Abstract
Amphetamine is a neurotoxic psychostimulant that causes dopamine depletion and neuronal death in the rodent striatum. In the present study, we sought to determine if toxic doses of the drug can also induce pathological changes in the mouse olfactory bulb. We found that injections of amphetamine (10 mg/kg × 4, given 2 h apart) caused significant decreases in dopamine levels in that structure. This dose of the drug also induced substantial increases in the number of terminal deoxynucleotidyl transferase-mediated deoxyribonucleotide triphosphate (dNTP) nick end labeling (TUNEL)-positive cells in the olfactory bulb indicative of elevated DNA fragmentation. These results show that the toxic effects of amphetamine involve the olfactory bulb in addition to the striatum. These observations need to be taken into consideration when discussing the clinical course of amphetamine addiction.
Keywords: neurotoxicity, striatum, TUNEL staining, DNA fragmentation
1. Introduction
Amphetamine is a psychostimulant which is abused by a large sector of the world population (Boniatti et al., 2007; Holmgren et al., 2007; Teter et al., 2006). Its abuse is accompanied by many medical and neuropsychiatric complications, which include impairments of attention, memory and decision-making (McKetin and Mattick, 1997; Ornstein et al., 2000; Rogers et al., 1999). The neurotoxic effects include long-term dopamine depletion (Wagner et al., 1980) and loss of dopamine transporters (Krasnova et al., 2001). In addition, injections of the drug also cause cell death in the cortex of amphetamine-treated animals as was shown by Fluoro-Jade staining and electron microscopy (Jakab and Bowyer, 2002; Ryan et al., 1990). More recently, Krasnova et al. (2005) reported that the use of amphetamine is associated with neuronal death in the mouse striatum.
It was, however, not clear to what extent amphetamine might also cause cell death in other brain regions such as the olfactory bulb, which is rich in dopamine neurons (Davila et al., 2003; Philpot et al., 1998). Therefore, we sought to determine if injections of amphetamine doses, that are known to cause dopamine depletion and cell death in the striatum, can also induce similar neuropathological changes in the mouse olfactory bulb.
2. Materials and methods
2.1. Animals and amphetamine treatment
Male CD-1 mice 10–12 weeks old, weighing 30–35 g and obtained from Charles River (Raleigh, NC) were used in the study. All animal procedures were performed according to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the local Animal Care Committee. The mice were injected with d-(+) amphetamine sulfate (10.0 mg/kg × 4 times, every 2 hours) or saline and euthanized at different time intervals after treatment.
2.2. High-performance liquid chromatography analysis
To examine amphetamine-induced depletion of monoaminergic terminals in the mouse brain, two groups of animals were sacrificed 1 week and 2 weeks after drug treatment. Olfactory bulb and striatal tissue samples (N = 6–10 per group) were dissected from each brain, weighed and extracted with 0.1 M perchloric acid, then centrifuged at 20,000g for 10 min. Concentrations of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) in the tissue extracts of amphetamine- and saline-treated mice were measured by high-performance liquid chromatography with electrochemical detection as described earlier (Krasnova et al., 2000) and expressed as ng/mg of tissue weight.
2.3. Terminal deoxynucleotidyl transferase-mediated deoxyribonucleotide triphosphate (dNTP) nick end labeling (TUNEL) histochemistry
To test if amphetamine causes cell death in the mouse brain, we performed TUNEL histochemistry that detects DNA fragmentation using the protocol previously described by us (Krasnova et al., 2005). At 3 and 4 days after amphetamine or saline injections, animals were euthanised, their brains were removed and frozen in isopentane on dry ice. The brains were then cut into 30gm coronal sections on the cryostat, and sections containing striatal and olfactory bulb areas were used for TUNEL staining. For statistical analyses, TUNEL-positive cells were counted in coronal sections using a Zeiss (Carl Zeiss MicroImaging, Inc., Thornwood, NY) microscope. Five sections were counted per brain region from each mouse, and 5–8 mice were used in each group.
2.4. Statistical Analysis
All data are presented as means ± SEM. Statistical analysis was performed using analysis of variance followed by Fisher’s protected least significant difference (StatView 4.02, SAS Institute, Cary, NC). Differences were considered significant at p ≤ 0.05.
3. Results
In order to test the effects of the drug on the olfactory bulb, we used amphetamine doses that have been shown to cause decreases in striatal dopamine levels (Krasnova et al., 2001). Injections of these doses of the drug do indeed cause substantial reductions in dopamine concentrations in both the olfactory bulb (Fig. 1A) and striatum (Fig 1B). The changes in dopamine levels corresponded to 80% and 20% decreases in the striatum and olfactory bulb, respectively, and lasted for the two weeks of observations. Amphetamine also causes marked decreases (about −60%) in DOPAC levels in the striatum (Fig 1B) but not in the olfactory bulb (Fig. 1A). Similarly, the drug also induced reductions (− 45%) in HVA levels in the striatum without changing those in the olfactory bulb (data not shown). DOPAC/dopamine ratios were increased in both brain regions at 1 week after amphetamine injections (Fig. 1A, B), but went back to normal in the olfactory bulb at 2 weeks post-drug (Fig. 1A). Amphetamine treatment had no effect on 5-HT and 5-HIAA levels in the olfactory bulb and striatum at any of the time-points studied (data not shown).
Figure 1.
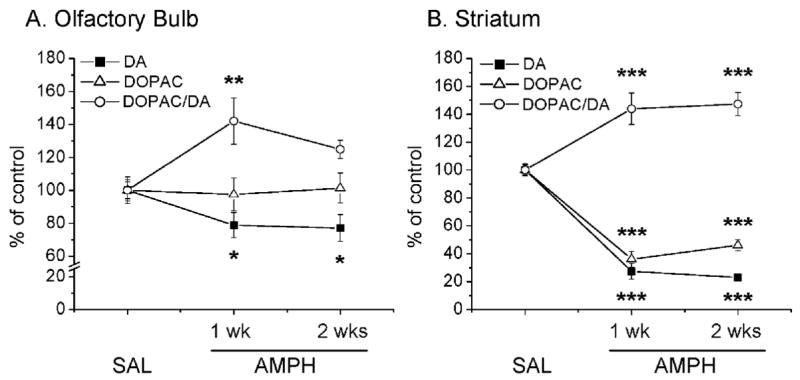
Effects of amphetamine (AMPH) administration on the dopamine (DA), DOPAC levels and DOPAC/DA ratios in the olfactory bulb (A) and striatum (B). N = 6–10 animals per group. Key to statistics: *, P≤0.05; **, P≤0.01; ***, P≤0.001 in comparison to saline-treated control.
Next, we performed TUNEL histochemistry to examine if amphetamine could cause cell death in the olfactory bulb. Previous study that investigated time-course of amphetamine-induced cell death in the striatum had shown that it peaked at 4 days after drug treatment (Krasnova et al., 2005). Therefore, we chose 3 and 4 day intervals to perform TUNEL staining in the olfactory bulb and striatum. Fig. 2 shows the effects of the drug on DNA fragmentation in the two brain regions. In agreement with previous findings (Deng et al., 2007), we found some TUNEL-positive cells in the olfactory bulb (Fig. 2A), but very few of those in the striata of control mice (Fig. 2B). Amphetamine caused significant increases in the number of TUNEL-positive cells in both the olfactory bulb (Fig. 2A) and the striatum (Fig. 2B). The number of drug-induced TUNEL-positive cells peaked at 3 days in the olfactory bulb (Fig. 2A) but at 4 days in the striatum (Fig. 2B).
Figure 2.
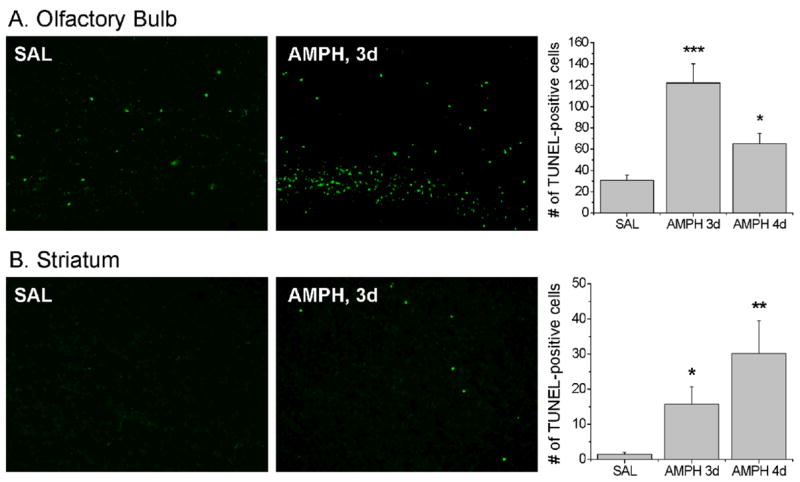
Effects of AMPH treatment on DNA fragmentation in the ofactory bulb (A) and striatum (B). TUNEL staining of coronal sections from saline- and AMPH-treated mice is shown. N = 5–8 animals per group. Key to statistics: *, P≤0.05; **, P≤0.01; ***, P≤0.001 in comparison to saline-treated control.
4. Discussion
Our main observations are that amphetamine can cause depletion of dopamine levels and significant increases in the number of TUNEL-positive cells in the mouse olfactory bulb. These findings are consistent with results of previous studies showing that the more potent psychostimulant, methamphetamine, can also cause degeneration of dopamine terminals and neuronal death in various regions of the rodent brain including the olfactory bulb (Deng et al., 2007). Although amphetamine and methamphetamine are both abused drugs that share similar chemical structure and cause dopamine release in the striatum, several studies have demonstrated differences in their neurochemical and behavioral effects (Peat et al., 1985; Ricaurte et al., 1980; Shoblock et al., 2003a,b; Wagner et al., 1980). Specifically, amphetamine, but not methamphetamine, can cause increase in glutamate levels in the nucleus accumbens; in contrast, methamphetamine causes elevation in glutamate concentrations in the prefrontal cortex whereas amphetamine has no effects (Shoblock et al., 2003b). In addition, amphetamine causes greater locomotor activity (Shoblock et al., 2003b) and induces more marked impairments in rat working memory (Shoblock et al., 2003a). Studies assessing the neurotoxic effects of the two drugs have shown that methamphetamine treatment results in depletion of dopamine and serotonin terminals in the brains of C57BL/6J mice (Ladenheim et al., 2000) and rats (Peat et al., 1985; Ricaurte et al., 1980), whereas amphetamine causes decreases in dopamine concentrations without affecting serotonin levels in these animals (Krasnova et al., 2001; Peat et al., 1985; Wagner et al., 1980). Thus, our data expand the literature comparing the neurotoxicity of the two drugs and provide documentation that they have similar toxic effects on the olfactory bulb.
Structurally, the olfactory bulb contains five layers. These include the subependymal, the combined mitral and granule cell, the external plexiform and the glomerular layers (Lledo et al., 2006). Because dopaminergic neurons are abundantly expressed in the glomerular layer of the olfactory bulb (Davila et al., 2003; Halasz et al., 1981), the present findings that amphetamine can cause dopamine depletion in this brain area suggests that the drug does indeed have effects beyond the nigrostriatal dopaminergic projections in the forebrain (Krasnova et al., 2001). These observations are consistent with the idea that the toxic effects of amphetamine are mediated by dopamine-dependent generation of oxygen-based radicals subsequent to psychostimulant-induced redistribution of the neurotransmitter from synaptic vesicles to the cytosol (Cadet et al., 2007; Fleckenstein et al., 2007). Amphetamine-induced increases in TUNEL-positive cells in the olfactory bulb are probably due to similar mechanisms, with secondary DNA breakdown being caused by dopamine-generated quinones, hydroxyl (Huang et al., 1997) or superoxide (Krasnova et al., 2001) radicals.
Evidence has accumulated to show the important role for dopamine in olfaction (Hsia et al., 1999). Specifically, dopamine inhibits transmission between olfactory epithelium and the glomeruli of the olfactory bulb, mediating the entry of olfactory information into the brain (Hsia et al., 1999). Therefore, dopamine depletion in the olfactory bulb after amphetamine treatment may induce impairments in olfaction because low dopamine levels would cause disinhibition of olfactory glomeruli leading to disorganized olfactory processing. This idea is in agreement with clinical studies showing that patients who suffer from Parkinson’s disease experience hyposmia that precedes motor deficits (Hawkes, 2003). While Parkinson’s disease patients suffer from degeneration of dopamine neurons in the substantia nigra, there is a significant increase in the number of dopaminergic cells in the olfactory bulb (Huisman et al., 2004). Because dopamine can inhibit olfactory transmission (Hsia et al., 1999), the increased dopamine levels in the olfactory bulb were hypothesized to underlie hyposmia in Parkinsonian patients (Huisman et al., 2004). Since amphetamine causes the opposite effects in the olfactory bulb decreasing dopamine levels, it suggests that drug-treated rodents and human abusers might suffer from hyperosmia. Clinical studies of olfaction in amphetamine abusers are needed to test this idea.
Because olfactory bulb is a part of the limbic system, it is also involved in motivation, emotions and memory (Rolls, 2005). Indeed, olfactory bulbectomy in rodents causes reward deficits similar to depressive symptoms in humans and is used as an animal model of depression (Song and Leonard, 2005). It is interesting that both depression and amphetamine withdrawal in humans are associated with diminished interest in reward stimuli and accumulated data suggest potential common neurobiological substrates underlying depressive states observed in these psychiatric conditions (Paterson and Markou, 2007). While olfactory dysfunction has been reported in patients with depression (Pause et al., 2001; Satoh et al., 1996), the association of this phenomena with depressive-like symptoms related to amphetamine withdrawal remains to be evaluated.
Acknowledgments
This study was supported by National Institute on Drug Abuse, Intramural Research Program.
References
- Boniatti MM, Zubaran C, Panarotto D, Delazeri GJ, Tirello JL, Feldens MO, Sperotto VF. The use of psychoactive substances among medical students in southern Brazil. Drug Alcohol Rev. 2007;26:279–285. doi: 10.1080/09595230701247715. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Jayanthi S, Lyles J. Neurotoxicity of substituted amphetamines: molecular and cellular mechanisms. Neurotox Res. 2007;11:183–202. doi: 10.1007/BF03033567. [DOI] [PubMed] [Google Scholar]
- Davila NG, Blakemore LJ, Trombley PQ. Dopamine modulates synaptic transmission between rat olfactory bulb neurons in culture. J Neurophysiol. 2003;90:395–404. doi: 10.1152/jn.01058.2002. [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL. Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry. 2007;61:1235–1243. doi: 10.1016/j.biopsych.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Halasz N, Johansson O, Hokfelt T, Ljungdahl A, Goldstein M. Immunohistochemical identification of two types of dopamine neuron in the rat olfactory bulb as seen by serial sectioning. J Neurocytol. 1981;10:251–259. doi: 10.1007/BF01257970. [DOI] [PubMed] [Google Scholar]
- Hawkes C. Olfaction in neurodegenerative disorder. Mov Disord. 2003;18:364–372. doi: 10.1002/mds.10379. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Holmgren P, Kugelberg FC, Jones AW, Ahlner J. Predominance of illicit drugs and poly-drug use among drug-impaired drivers in Sweden. Traffic Inj Prev. 2007;8:361–367. doi: 10.1080/15389580701329344. [DOI] [PubMed] [Google Scholar]
- Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol. 1999;82:1082–1085. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]
- Huang NK, Wan FJ, Tseng CJ, Tung CS. Amphetamine induces hydroxyl radical formation in the striatum of rats. Life Sci. 1997;61:2219–2229. doi: 10.1016/s0024-3205(97)00924-7. [DOI] [PubMed] [Google Scholar]
- Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson’s disease. Mov Disord. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Bowyer JF. Parvalbumin neuron circuits and microglia in three dopamine-poor cortical regions remain sensitive to amphetamine exposure in the absence of hyperthermia, seizure and stroke. Brain Res. 2002;958:52–69. doi: 10.1016/s0006-8993(02)03439-x. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Bychkov ER, Lioudyno VI, Zubareva OE, Dambinova SA. Intracerebroventricular administration of substance P increases dopamine content in the brain of 6-hydroxydopamine-lesioned rats. Neuroscience. 2000;95:113–117. doi: 10.1016/s0306-4522(99)00400-5. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Cadet JL. Amphetamine induces apoptosis of medium spiny striatal projection neurons via the mitochondria-dependent pathway. Faseb J. 2005;19:851–853. doi: 10.1096/fj.04-2881fje. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Ladenheim B, Jayanthi S, Oyler J, Moran TH, Huestis MA, Cadet JL. Amphetamine-induced toxicity in dopamine terminals in CD-1 and C57BL/6J mice: complex roles for oxygen-based species and temperature regulation. Neuroscience. 2001;107:265–274. doi: 10.1016/s0306-4522(01)00351-7. [DOI] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- McKetin R, Mattick RP. Attention and memory in illicit amphetamine users. Drug Alcohol Depend. 1997;48:235–242. doi: 10.1016/s0376-8716(97)00132-4. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23:113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Animal models and treatments for addiction and depression co-morbidity. Neurotox Res. 2007;11:1–32. doi: 10.1007/BF03033479. [DOI] [PubMed] [Google Scholar]
- Pause BM, Miranda A, Goder R, Aldenhoff JB, Ferstl R. Reduced olfactory performance in patients with major depression. J Psychiatr Res. 2001;35:271–277. doi: 10.1016/s0022-3956(01)00029-2. [DOI] [PubMed] [Google Scholar]
- Peat MA, Warren PF, Bakhit C, Gibb JW. The acute effects of methamphetamine, amphetamine and p-chloroamphetamine on the cortical serotonergic system of the rat brain: evidence for differences in the effects of methamphetamine and amphetamine. Eur J Pharmacol. 1985;116:11–16. doi: 10.1016/0014-2999(85)90179-7. [DOI] [PubMed] [Google Scholar]
- Philpot BD, Men D, McCarty R, Brunjes PC. Activity-dependent regulation of dopamine content in the olfactory bulbs of naris-occluded rats. Neuroscience. 1998;85:969–977. doi: 10.1016/s0306-4522(97)00667-2. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav. 2005;85:45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Linder JC, Martone ME, Groves PM. Histological and ultrastructural evidence that D-amphetamine causes degeneration in neostriatum and frontal cortex of rats. Brain Res. 1990;518:67–77. doi: 10.1016/0006-8993(90)90955-b. [DOI] [PubMed] [Google Scholar]
- Satoh S, Morita N, Matsuzaki I, Konishi T, Nakano T, Minoshita S, Arizono H, Saito S, Ayabe AS. Relationship between odor perception and depression in the Japanese elderly. Psychiatry Clin Neurosci. 1996;50:271–275. doi: 10.1111/j.1440-1819.1996.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Maisonneuve IM, Glick SD. Differences between d-methamphetamine and d-amphetamine in rats: working memory, tolerance, and extinction. Psychopharmacology (Berl) 2003a;170:150–156. doi: 10.1007/s00213-003-1522-y. [DOI] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003b;165:359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev. 2005;29:627–647. doi: 10.1016/j.neubiorev.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Johanson CE, Schuster CR, Seiden LS. Amphetamine induces depletion of dopamine and loss of dopamine uptake sites in caudate. Neurology. 1980;30:547–550. doi: 10.1212/wnl.30.5.547. [DOI] [PubMed] [Google Scholar]


