, Piepgrass WT, Lin Y-L, Thomas MV, Puleo DA. Localized intermittent delivery of simvastatin hydroxyacid stimulates bone formation in rats. J. Periodontol. 2008 doi: 10.1902/jop.2008.080004. (in press) [DOI] [PubMed] [Google Scholar]
- 14.Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN. Bone-graft substitutes: Facts, fictions & applications. American Academy of Orthopaedic Surgeons; 2002. [DOI] [PubMed] [Google Scholar]
- 15.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Moore EE, Feliciano DV, Mattox KL. Trauma. New York: McGraw-Hill Professional; 2003. [Google Scholar]
- 17.Boman HG. Antibacterial peptides: key components needed in immunity. Cell. 1991;65:205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- 18.Boman HG, Faye I, Gudmundsson GH, Lee JY, Lidholm DA. Cell-free immunity in Cecropia. A model system for antibacterial proteins. Eur. J. Biochem. 1991;201:23–31. doi: 10.1111/j.1432-1033.1991.tb16252.x. [DOI] [PubMed] [Google Scholar]
- 19.Vaara M, Vaara T. Ability of cecropin B to penetrate the enterobacterial outer membrane. Antimicrob. Agents Chemother. 1994;38:2498–2501. doi: 10.1128/aac.38.10.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelman ER, Mathiowitz E, Langer R, Klagsbrun M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials. 1991;12:619–626. doi: 10.1016/0142-9612(91)90107-l. [DOI] [PubMed] [Google Scholar]
- 21.Langer R. Drug delivery and targeting. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 22.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 23.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 24.Wei G, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25:345–352. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 25.Kretlow JD, Klouda L, Mikos AG. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007;59:263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Pettway GJ, McCauley LK, Ma PX. Pulsatile release of parathyroid hormone from an implantable delivery system. Biomaterials. 2007 doi: 10.1016/j.biomaterials.2007.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye WP, Chien YW. Dual-controlled drug delivery across biodegradable copolymer. I. Delivery kinetics of levonorgestrel and estradiol through (caprolactone/lactide) block copolymer. Pharm. Dev. Technol. 1996;1:1–9. doi: 10.3109/10837459609031412. [DOI] [PubMed] [Google Scholar]
- 28.Murphy WL, Mooney DJ. Controlled delivery of inductive proteins, plasmid DNA and cells from tissue engineering matrices. J. Periodontal Res. 1999;34:413–419. doi: 10.1111/j.1600-0765.1999.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 29.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 30.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J. Control. Release. 2005;101:111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Jeon JH, Thomas MV, Puleo DA. Bioerodible devices for intermittent release of simvastatin acid. Int. J. Pharm. 2007;340:6–12. doi: 10.1016/j.ijpharm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raiche AT, Puleo DA. Association polymers for modulated release of bioactive proteins. IEEE Eng. Med. Biol. Mag. 2003;22:37–41. doi: 10.1109/memb.2003.1256270. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Lee PI. Programmable drug delivery from an erodible association polymer system. Pharm. Res. 1993;10:1144–1152. doi: 10.1023/a:1018960016756. [DOI] [PubMed] [Google Scholar]
- 34.Jeon JH, Puleo DA. Formulations for intermittent release of parathyroid hormone (1-34) and local enhancement of osteoblast activities. 2008 doi: 10.1080/10837450802282488. in review. [DOI] [PubMed] [Google Scholar]
- 35.Raiche AT, Puleo DA. In vitro effects of combined and sequential delivery of two bone growth factors. Biomaterials. 2004;25:677–685. doi: 10.1016/s0142-9612(03)00564-7. [DOI] [PubMed] [Google Scholar]
- 36.Scutt A, Duvos C, Lauber J, Mayer H. Time-dependent effects of parathyroid hormone and prostaglandin E2 on DNA synthesis by periosteal cells from embryonic chick calvaria. Calcif. Tissue. Int. 1994;55:208–215. doi: 10.1007/BF00425877. [DOI] [PubMed] [Google Scholar]
- 37.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–3638. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt IU, Dobnig H, Turner RT. Intermittent parathyroid hormone treatment increases osteoblast number, steady state messenger ribonucleic acid levels for osteocalcin, and bone formation in tibial metaphysis of hypophysectomized female rats. Endocrinology. 1995;136:5127–5134. doi: 10.1210/endo.136.11.7588250. [DOI] [PubMed] [Google Scholar]
- 39.Maeda T, Matsunuma A, Kurahashi I, Yanagawa T, Yoshida H, Horiuchi N. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. J. Cell Biochem. 2004;92:458–471. doi: 10.1002/jcb.20074. [DOI] [PubMed] [Google Scholar]
- 40.Lin SY, Kawashima Y. Drug release from tablets containing cellulose acetate phthalate as an additive or enteric-coating material. Pharm. Res. 1987;4:70–74. doi: 10.1023/a:1016442330193. [DOI] [PubMed] [Google Scholar]
- 41.Giunchedi P, Conti B, Genta I, Conte U, Puglisi G. Emulsion spray-drying for the preparation of albumin-loaded PLGA microspheres. Drug. Dev. Ind. Pharm. 2001;27:745–750. doi: 10.1081/ddc-100107331. [DOI] [PubMed] [Google Scholar]
- 42.Port CD, Garvin PJ, Ganote CE. The effect of Pluronic F-38 (Polyoxamer 108) administered intravenously to rats. Toxicol. Appl. Pharmacol. 1978;44:401–411. doi: 10.1016/0041-008x(78)90200-4. [DOI] [PubMed] [Google Scholar]
- 43.Johnston TP, Miller SC. Toxicological evaluation of poloxamer vehicles for intramuscular use. J. Parenter. Sci. Technol. 1985;39:83–89. [PubMed] [Google Scholar]
- 44.Magnusson G, Olsson T, Nyberg JA. Toxicity of Pluronic F-68. Toxicol. Lett. 1986;30:203–207. doi: 10.1016/0378-4274(86)90156-6. [DOI] [PubMed] [Google Scholar]
- 45.Al-Maaieh A, Flanagan DR. New drug salt formation in biodegradable microspheres. Int. J. Pharm. 2005;303:153–159. doi: 10.1016/j.ijpharm.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 46.Srinivasan C, Katare YK, Muthukumaran T, Panda AK. Effect of additives on encapsulation efficiency, stability and bioactivity of entrapped lysozyme from biodegradable polymer particles. J. Microencapsul. 2005;22:127–138. doi: 10.1080/02652040400026400. [DOI] [PubMed] [Google Scholar]
- 47.Zhao H, Gagnon J, Hafeli UO. Process and formulation variables in the preparation of injectable and biodegradable magnetic microspheres. Biomagn. Res. Technol. 2007;5:2. doi: 10.1186/1477-044X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Disthabanchong S, Martin KJ, McConkey CL, Gonzalez EA. Metabolic acidosis up-regulates PTH/PTHrP receptors in UMR 106-01 osteoblast-like cells. Kidney Int. 2002;62:1171–1177. doi: 10.1111/j.1523-1755.2002.kid568.x. [DOI] [PubMed] [Google Scholar]
- 49.Kohn DH, Sarmadi M, Helman JI, Krebsbach PH. Effects of pH on human bone marrow stromal cells in vitro: implications for tissue engineering of bone. J. Biomed. Mater. Res. 2002;60:292–299. doi: 10.1002/jbm.10050. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald BR, Gallagher JA, Russell RG. Parathyroid hormone stimulates the proliferation of cells derived from human bone. Endocrinology. 1986;118:2445–2449. doi: 10.1210/endo-118-6-2445. [DOI] [PubMed] [Google Scholar]
- 51.Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S, et al. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest. 1997;99:2961–2970. doi: 10.1172/JCI119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakatani Y, Tsunoi M, Hakeda Y, Kurihara N, Fujita K, Kumegawa M. Effects of parathyroid hormone on cAMP production and alkaline phosphatase activity in osteoblastic clone MC3T3-E1 cells. Biochem. Biophys. Res. Commun. 1984;123:894–898. doi: 10.1016/s0006-291x(84)80219-3. [DOI] [PubMed] [Google Scholar]
- 53.Jongen JW, Bos MP, van der Meer JM, Herrmann-Erlee MP. Parathyroid hormone-induced changes in alkaline phosphatase expression in fetal calvarial osteoblasts: differences between rat and mouse. J. Cell. Physiol. 1993;155:36–43. doi: 10.1002/jcp.1041550106. [DOI] [PubMed] [Google Scholar]
- 54.Garrett IR, Gutierrez G, Mundy GR. Statins and bone formation. Curr. Pharm. Des. 2001;7:715–736. doi: 10.2174/1381612013397762. [DOI] [PubMed] [Google Scholar]
- 55.Kim HD, Valentini RF. Human osteoblast response in vitro to platelet-derived growth factor and transforming growth factor-beta delivered from controlled-release polymer rods. Biomaterials. 1997;18:1175–1184. doi: 10.1016/s0142-9612(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 56.Sohier J, Vlugt TJ, Cabrol N, Van Blitterswijk C, de Groot K, Bezemer JM. Dual release of proteins from porous polymeric scaffolds. J. Control. Release. 2006;111:95–106. doi: 10.1016/j.jconrel.2005.11.016. [DOI] [PubMed] [Google Scholar]