Abstract
In the fruit fly Drosophila melanogaster, CRYPTOCHROME (CRY) functions as a photoreceptor to entrain circadian oscillators to light-dark cycles and as a transcription factor to maintain circadian oscillator function in certain peripheral tissues. Given the importance of CRY to circadian clock function, we expected this protein to be expressed in all oscillator cells, yet CRY cellular distribution and subcellular localization has not been firmly established. Here we investigate CRY spatial expression in the brain using a newly developed CRY antibody and a novel set of cry deletion mutants. We find that CRY is expressed in s-LNvs, l-LNvs and a subset of LNds and DN1s, but not DN2s and DN3s. CRY is present in both the nucleus and cytoplasm of these neurons, and its subcellular localization does not change over the circadian cycle. Although CRY is absent in DN2s and DN3s, cry promoter activity and/or cry mRNA accumulation can be detected in these neurons, suggesting that CRY levels are regulated post-transcriptionally. Oscillators in DN2s and DN3s entrain to environmental light-dark cycles, which implies that they are entrained indirectly by retinal photoreceptors, extra-retinal photoreceptors or other CRY expressing cells.
Keywords: circadian clock, cryptochrome, Drosophila, immunolocalization, behavior, mutant
Introduction
The fruit fly Drosophila melanogaster keeps circadian time via interlocked period/timeless (per/tim) and Clock (Clk) transcriptional feedback loops (reviewed in Hardin 2005). These feedback loops operate in the brain and many peripheral tissues to control behavioral, physiological and metabolic rhythms. Several groups of oscillator neurons in the lateral and dorsal brain control rhythms in locomotor activity (Helfrich-Forster 2005; Shafer et al. 2006). Oscillator neurons in the lateral brain include the dorsal lateral neurons (LNds), the large ventral lateral neurons (l-LNvs), small ventral lateral neurons (s-LNvs), and the lateral posterior neurons (LPNs). The s-LNvs are further subdivided into four cells that express the neuropeptide Pigment Dispersing Factor (PDF), and a fifth s-LNv that lacks PDF expression (the 5th s-LNv). Three additional clusters of oscillator neurons reside in the dorsal brain: dorsal neurons 1s (DN1s), DN2s and DN3s. The s-LNvs are sufficient for locomotor activity rhythms in constant darkness (DD), whereas in 12h light: 12h dark (LD) conditions s-LNvs control the morning peak of activity, and the 5th s-LNv and the LNds control the evening peak of activity (Grima et al. 2004; Rieger et al. 2006; Stoleru et al. 2004). Peripheral tissues that contain oscillators include sensory organs (e.g. compound eyes, ocelli, antennae, proboscis), testes, the gut, and Malpighian tubules (reviewed in Bell-Pedersen et al. 2005; Hall 2003). Oscillators in olfactory sensory neurons control rhythms in olfactory physiology (Krishnan et al. 1999; Tanoue et al. 2004), but rhythmic outputs from other peripheral oscillator tissues have not been characterized.
Circadian oscillators in the brain and peripheral tissues are entrained by environmental light-dark cycles. Photic entrainment in Drosophila is mediated by the blue light photoreceptor CRYPTOCHYROME (CRY), a conserved pterin/flavin containing protein found in other eukaryotes (Cashmore et al. 1999), and photoreceptors in compound eyes, ocelli and the Hofbauer-Buchner eyelet (Helfrich-Forster et al. 2001; Rieger et al. 2003; Veleri et al. 2007). CRY entrains the circadian oscillator by undergoing a light-dependent conformational change that promotes CRY-TIM binding, TIM ubiquitination, and ultimately the proteosome-mediated degradation of TIM (Busza et al. 2004; Ceriani et al. 1999; Koh et al. 2006; Lin et al. 2001; Peschel et al. 2006). Eliminating TIM destabilizes PER, which delays or advances the oscillator depending on whether light is presented during the early evening or late night, respectively. Consequently, mutants that alter or eliminate CRY show severe defects in their entrainment to light (Dolezelova et al. 2007; Emery et al. 2000b; Stanewsky et al. 1998).
In addition to its role as a circadian photoreceptor, CRY is necessary for circadian oscillator function in some peripheral tissues. In antennae, rhythms in per and tim expression and odor-dependent electroantennagram (EAG) responses are severely disrupted in cryb mutant flies (Krishnan et al. 2001; Levine et al. 2002). Likewise, rhythmic PER and TIM accumulation in compound eyes and Malpighian tubules are abolished in cryb flies (Ivanchenko et al. 2001; Stanewsky et al. 1998). Genes activated by CLK-CYC are expressed at high levels in cryb flies and low levels in flies that overexpress CRY, which suggests that CRY represses CLK-CYC dependent transcription (Collins et al. 2006). Such a role in transcriptional repression is consistent with CRY function in mammals, where transcriptional activation by CLOCK-BMAL1 (the mammalian homolog of CLK-CYC) is repressed by mCry1 and mCry2 (Kume et al. 1999).
The dual roles of CRY as a circadian photoreceptor and, in many peripheral tissues, a transcriptional repressor implies that cry expression will encompass all oscillator cells. Several methods have been used to track cry expression in the brains (primarily) of adults including cry promoter driven Gal4 (cry-Gal4) reporter genes (Emery et al. 2000b; Klarsfeld et al. 2004; Zhao et al. 2003), cry RNA in situ hybridization (Egan et al. 1999; Zhao et al. 2003), and CRY immunohistochemistry (Klarsfeld et al. 2004), but disparate results within and between these methods leave no clear consensus on cry spatial expression. For instance, cry-Gal4 lines containing different amounts of cry upstream sequences are expressed in virtually all oscillator neurons in the brain and many peripheral tissues (Zheng et al., submitted), whereas cry-Gal4 lines that contain the first intron have a more restricted distribution (Emery et al. 2000b; Zheng et al., submitted). Moreover, different insertion sites of the same cry-Gal4 construct can produce substantial differences in spatial expression (Zhao et al. 2003). Among all cry-Gal4 lines the only common sites of expression are the LNvs and LNds, though most cry-Gal4 lines are also expressed in subsets of DN1s and DN3s (Emery et al. 2000b; Helfrich-Forster et al. 2007; Klarsfeld et al. 2004; Shafer et al. 2006; Stoleru et al. 2004; Zhao et al. 2003). In situ hybridization experiments varied substantially in the number and location of cry positive cells, though at maximum cry signal was detected in LNvs, LNds, DN2s and a subset of DN1s (Zhao et al. 2003). A novel peptide antibody detected strong CRY immunostaining in s-LNvs and two DN1s from adult brains, and weaker staining in l-LNvs, LNds and additional DN1s (Klarsfeld et al. 2004). In these neurons, CRY was detected in both the cytoplasm and nucleus, but time dependent changes in subcellular localization were not assessed. A weakness of previous cry mRNA and protein localization studies was the lack of a negative control in the form of a cry null mutant, though such a mutant was recently generated (Dolezelova et al. 2007).
Here we generate a novel CRY antiserum and cry deletion mutants to investigate CRY spatial expression and subcellular localization in adult brains. CRY immunostaining is detected in the LNvs and a subset of LNds and DN1s, but not DN2s or DN3s from wild-type flies, or any oscillator cells in cryb mutant and cry deletion controls. CRY is detected in both the nucleus and cytoplasm at all times of the circadian cycle even though its light dependent molecular target, TIM, translocates from the cytoplasm to the nucleus during the night. These results reveal that CRY is only expressed in a subset of brain oscillator cells, indicating that some oscillator cells entrain to light indirectly. CRY is absent in DN2s and DN3s even though cry promoter activity and/or cry mRNA are detected in these neurons, suggesting that cry is under post-transcriptional control in DN2s and DN3s.
Materials and Methods
Fly stocks and transgenic flies
Flies that were wild-type with regard to clock function were either Canton-S or w1118. The cyc01, per01, tim-Gal4, cryb, and hscry strains have been described previously (Emery et al. 1998; Hazelrigg et al. 1984; Konopka and Benzer 1971; Park et al. 2000; Rutila et al. 1998; Stanewsky et al. 1998). cryb+ flies, which are wild-type flies having the same genetic background as cryb mutants, were generously provided by Dr. R. Stanewsky (School of Biological and Chemical Sciences Queen Mary College, University of London). Flies bearing the P-element P{XP}cryd10630 (BL 19331) were obtained from the Bloomington Stock Center (Wilson et al. 2008). All fly strains were reared on medium containing corn meal, molasses, yeast, agar, and Tegosept (a mold inhibitor) at 25°C.
Generation and characterization of cry excisions
To generate cry deletions via imprecise excision, P{XP}cryd10630, a w+ transposable element insert 57bp upstream of the cry transcription start site, was excised as described (Greenspan 2004). The approximate size of deletions due to imprecise excision was determined by PCR. To precisely determine the deletion endpoints in three different excision lines, PCR products containing the deleted sequences were cloned into pCR-Blunt II-TOPO vector (Invitrogen). For A2, the primer combination used was cryjumpF 5'-ATCTTTTCTCGCAGCCATTAT-3' and cryjumpR5 5'-CGATATTCAGCTCCCGACAT-3', and for B1 and I1 the primers used were cryjumpF3 5'-CCTGGCGTATGGGTAAGTG-3' with cryjumpR5. Inserts for each of the deletions were sequenced on both strands.
Fly heat shock
hs-cry flies were placed in an empty vial, pushing the cotton lid down so that the flies were close to the bottom. The vial was incubated on a 37°C water bath for 30 minutes. Immediately after that, flies were transferred to a 25 °C dark incubator, recovered for 2hr, collected and stored at −80 °C.
CRY antibody generation
Full length CRY was expressed in E. coli, purified and used to produce antisera in Guinea pigs. A DNA fragment containing the complete cry coding region was amplified from a head cDNA library (Hamilton'91) using the crypETs1 (5' GGAATTCACATATGGCCACGCGAGG 3') and crypETa1 (GGAATTCACTCGAGTCAAACCACCACGTC 3') primers. This fragment was inserted into the NcoI and XhoI sites of pET28a (+) to generate pET28a-cry, which expresses an N-terminal HIS-tagged CRY fusion protein. pET28a-cry was transformed into BL21 cells and protein expression was induced using IPTG. CRY fusion protein was purified from cell lysates using a copper affinity column (Amersham) as described (Houl et al. 2006). A single band at the predicted molecular weight of the CRY fusion protein (∼60kDa) was detected, and used to immunize guinea pigs for antibody production (Cocalico Biological Inc).
Whole mount immunofluorescence
Flies were entrained in a 12 hour Light: 12 hour Dark cycle (LD) for at least three days, released into constant darkness (DD), and collected at the indicated time points. Adult brains were dissected in 1XPBS pH7.4 followed by fixation with 5% formaldehyde in 0.1M Phosphate buffer during 20 min at room temperature. The specimens were then washed twice with 1xPBS (10 minutes each), and once with PBT (1XPBS pH7.4, 0.05% Triton-X100) for 10 minutes. Blocking was performed with 5% goat serum, 2% BSA in PBT at room temperature for 3hr or at 4°C O/N. Primary antibody dilutions were prepared in 2% goat serum in PBT, added to the brains, and incubated at 4°C for at least 48 hours. Dilutions used for the primary antibodies were 1/300 for rabbit anti PER that was preadsorbed against per01 (Cheng and Hardin 1998), 1/1000 for rabbit anti PDF, and 1/100 for guinea pig anti CRY. Brains were left for 2 hr at room temperature before washing off the primary antibody with PBT for at least 6 times (30 minutes each) at room temperature. Specimens were incubated with fluorescently labeled secondary antibody (1:300) diluted in PBT at 4°C overnight. Secondary antibodies used were: Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 488 and 568 goat anti-guinea pig (Molecular Probes, Invitrogen), Cy3 goat anti-rabbit and Cy3 goat anti-guinea pig (Jackson ImmunoResearch Laboratories Inc.). After equilibration for 2 hr at room temperature, brains were washed with PBT for 30 minutes at least 6 times at room temperature and mounted on glass slides using Vectashield (Vectorlab). Visualization of whole mounts was performed using an Olympus FV100 confocal microscope. Serial Z-stack images were obtained at 1−2um intervals. Images were processed using Adobe Photoshop. Images shown in the figures are overlays of several confocal stacks or single optical sections.
Behavioral analysis
Locomotor activity was assessed using Drosophila Activity Monitors (TriKinetics Inc.). Flies were entrained in LD cycles at 25°C for 3 days and then kept in constant darkness or constant light for at least 7 days. The data collected was analyzed with Fly Activity Analysis Suite for Mac OSX (FaasX), version 0.7.1 (Copyright 2002−2003 Boudinot, CNRS-INAF). Flies having powers >10 and widths >2 were considered rhythmic.
Real time PCR
cry mRNA levels were assayed by Quantitative Real Time PCR (RT-PCR). Flies were entrained in a 12hour light: 12hour dark cycle for at least three days and collected at Zeitgeiber Time (ZT) 5 and ZT17 (where ZT0 corresponds to lights on and ZT12 to lights off). Total RNA was isolated from fly heads using TRIZOL (Invitrogen). To eliminate genomic DNA contamination, each sample was treated with DNase (Promega). First-strand cDNA was synthesized from 2ug of RNA using random primers and Superscript™ II (Invitrogen) in an Eppendorf Mastercycler® gradient (Eppendorf). Ribosomal protein 49 (rp49) was used as the internal control. Reactions were carried out on an ABI Prism® 7000 Sequence Detector System (ABI). The sequences of the primers and probes used for the different messages are: cry forward 5'-CACCGCTGACCTACCAAA-3', cry reverse 5'-GGTGGAAGCCCAATAATTTGC-3', cry probe 5'6 FAM- TGTTCCTGCACACGGT-3'MGBNFQ; rp49 forward 5'-CTGCCCACCGGATTCAAG-3', rp49 reverse 5'-CGATCTCGCCGCAGTAAAC-3', rp49 probe 5'VIC-CCTCCAGCTCGCGCACGTTG-3'MGBNFQ. For mRNA quantification, a standard curve was prepared for cry and rp49. Target mRNAs and rp49 relative values were calculated based on the standard curve and the studied mRNA values were normalized to rp49.
Western blots
During the initial western blot experiments, head extracts were prepared as described (Emery et al. 1998), and blots were developed for chemiluminescence using ECL (Amersham). Subsequently, head extracts were prepared by homogenizing heads from ∼50 flies in 3 volumes of EB4 buffer (5mM Tris-HCl pH 7.5, 50mM KCl, 10mM HEPES pH 7.5, 10% Glycerol, 1mM EDTA, 1mM DTT, 0.05%Triton X100, 0.5mM PMSF, 0.01mg/ml Aprotinin, 0.01mg/ml Leupepetin, 0.002 mg/ml Pepstatin) using sterile pestles, centrifuged at 4°C for 15 minutes, and protein levels in the supernatant were quantified using the DC Protein Assay (Bio-Rad) or the Coomassie (Bradford) Protein Assay Kit (Pierce). 40−60 μg of protein per sample was separated on a 12% acrylamide gel, transferred to nitrocellulose, and blots were developed for chemiluminescence using Advanced Plus ECL (Amersham). Nitrocellulose membranes were blocked in 5% skim milk (or 2% blocking powder if using Advanced Plus ECL) for 1 hr at room temperature or overnight at 4°C, and incubated with 1:5000 dilution of CRY antiserum (or a dilution of 1:7500 for Advanced Plus ECL) overnight at room temperature. Blots were washed six times for 1 hr using 0.1% Tween 20 in TBS (TBST), and incubated with secondary antibody (HRP goat anti-guinea pig, SIGMA) diluted 1/1000 (for detection using ECL) or 1/5000 (for detection using Advanced Plus ECL) in blocking solution for 1 hr at room temperature. Blots were then washed six times for 1 hr in TBST, developed, and exposed to film.
Results
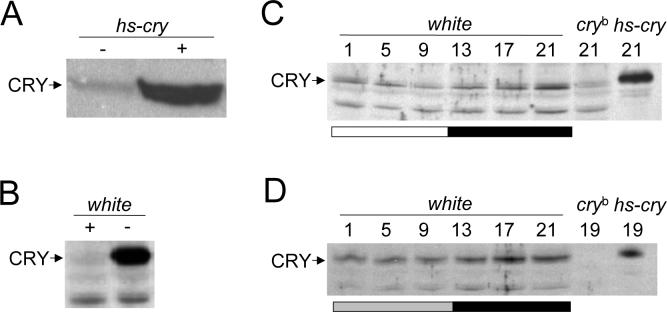
Generation of a CRY antibody (* Place Fig. 1 here, 3 inches high)
Figure 1.
GP23 antiserum detects CRY in fly head extracts. A. Western containing head extracts from hs-cry flies that were heat induced (+) or uninduced (−) at 37°C for 30 min. A ∼60 kDa band corresponding to CRY is detected. B. Western containing head extracts from wild-type flies that received (+) or did not receive (−) a 6hr light pulse at CT12 on the third day of DD before being collected at CT18. C. Western blot of white, cryb and heat induced hs-cry flies collected at the times shown during a 12hr light: 12hr dark cycle and probed with GP23. The CRY and non-specific (ns) bands are shown. D. Western blot of white, cryb and heat induced hs-cry flies collected at the times shown during the first day of DD and probed with GP23. The CRY band is shown. In panels B-D, proteins were extracted using the lab's standard procedure (see Material and Methods). All experiments were replicated at least three times with similar results.
Full length HIS-tagged CRY protein was produced in bacteria, purified, and used to immunize Guinea pigs. One antiserum, designated GP23, detected a protein of approximately 60 KDa on western blots that was induced to high levels in hs-cry flies upon heat shock, which suggests that this protein corresponds to CRY (Fig. 1A). CRY is destabilized by both light and the cryb mutation (Emery et al. 1998; Stanewsky et al. 1998). After entrainment in LD, wild-type flies were given a 6hr light pulse on the third day of DD and collected at CT18 along with untreated controls. GP23 detected a strong band at ∼60kDa in control flies kept in DD, but this band was virtually eliminated after light treatment (Fig. 1B), consistent with detection of CRY. Likewise, when flies were collected every four hours during LD, the band corresponding to CRY rose to high levels during the night and fell to low levels by the end of the day (Fig. 1C). This band was detected at very low levels in cryb flies (Fig. 1C), further demonstrating that it corresponds to CRY. Consistent with a very long half-life for CRY in DD, the band detected by GP23 accumulates during the first day of DD (Fig. 1D).
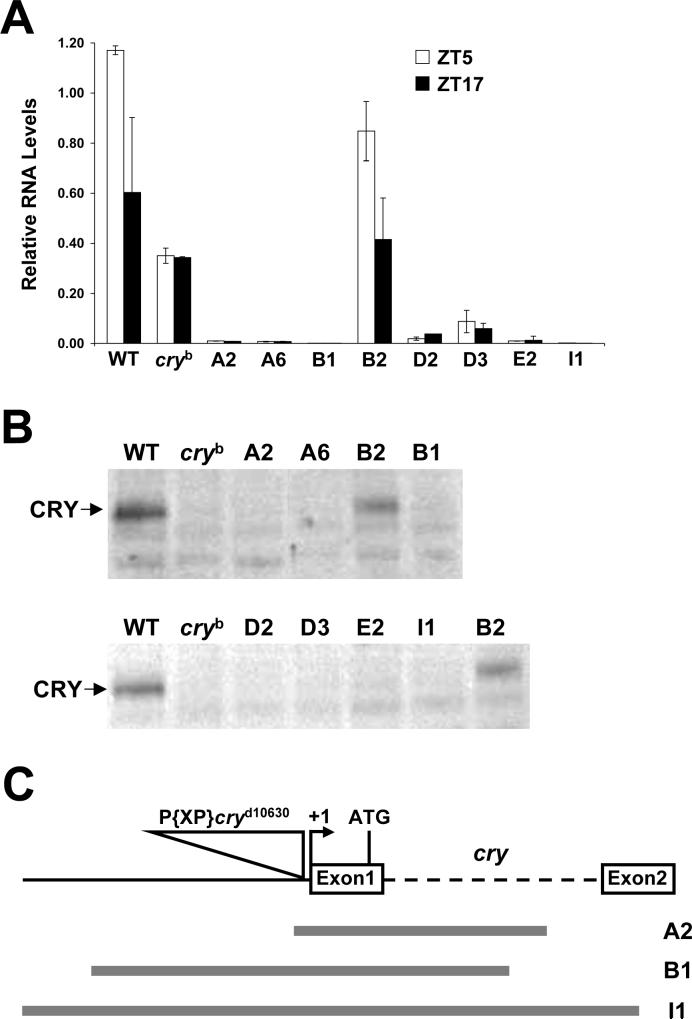
Generation and confirmation of cry null mutants (* Place Fig. 2 here, 1 column wide)
Figure 2.
Expression and structure of cry deletion mutants. A. qPCR of cry mRNA from the heads of w (WT), cryb and cry excision flies (A1, A6, B1, B2, D2, D3, E2, I1) collected at ZT5 (white bars) or ZT17 (black bars) under LD conditions. Relative cry mRNA levels were quantified as described in Materials and Methods. Error bars are ±SD. B. Western blots of head extracts from w (WT), cryb and excision flies (A1, A6, B1, B2, D2, D3, E2, I1) collected at CT19 during the first day of DD were probed with GP23. In panels A and B, B2 is a precise excision control. C. Structural characterization of cry deletion mutants. Diagram of the cry gene (top) showing the first two exons (white boxes), intron 1 (dashed line), upstream sequence (black line), the P{XP}cryd10630 insert (triangle), the transcription start site (+1) and the translation start site (ATG). The extent of deleted sequences the A2, B1 and I1 strains are shown as gray lines.
Most functional studies of CRY are based on the cryb mutant, which produces low levels of CRY that retain some CRY activity (Dolezelova et al. 2007; Emery et al. 1998; Stanewsky et al. 1998). However, cryb may not serve as a useful negative control for immunolocalization studies since CRY antisera may detect the low levels of CRYb protein. To avoid this potential problem, mutants having no detectable CRY expression were generated via imprecise P-element excisions (see Materials and Methods). Of 16 precise excision and 30 cry deletion lines, one precise excision line (B2) and seven deletion lines were selected for further characterization. The abundance of cry mRNA was measured in these eight lines at times when cry mRNA was at peak (ZT5) and trough (ZT17) levels. In the wild-type and B2 controls, cry mRNA is expressed at ∼2-fold higher levels at ZT5 than ZT17 (Fig. 2A). In contrast, cry mRNA from the seven deletion strains ranged from undetectable to 4-fold lower than cry mRNA in wild-type and B2 flies at ZT17 or cry mRNA in cryb flies at ZT5 and ZT17 (Fig. 2A). Consistent with these cry mRNA results, CRY levels were very low or undetectable in the seven deletion strains and cryb, but were high in wild-type and B2 flies (Fig. 2B). These results argue that the seven cry deletion strains examined are either null or severely hypomorphic.
Three lines that produce extremely low levels of cry mRNA, A2, B1 and I1, were characterized in detail. The A2 deletion removed 50bp of cry upstream sequence and 963bp of cry transcribed sequence, the B1 deletion removed 835bp cry upstream sequence and 881bp of cry transcribed sequence, and the I1 deletion removed 1209bp of cry upstream sequence and 1279bp of cry transcribed sequence (Fig. 2C). In each case, the deleted sequences remove the cry transcription and translation initiation sites, consistent with the loss of cry mRNA and protein expression in these strains. We will refer to these new cry alleles as cryΔA2, cryΔB1 and cryΔI1. (* Place Table 1 here, 1 column wide)
Table 1.
Locomotor activity rhythms of cry excision strains under LL conditions. Period and power were calculated for all rhythmic animals as described in Materials and Methods. Activity was calculated for all flies that survived to the end of the experiment, as described in Materials and Methods. Homozygous deletions are shown in bold. n.a.; not applicable.
| Genotype | N | % Rhythmic (N) | Tau +/−SEM | Power | Activity |
|---|---|---|---|---|---|
| white | 16 | 0 | n.a. | n.a. | 22.01+/−2.5 |
| cryb | 14 | 85.7 (12) | 25.4+/−0.2 | 77.1+/−10.1 | 14.75+/−1.5 |
| B2/+ | 10 | 0 (0) | n.a. | n.a. | 19.41+/−1.7 |
| B2/B2 | 17 | 0 (0) | n.a. | n.a. | 24.3+/−2 |
| A2/+ | 10 | 0 (0) | n.a. | n.a. | 22.01+/−1.4 |
| A2/A2 | 12 | 83.3 (10) | 25.01+/−0.4 | 42.8+/−7.4 | 30+/−1.4 |
| B1/+ | 8 | 0 (0) | n.a. | n.a. | 30.2 |
| B1/B1 | 17 | 70.6 (12) | 26.3+/−0.7 | 50.3+/−8 | 33.7+/−2.4 |
| I1/+ | 11 | 0 (0) | n.a. | n.a. | 22.8+/−1.76 |
| I1/I1 | 20 | 90 (18) | 25.1+/−0.4 | 51.1+/−7.1 | 25.9+/−2.4 |
The loss of CRY function in cryb flies enables rhythmic behavioral activity with slightly long periods in constant light and disrupts phase shifts to pulses of light (Emery et al. 2000a; Emery et al. 2000b; Stanewsky et al. 1998). Nevertheless, robust circadian activity rhythms with ∼24hr periods in cryb flies indicates that cry is not required for oscillator function in neurons that mediate locomotor activity rhythms (Stanewsky et al. 1998). To determine whether the behavioral phenotypes of the cryΔA2, cryΔB1 and cryΔI1 mutants are similar to, or more severe than, cryb, we tested behavioral rhythms under LL and DD conditions after entrainment in LD. Wild-type and B2 control flies were arrhythmic in LL, while ∼85% of cryb flies were rhythmic with a period of 25.4hr (Table 1). Like cryb flies, ∼70% to 90% of cryΔA2, cryΔB1 and cryΔI1 flies exhibited rhythmic locomotor activity under LL having periods of ∼25hr (Table 1). Under DD conditions, cryΔA2, cryΔB1 and cryΔI1 flies were mostly rhythmic with periods of ∼24.5−25.5hr., which was similar to the B2 control flies having the same genetic background, but longer period than either the wild-type or cryb controls (Table 2). The similarity in LL and DD locomotor activity phenotypes between cryb and the cry deletion lines suggests that cryΔA2, cryΔB1 and cryΔI1 are severely hypomorphic or null. (* Place Table 2 here, 1 column wide)
Table 2.
Locomotor activity rhythms of cry excision strains under DD conditions. Period and power were calculated for all rhythmic animals as described in Materials and Methods. Activity was calculated for all flies that survived to the end of the experiment, as described in Materials and Methods. Homozygous deletion strains are shown in bold.
| Genotype | N | %Rhythmic (N) | Tau | Power | Activity |
|---|---|---|---|---|---|
| white | 25 | 96 (24) | 23.8+/−0.05 | 93.1+/−7.3 | 26.0+/−1.4 |
| cryb | 19 | 74 (14) | 23.9+/−0.09 | 44.5+/−5.9 | 14.1+/−1.7 |
| B2/+ | 18 | 83.3 (15) | 24.4+/−0.13 | 61.7+/−8.5 | 24.4+/−2.2 |
| B2/B2 | 29 | 55.2 (16) | 24.8+/−0.11 | 64.77+/−7.9 | 25.7+/−1.7 |
| B1/+ | 18 | 66.7 (12) | 24.4+/−0.07 | 79+/−18.7 | 21.7+/−1.4 |
| B1/B1 | 19 | 63.2 (12) | 25.5+/−0.41 | 93.8+/−15.5 | 30.0+/−2.8 |
| I1/+ | 26 | 88.5 (23) | 24.3+/−0.08 | 50.6+/−6 | 18.8+/−1.6 |
| I1/I1 | 32 | 62.5 (20) | 24.7+/−0.11 | 61.0+/−4.7 | 27.3+/−1.9 |
| A2/+ | 14 | 85.7 (12) | 24.6+/−0.18 | 58.2+/−9.8 | 23.3+/−2.1 |
| A2/A2 | 15 | 13.3 (2) | 24.3+/−0.5 | 18.5+/−3.9 | 23.9+/−2 |
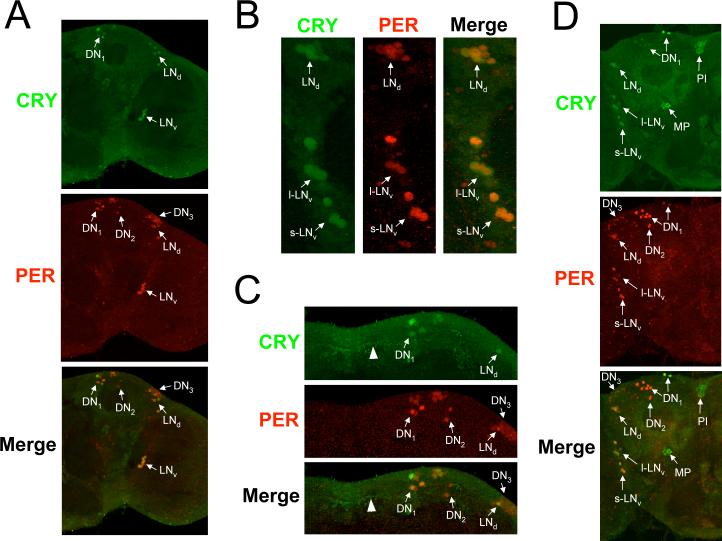
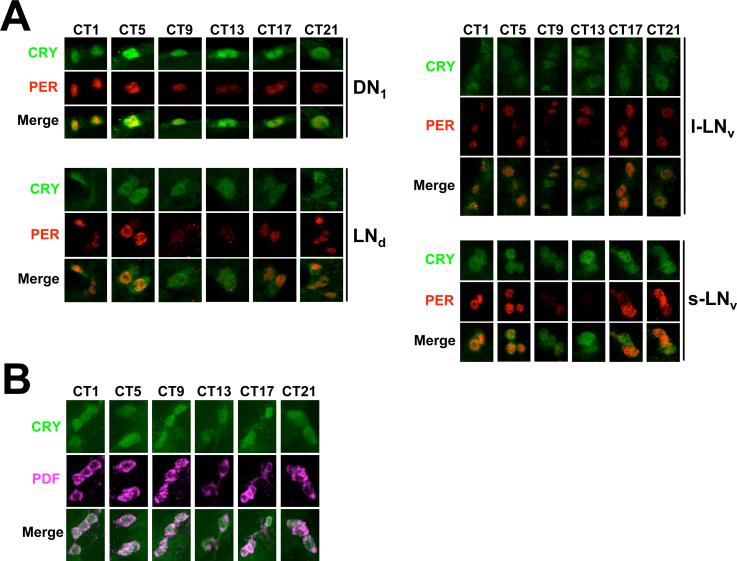
CRY localization in adult brains (* Place Fig. 3 here, 5 inches high)
Figure 3.
CRY expression in adult brains. Brains were dissected from w flies collected at CT19 on the third day of DD, immunostained with CRY (GP23) and PER antisera, and imaged via confocal microscopy. A minimum of six brains were analyzed with similar results. A. Images of a 2μm z-stack projection through the left hemisphere are shown, where dorsal is at the top. Colocalization of CRY (green) and PER (red) immunofluorescence is seen in the merged image as yellow. Arrows indicate CRY and/or PER immunostaining in oscillator neurons. s-LNv, small ventrolateral neurons; l-LNvs, large ventrolateral neurons; LNds, dorsolateral neurons; DN1, dorsal neuron1s; DN2, dorsal neuron 2s; DN3, dorsal neuron 3s. B. A compressed stack of six 1 μm optical sections through the lateral protocerebrum is shown. Colocalization of CRY (green) and PER (red) immunofluorescence is seen in the merged image as yellow. Arrows indicate CRY and/or PER immunostaining in oscillator neurons. Oscillator neurons are labeled as in panel A. C. A compressed stack of six 1μm optical sections through the dorsal protocerebrum is shown. Colocalization of CRY (green) and PER (red) immunofluorescence is seen in the merged image as yellow. Arrows indicate CRY and/or PER immunostaining in oscillator neurons. Oscillator neurons are labeled as in panel A. D. Images of a 2μm z-stack projection through the right hemisphere are shown, where dorsal is at the top. CRY and PER immunostaining and oscillator cells are as described in panels A-C, CRY-IR is also detected in the Pars Intercerebralus (PI) and the medial protocerebrum (MP).
In situ hybridization and promoter analyses show that cry is expressed almost exclusively in brain oscillator cells, though the number and identity oscillator cells that express cry vary among studies (Emery et al. 2000b; Zhao et al. 2003; Zheng et al., submitted). To determine whether GP23 can detect CRY expression in oscillator cells, we used this antibody along with antiserum against the clock cell marker PER to immunostain brains from flies collected at CT19 on the third day of DD. Strong CRY immunoreactivity (CRY-IR) was detected in many, but not all PER expressing brain neurons (Fig. 3A-C). A magnified image of the lateral brain region shows CRY-IR in all of the s-LNvs, l-LNvs, and some LNds (Fig. 3B). In the dorsal brain region, CRY-IR is detected in a majority of the DN1s, but not in DN2s or DN3s (Fig 3C). Two of the DN1s show more intense CRY-IR than other DN1s, and likely correspond to DN1a neurons based on their position (Shafer et al. 2006). CRY-IR was uniform within all CRY positive oscillator neurons, suggesting that CRY is present in both the cytoplasm and the nucleus at this time. Projections from oscillator neurons also showed CRY-IR (Fig. 3C), consistent with previous results (Klarsfeld et al. 2004). However, CRY-IR could only be detected in neuronal projections from flies kept for at least 3 days in DD, suggesting that such staining is due to inordinately high levels of CRY that have accumulated over several days. CRY-IR is also detected in non-PER expressing cells in the Pars Intercerebralis (PI) and medial protocerebrum (Fig. 3D). These cells were quite large, and CRY-IR was cytoplasmic. (* Place Fig. 4 here, 5 inches high)
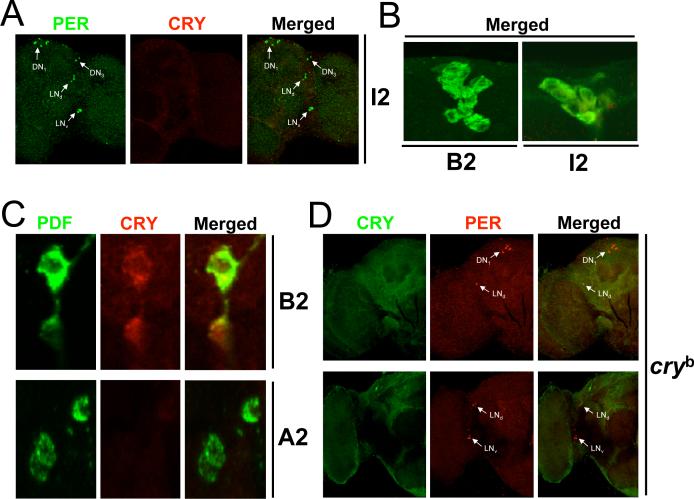
Figure 4.
CRY expression in cryb and cry deletion strains. Brains were dissected from cry deletion strains cryΔI2 and cryΔA2, cryb mutant flies, and cry B2 (precise excision) flies collected at CT19 on the third day of DD, immunostained with CRY (GP23) and PER or PDF antisera, and imaged via confocal microscopy. A minimum of four cryΔI2, four cryΔA2, and six cryb brains were analyzed with similar results. A. A 2μm z-stack projection through the left hemisphere of a cryΔI2 fly is shown. Colocalization of PER (green) and CRY (red) immunofluorescence is seen in the merged image as yellow. Arrows indicate PER and/or CRY immunostaining in oscillator neurons, which are labeled as in panel A of Fig. 3. B. A compressed stack of 6−8 1μm optical sections through the Pars Intercerebralus is shown. A merged image of CRY (green) and PER (red) immunofluorescence is shown. C. A compressed stack of 3−6 1μm optical sections through the lateral protocerebrum of cry B2 and cryΔA2 flies is shown. Colocalization of PDF (green) and CRY (red) immunofluorescence in s-LNvs is seen in the merged image as yellow. D. A compressed stack of 3−4 2μm optical sections from the right brain hemispheres of cryb flies is shown. Colocalization of CRY (green) and PER (red) immunofluorescence in the merged image is shown as yellow. Arrows indicate PER and/or CRY immunostaining in oscillator neurons, which are labeled as in panel A of Fig. 3.
To confirm that CRY-IR in adult brains corresponds to CRY, GP23 and PER were used to immunostain brains from cryΔI1 adults collected on the fourth day of DD. No CRY-IR was detected in neurons that were immunostained with PER (Fig. 4A). However, large PER negative cells in the PI and medial protocerebrum of cryΔI1 flies continued to show CRY-IR similar to that in wild-type flies (Fig. 4B), indicating that this immunostaining is non-specific. Brains from cryΔA2 and control B2 excision flies collected on the third day of DD were immunostained with PDF and GP23 antisera to confirm the lack of CRY-IR in LNvs using a separate cry deletion strain. Although CRY-IR was seen in LNvs from B2 controls, no CRY-IR was detected in LNvs from cryΔA2 flies (Fig. 4C). In addition, no CRY-IR was detected in oscillator cells from cryb flies collected on the third day of DD (Fig. 4D). The lack of CRY-IR in cryΔA2, cryΔI1, and cryb flies demonstrates that GP23 specifically detects CRY.
Localization of CRY in brain oscillator neurons during the circadian cycle (* Place Fig. 5 here, 5 inches high)
Figure 5.
CRY subcellular localization over the circadian cycle. A. Brains were dissected from w flies collected every 4 hours starting at CT1 on the third day of DD, immunostained with CRY (GP23) and PER or PDF antisera, and imaged via confocal microscopy. A minimum of five brains were analyzed for each region with similar results. A. 1μm optical sections of DN1s in the dorsal protocerebrum and LNds, l-LNvs and s-LNvs in the lateral protocerebrum. Colocalization of CRY (green) and PER (red) immunofluorescence is seen in the merged images as yellow. B. A compressed stack of 2−31μm optical sections showing s-LNvs in the lateral protocerebrum. Colocalization of PDF (magenta) and CRY (green) immunofluorescence is seen in the merged image as pink.
Since TIM localization shifts from the cytoplasm to the nucleus during the night and is degraded during the morning (Hunter-Ensor et al. 1996; Meyer et al. 2006; Myers et al. 1996; Shafer et al. 2002; Zeng et al. 1996), it is possible that CRY undergoes similar changes in subcellular localization so that it can always access TIM. To test this possibility, brains from flies collected every 4hr during the third day of DD were immunostained with PER and CRY antisera. Consistent with previous results (Shafer et al. 2002; Veleri et al. 2003; Yang and Sehgal 2001), high amplitude rhythms in PER abundance and nuclear localization were seen in s-LNvs, LNds and DN1s, but not l-LNvs (Fig. 5A). However, CRY abundance and subcellular localization to both the nucleus and cytoplasm were constant in s-LNvs, l-LNvs, LNds, and DN1s. Constant levels of CRY in l-LNvs and s-LNvs is supported by experiments using PDF as a cytoplasmic marker; CRY abundance and subcellular localization do not cycle in PDF positive cells during the third day of DD (Fig. 5B). These results demonstrate that in brain oscillator neurons, the levels and subcellular localization of CRY remain constant in DD.
Discussion
CRY expression in brain oscillator cells
Here we employed a novel CRY antibody and newly generated cry deletion mutants to define the pattern of CRY expression in adult brains. CRY immunostaining was detected in most, but not all, groups of oscillator neurons in the adult brain. Specifically, CRY is present in all of the s-LNvs, l-LNvs, but only about half of the LNds and DN1s, and none of the DN2s or DN3s (Fig. 3AD). Our CRY immunostaining results are consistent with those of Klarsfeld et al., in which CRY-IR could be detected in LNvs, and some LNds and DN1s on the third day of DD, but not DN2s or DN3s (Klarsfeld et al. 2004). We also detect CRY immunostaining in s-LNv projections into the dorsal brain. The significance of this observation is not known, but it is likely this staining is simply a consequence of high CRY accumulation in DD over several days since we did not observe such staining under LD cycling conditions (data not shown).
The lack of CRY expression in half the DN1s, and all DN2s and DN3s suggests that the oscillator in these neurons is light-entrained indirectly by other CRY expressing neurons, retinal photoreceptors in the compound eye or ocelli, or extraretinal photoreceptors in the Hofbauer-Buchner eyelet. When retinal and extraretinal photoreceptors are removed using the gl60j mutant, oscillator function persists in DN2s, demonstrating that DN2 oscillators are not light-entrained by retinal or extraretinal photoreceptors (Helfrich-Forster et al. 2001). However, in cryb flies oscillator function is lost in DN2s, suggesting that these neurons entrain indirectly through other CRY expressing neurons (Helfrich-Forster et al. 2001). Since s-LNvs are not able to reset oscillator phase in DN2s (Stoleru et al. 2005), other CRY expressing neurons must entrain DN2s.
We expected cry mRNA to be expressed in all CRY-expressing brain cells. However, different cry promoter driven Gal4 lines (with or without cry intron 1) give variable patterns of GFP reporter gene (i.e. UAS-GFP) expression, with some lines expressing exclusively in a single cluster of brain oscillator neurons, and others expressing in most or all of the brain oscillator neuron clusters along with different groups of non-oscillator cells (Emery et al. 2000b; Klarsfeld et al. 2004; Zhao et al. 2003). Recent analysis of different portions of the cry promoter (with or without the first intron) driving Gal4 show consistent expression of a β-galactosidase (i.e. UAS-lacZ) reporter gene within all six clusters of brain oscillator neurons (Zheng et al., submitted). In situ hybridization analysis detects cry mRNA in all three groups of LNs, DN1s and DN2s, but not DN3s (Zhao et al. 2003). Since cry-Gal4 driven reporter genes and cry RNA in situ hybridizations detect cry expression in s-LNvs, l-LNvs, LNds and DN1s, it is not surprising that CRY is present in these neuronal clusters. However, we do not detect CRY in DN2s even though cry expression can be detected via reporter gene and RNA in situ analyses. Similarly, CRY is not detected in a group of non-oscillator cells in the dorsal margin of the optic lobe (DOL cells) that express cry mRNA and cry promoter dependent reporter genes (Zhao et al. 2003). These results suggest that CRY is either not translated or is unstable in DN2s and DOLs. Although cry-Gal4 driven reporter gene expression can be detected in DN3s (Klarsfeld et al. 2004; Zheng et al., submitted), no cry mRNA is detected by in situ hybridization (Zhao et al. 2003), indicating that cry mRNA is unstable in these cells. Taken together, these studies indicate that cry expression is regulated at the transcriptional or post-transcriptional level depending on the cluster of brain oscillator neurons.
CRY-IR was consistently observed in non-oscillator cells within the Pars Intercerebralis (PI), a region of the dorsal protocerebrum containing neurosecretory cells (de Velasco et al. 2007), and the medial protocerebrum. Unlike brain oscillator cells, CRY-IR in these cells was very intense and always cytoplasmic. However, in contrast to a loss of CRY-IR in oscillator cells from cryb and cry deletion flies, CRY-IR remained in the cytoplasm of cells in the PI and medial brain (Fig. 4B), suggesting that CRY-IR in these cells is not due to CRY. These results suggest that the GP23 antibody cross-reacts with a protein present in these cells.
Subcellular localization of CRY during the circadian cycle
During the circadian cycle, TIM accumulates in the cytoplasm during the early evening, then moves into the nucleus until it is degraded during the early morning (in LD) or the subjective early morning (in DD) (Hunter-Ensor et al. 1996; Meyer et al. 2006; Myers et al. 1996; Shafer et al. 2002; Zeng et al. 1996). However, CRY does not move from the cytoplasm to the nucleus each day, but is present in both the nucleus and cytoplasm at all times of the circadian cycle (Fig. 5A, B). CRY nuclear and cytoplasmic localization provides constant access to TIM, thus allowing for phase resetting in response to light at any time during the circadian cycle. Since CRY controls oscillator function in some peripheral tissues by rhythmically repressing CLK-CYC activity in the nucleus (Collins et al. 2006), it is possible that rhythms in CRY nuclear translocation could occur in peripheral tissues.
Acknowledgements
We thank P. Emery, R. Stanewsky, J. Hall for providing antibodies or Drosophila strains, and C. Helfrich-Forster for communicating results prior to publication. We also thank A. Kralcheva for generating the CRY expression plasmid. This work was supported by NIH grants NS051280 (PEH) and NS042185 (GR).
References
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science. 2004;304:1503–1506. doi: 10.1126/science.1096973. [DOI] [PubMed] [Google Scholar]
- Cashmore AR, Jarillo JA, Wu YJ, Liu D. Cryptochromes: blue light receptors for plants and animals. Science. 1999;284:760–765. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- Ceriani MF, Darlington TK, Staknis D, Mas P, Petti AA, Weitz CJ, Kay SA. Light-dependent sequestration of TIMELESS by CRYPTOCHROME. Science. 1999;285:553–556. doi: 10.1126/science.285.5427.553. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hardin PE. Drosophila photoreceptors contain an autonomous circadian oscillator that can function without period mRNA cycling. J Neurosci. 1998;18:741–750. doi: 10.1523/JNEUROSCI.18-02-00741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Mazzoni EO, Stanewsky R, Blau J. Drosophila CRYPTOCHROME is a circadian transcriptional repressor. Curr Biol. 2006;16:441–449. doi: 10.1016/j.cub.2006.01.034. [DOI] [PubMed] [Google Scholar]
- de Velasco B, Erclik T, Shy D, Sclafani J, Lipshitz H, McInnes R, Hartenstein V. Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev Biol. 2007;302:309–323. doi: 10.1016/j.ydbio.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Dolezelova E, Dolezel D, Hall JC. Rhythm defects caused by newly engineered null mutations in Drosophila's cryptochrome gene. Genetics. 2007;177:329–345. doi: 10.1534/genetics.107.076513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan ES, Franklin TM, Hilderbrand-Chae MJ, McNeil GP, Roberts MA, Schroeder AJ, Zhang X, Jackson FR. An Extraretinally Expressed Insect Cryptochrome with Similarity tio the Blue Light Photoreceptors of Mammals and Plants. J Neurosci. 1999;19:3665–3673. doi: 10.1523/JNEUROSCI.19-10-03665.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Hall JC, Rosbash M. A unique circadian-rhythm photoreceptor. Nature. 2000a;404:456–457. doi: 10.1038/35006558. [DOI] [PubMed] [Google Scholar]
- Emery P, Stanewsky R, Helfrich-Forster C, Emery-Le M, Hall JC, Rosbash M. Drosophila CRY is a deep brain circadian photoreceptor. Neuron. 2000b;26:493–504. doi: 10.1016/s0896-6273(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Greenspan RJ. Fly pushing: The theory and practice of Drosophila genetics. Cold Spring Harbor Press; Cold Spring Harbor: 2004. [Google Scholar]
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Hall JC. Genetics and molecular biology of rhythms in Drosophila and other insects. Adv Genet. 2003;48:1–280. doi: 10.1016/s0065-2660(03)48000-0. [DOI] [PubMed] [Google Scholar]
- Hardin PE. The circadian timekeeping system of Drosophila. Curr Biol. 2005;15:R714–722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Hazelrigg T, Levis R, Rubin GM. Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984;36:469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C. Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 2005;4:65–76. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, Shafer OT, Wulbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- Helfrich-Forster C, Winter C, Hofbauer A, Hall JC, Stanewsky R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron. 2001;30:249–261. doi: 10.1016/s0896-6273(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Houl JH, Yu W, Dudek SM, Hardin PE. Drosophila CLOCK Is Constitutively Expressed in Circadian Oscillator and Non-Oscillator Cells. J Biol Rhythms. 2006;21:93–103. doi: 10.1177/0748730405283697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter-Ensor M, Ousley A, Sehgal A. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell. 1996;84:677–685. doi: 10.1016/s0092-8674(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Ivanchenko M, Stanewsky R, Giebultowicz JM. Circadian photoreception in Drosophila: functions of cryptochrome in peripheral and central clocks. J Biol Rhythms. 2001;16:205–215. doi: 10.1177/074873040101600303. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K, Zheng X, Sehgal A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science. 2006;312:1809–1812. doi: 10.1126/science.1124951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan B, Dryer SE, Hardin PE. Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature. 1999;400:375–378. doi: 10.1038/22566. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Levine JD, Lynch MK, Dowse HB, Funes P, Hall JC, Hardin PE, Dryer SE. A new role for cryptochrome in a Drosophila circadian oscillator. Nature. 2001;411:313–317. doi: 10.1038/35077094. [DOI] [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Levine JD, Funes P, Dowse HB, Hall JC. Advanced analysis of a cryptochrome mutation's effects on the robustness and phase of molecular cycles in isolated peripheral tissues of Drosophila. BMC Neurosci. 2002;3:5. doi: 10.1186/1471-2202-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, Song W, Meyer-Bernstein E, Naidoo N, Sehgal A. Photic signaling by cryptochrome in the Drosophila circadian system. Mol Cell Biol. 2001;21:7287–7294. doi: 10.1128/MCB.21.21.7287-7294.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P, Saez L, Young MW. PER-TIM interactions in living Drosophila cells: an interval timer for the circadian clock. Science. 2006;311:226–229. doi: 10.1126/science.1118126. [DOI] [PubMed] [Google Scholar]
- Myers MP, Wager-Smith K, Rothenfluh-Hilfiker A, Young MW. Light-induced degradation of TIMELESS and entrainment of the Drosophila circadian clock. Science. 1996;271:1736–1740. doi: 10.1126/science.271.5256.1736. [DOI] [PubMed] [Google Scholar]
- Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci U S A. 2000;97:3608–3613. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschel N, Veleri S, Stanewsky R. Veela defines a molecular link between Cryptochrome and Timeless in the light-input pathway to Drosophila's circadian clock. Proc Natl Acad Sci U S A. 2006;103:17313–17318. doi: 10.1073/pnas.0606675103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger D, Shafer OT, Tomioka K, Helfrich-Forster C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci. 2006;26:2531–2543. doi: 10.1523/JNEUROSCI.1234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger D, Stanewsky R, Helfrich-Forster C. Cryptochrome, compound eyes, Hofbauer-Buchner eyelets, and ocelli play different roles in the entrainment and masking pathway of the locomotor activity rhythm in the fruit fly Drosophila melanogaster. J Biol Rhythms. 2003;18:377–391. doi: 10.1177/0748730403256997. [DOI] [PubMed] [Google Scholar]
- Rutila JE, Suri V, Le M, So WV, Rosbash M, Hall JC. CYCLE is a second bHLHPAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- Shafer OT, Helfrich-Forster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Rosbash M, Truman JW. Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J Neurosci. 2002;22:5946–5954. doi: 10.1523/JNEUROSCI.22-14-05946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Veleri S, Brandes C, Helfrich-Forster C, Hall JC, Stanewsky R. A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr Biol. 2003;13:1758–1767. doi: 10.1016/j.cub.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Veleri S, Rieger D, Helfrich-Forster C, Stanewsky R. Hofbauer-Buchner eyelet affects circadian photosensitivity and coordinates TIM and PER expression in Drosophila clock neurons. J Biol Rhythms. 2007;22:29–42. doi: 10.1177/0748730406295754. [DOI] [PubMed] [Google Scholar]
- Wilson RJ, Goodman JL, Strelets VB. FlyBase: integration and improvements to query tools. Nucleic Acids Res. 2008;36:D588–593. doi: 10.1093/nar/gkm930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sehgal A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron. 2001;29:453–467. doi: 10.1016/s0896-6273(01)00218-5. [DOI] [PubMed] [Google Scholar]
- Zeng H, Qian Z, Myers MP, Rosbash M. A light-entrainment mechanism for the Drosophila circadian clock. Nature. 1996;380:129–135. doi: 10.1038/380129a0. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kilman VL, Keegan KP, Peng Y, Emery P, Rosbash M, Allada R. Drosophila Clock Can Generate Ectopic Circadian Clocks. Cell. 2003;113:755–766. doi: 10.1016/s0092-8674(03)00400-8. [DOI] [PubMed] [Google Scholar]