Abstract
Fatty acids (FAs) are acquired from free FA associated with albumin and lipoprotein triglyceride that is hydrolyzed by lipoprotein lipase (LpL). Hypertrophied hearts shift their substrate usage pattern to more glucose and less FA. However, FAs may still be an important source of energy in hypertrophied hearts. The aim of this study was to examine the importance of LpL-derived FAs in hypertensive hypertrophied hearts. We followed cardiac function and metabolic changes during 2 wk of angiotensin II (ANG II)-induced hypertension in control and heart-specific lipoprotein lipase knockout (hLpL0) mice. Glucose metabolism was increased in ANG II-treated control (control/ANG II) hearts, raising it to the same level as hLpL0 hearts. FA uptake-related genes, CD36 and FATP1, were reduced in control/ANG II hearts to levels found in hLpL0 hearts. ANG II did not alter these metabolic genes in hLpL0 mice. LpL activity was preserved, and mitochondrial FA oxidation-related genes were not altered in control/ANG II hearts. In control/ANG II hearts, triglyceride stores were consumed and reached the same levels as in hLpL0/ANG II hearts. Intracellular ATP content was reduced only in hLpL0/ANG II hearts. Both ANG II and deoxycorticosterone acetate-salt induced hypertension caused heart failure only in hLpL0 mice. Our data suggest that LpL activity is required for normal cardiac metabolic compensation to hypertensive stress.
Keywords: hypertrophy, heart failure, fatty acids, triglyceride, angiotensin II, deoxycorticosterone acetate
circulating fatty acids (FAs) are the major energy source of normal adult hearts. Approximately 60–90% of cardiac energy is derived from FA oxidation, and the remainder is from glucose and lactate oxidation. Exogenous FAs are obtained from free FAs (FFAs) that circulate in association with albumin and from the hydrolysis of triglyceride-rich lipoproteins via the actions of the enzyme lipoprotein lipase (LpL) (5, 27). The majority of FAs acquired by normal human hearts are derived from esterified FAs contained in triglycerides and phospholipids (6).
During the development of pathophysiological conditions such as ischemia, hypertrophy, and heart failure, the substrate usage pattern for cardiac energy shifts to decreased FA oxidation and increased glucose oxidation (8, 10, 22, 26, 34, 38, 46). Increased glucose oxidation is believed to be beneficial, since it allows the heart to produce ATP using less oxygen than that needed for FA oxidation (16). However, recent studies have suggested that increased glucose oxidation alone is insufficient for cardiac energy demands during stress. Peroxisome proliferator-activated receptor-γ coactivator-1α knockout mice, which have suppressed FA oxidation, developed accelerated cardiac dysfunction when hypertrophy was induced by transverse aortic constriction (2). The importance of continued cardiac use of FA was also suggested by the lack of downregulation of FA oxidation in canine models of ischemic heart failure (9).
In addition to exogenous FAs such as lipoprotein triglyceride-derived FAs and adipose-derived FAs, endogenous stored triglyceride-derived FAs can become an important energy source for the heart; this appears to be true during ischemia (43, 48). Exogenous and endogenous FAs are interrelated for energy production. When the concentration of exogenous FAs was reduced in isolated perfused hearts, more FA-derived energy was produced by use of endogenous triglyceride (11, 12, 39).
We created mice with a cardiomyocyte-specific deletion of LpL, denoted hLpL0 mice, and reported that hLpL0 mice have greater cardiac glucose utilization but developed cardiac dysfunction with age (4). In the current study, we examined how loss of LpL activity affects cardiac metabolism and function during the development of hypertensive hypertrophy.
METHODS
Mice.
hLpL0 mice were created using the Cre-LoxP system, and their genotypes were identified from tail tip DNA by PCR analysis, as previously described (3). The characteristics of hLpL0 hearts have already been reported: cardiac LpL activity decreased 80%, cardiac very low density lipoprotein-triglyceride uptake decreased 50–75%, FA oxidation decreased 40–50%, and glucose uptake and oxidation increased (3, 4). hLpL0 hearts developed systolic dysfunction with age, although young hLpL0 hearts were morphologically and functionally normal (3, 4). The current studies used 10- to 12-wk-old male hLpL0 mice and littermate LpL floxed male mice as controls.
All experimental procedures were approved by the Columbia University Institutional Animal Care and Use Committee. Mice were fed normal chow diet containing, by weight, 3.8% fat, 27.9% carbohydrate, and 22.4% protein and were housed in an approved animal facility under a 12:12-h light-dark cycle.
ANG II treatment.
Saline or ANG II (4.0 mg·kg−1·day−1; Calbiochem, La Jolla, CA) was administered to control and hLpL0 mice for 2 wk via subcutaneous implantation of microosmotic pumps (Alzet, model 1002; DURECT, Cupertino, CA), under ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip) anesthesia. This dose was empirically derived to consistently increase blood pressure to >150 mmHg.
Deoxycorticosterone acetate-salt treatment.
Deoxycorticosterone acetate (DOCA)-salt induced hypertension was also created in both control and hLpL0 mice by the method of Johns et al. (18). These mice are denoted control/DOCA and hLpL0/DOCA. In treated groups, DOCA pellets (21-day release; Innovation Research of America, Sarasota, FL) were implanted subcutaneously. Heminephrectomy was performed in both treated and nontreated groups under similar anesthesia as before. For 3 wk following surgery, the drinking water given to treated mice contained 1% NaCl; regular water was given to nontreated groups.
Blood pressure measurement.
Systolic blood pressure was measured using a noninvasive tail-cuff blood pressure machine, MC4000 Blood Pressure Analysis System (Hatteras Instruments, Cary, NC). Conscious mice were placed on the warmed platform of the machine for 2 min, which was maintained at 37°C, and blood pressures were taken at least three times per mouse within 5 min.
Echocardiography.
Transthoracic echocardiography was performed using a high-resolution imaging system with a 30-MHz imaging transducer (Vevo 770; VisualSonics, Toronto, ON, Canada). The mice were anesthetized with 1.5–2% isoflurane and thereafter maintained on 1–1.5% isoflurane throughout the procedure. Each procedure was done within 5 min using short-axis views at the level of papillary muscles, and each parameter was measured using M-mode view. Percent fractional shortening (%FS) and left ventricular mass (LVM) were calculated as follows: %FS = (LVDd − LVDs)/LVDd × 100,LVM = [1.04 × (IVST + PWT + LVDd)3 − LVDd3] × 0.8 where LVDd is left ventricular diastolic dimension, LVDs is left ventricular systolic dimension, IVST is interventricular septal wall thickness, and PWT is posterior wall thickness (13).
Cardiac and plasma lipid measurements.
Bloods were taken, and hearts were resected when mice were killed after a 6-h fast. Plasma triglycerides were measured enzymatically using Infinity triglycerides single liquid stable reagent (Thermo Fisher Scientific, Waltham, MA). FA measurement utilized the NEFA C test kit (Wako Chemical, Richmond, VA). Lipid extraction from the heart was performed using Folch et al.'s (15) method. After extraction, lipids were measured using the same kits as above.
Cardiac gene expression.
Cardiac mRNA levels were evaluated by quantitative real-time PCR. Briefly, total RNA was extracted using the PureLink Micro-to-Midi total RNA purification system (Invitrogen, Carlsbad, CA), and cDNA was prepared from 1 μg of the extracted total RNA using the oligonucleotide-primed cDNA synthesis system, ThermoScript RT-PCR system (Invitrogen). The cDNA was amplified with SYBR Green fluorescence dye (Applied Biosystems, Foster City, CA) in an Mx3000 sequence detection system (Stratagene, Cedar Creek, TX). All results were normalized against ribosomal protein S18. Primer used for S18 was as follows: forward GGAGAACTCACGGAGGACGA/reverse CCAGTGGTCTTGGTGTGCTG. Sequences are listed 5′ to 3′.
In vivo assessment of cardiac glucose uptake.
In vivo cardiac glucose uptake was investigated as described previously (4). Briefly, after a 6-h fast, radiolabeled glucose, 1 μCi of 2-deoxy-d-[1,2,3H(N)] glucose (2-DG) (PerkinElmer Life and Analytical Science, Waltham, MA), was injected intravenously via the tail vein. Blood (20 μl) was collected 0.5, 5, 30, and 60 min following the injection, and the radioactive counts were measured. At 60 min after the injection, hearts were perfused with PBS (in mM: 2.67 KCl, 1.47 KH2PO4, 138 NaCl, and 8.10 NaHPO4, pH 7.2), resected, and homogenized, and the radioactivity was counted (50).
Cardiac LpL assay.
Frozen hearts obtained after a 6-h fast were homogenized and used for LpL activity measurement as described (51).
Cardiac glycogen measurement.
Cardiac glycogen was measured as glucose after hydrolysis with 1 N HCl (35). Glycogen-derived glucose was measured using the Amplex Red Glucose Assay Kit (Invitrogen) after neutralizing with 1 N NaOH.
Intracellular cardiac ATP measurement.
Samples of frozen hearts from 6-h-fasted mice were used to measure ATP as described (19). Briefly, the hearts were homogenized with 0.3 N HClO4 and centrifuged at 4°C. Subsequently, the supernatant was isolated and neutralized to pH 7.0 by adding 3 and 0.6 N KOH. After the second centrifugation, the supernatant was used to measure ATP. An ATP bioluminescent assay kit (Sigma-Aldrich, St. Louis, MO) and Monolight 2010 (Analytical Luminescence Laboratory, San Diego, CA) were used for ATP measurement.
Histology.
The hearts were fixed in 10% buffered formalin solution for 24 h. The hearts were then embedded in paraffin, and 3-μm cross sections were cut at the level of papillary muscles and stained with hematoxylin and eosin (H&E) and Masson's trichrome.
Statistics.
The results are presented as means ± SE. One-way ANOVA followed by Bonferroni/Dunn method was used for comparison among the four groups, and paired t-test was used for comparison within each group. Data from cardiac glucose uptake studies and plasma triglyceride and FFA levels were analyzed using the Mann-Whitney's U-test because the data were not normally distributed. Statistical significance was considered at P < 0.05.
RESULTS
ANG II treatment increased systolic blood pressure and cardiac mass in both hLpL0 and control mice.
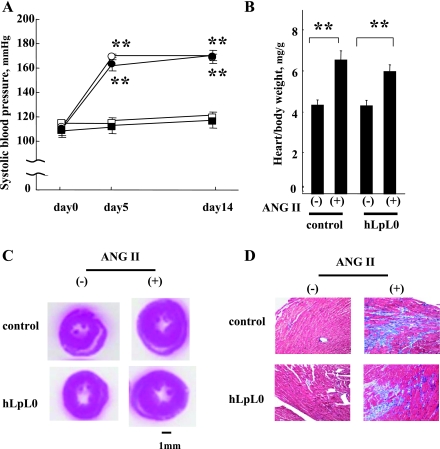
Untreated control and hLpL0 mice had similar baseline blood pressures (Fig. 1A). At day 5 of the ANG II treatment, systolic blood pressure increased by ∼55 mmHg in both hLpL0 and control mice and was maintained until day 14. Heart weight (HW)-to-body weight (BW) ratio increased similarly in both ANG II-treated groups (Fig. 1B). Cross sections of H&E-stained hearts after the ANG II treatment showed hypertrophied left ventricular walls in both control and hLpL0 mice (Fig. 1C). Collagen content was increased similarly in both control/ANG II and hLpL0/ANG II hearts (Fig. 1D).
Fig. 1.
Effect of ANG II on blood pressure and myocardial histology. A: systolic blood pressure before (day 0) and after ANG II treatment in each group. Open symbols represent control mice, and filled symbols represent hLpL0 mice. ANG II-treated mice are shown in circles and nontreated mice in squares; n = 5–7. B: heart weight-to-body weight ratio at day 14; n = 5–7. **P < 0.01 vs. corresponding nontreated group. C: cardiac cross sections of hematoxylin and eosin (H&E) staining from 2 wk ANG II-treated mice and nontreated mice. D: trichrome staining (for collagen in blue) in hearts from 2 wk ANG II-treated mice and nontreated mice, ×400 magnification.
ANG II treatment and plasma profile.
ANG II treatment increased plasma triglyceride in control and hLpL0 mice (control vs. control/ANG II 1.5 ± 0.2 vs. 9.2 ± 2.6 mM, n = 6–8, P < 0.05; hLpL0 vs. hLpL0/ANG II 2.2 ± 0.4 vs. 9.9 ± 4.3 mM, n = 6–8, P < 0.05.) There was a wide variation in response. Plasma FFA concentrations were not significantly increased by the ANG II treatment [control vs. control/ANG II 0.5 ± 0.1 vs. 0.9 ± 0.5 mM, n = 6–8, not significant (NS); hLpL0 vs. hLpL0/ANG II 0.7 ± 0.1 vs. 1.2 ± 0.8 mM, n = 6–8, NS]. Plasma total cholesterol tended to increase with ANG II treatment in both control and hLpL0 mice [control vs. control/ANG II 2.9 ± 0.1 vs. 5.8 ± 1.1 mM, n = 6–8, NS; hLpL0 vs. hLpL0/ANG II 3.0 ± 0.2 vs. 7.7 ± 2.4 mM, n = 6–8, NS]. Plasma glucose was not different between the four groups of mice (control vs. control/ANG II 6.7 ± 0.3 vs. 6.6 ± 0.4 mM, n = 6–8, NS; hLpL0 vs. hLpL0/ANG II 6.8 ± 0.2 vs. 6.3 ± 0.4 mM, n = 6–8, NS).
ANG II treatment changed glucose metabolism in control hearts but not in hLpL0 hearts.
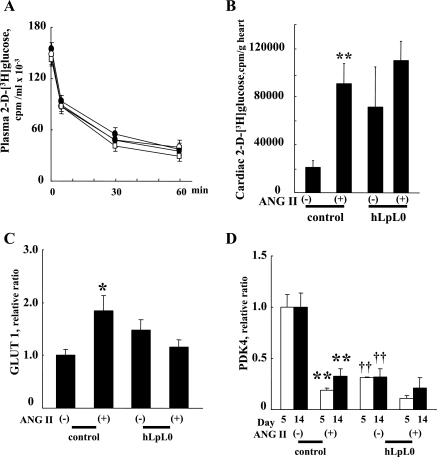
hLpL0 and control mice have identical plasma glucose and insulin levels (3), and plasma 2-DG clearance did not differ among the groups (Fig. 2A). Therefore, the direct measurements of glucose uptake were not altered by differences in plasma decay rate. Compared with control hearts, hLpL0 hearts had a 3.4-fold greater glucose uptake in the absence of ANG II. Control hearts showed a 4.3-fold increase in 2-DG uptake after ANG II treatment (Fig. 2B). Although there was more variability in the hLpL0 group, glucose uptake was not significantly increased with hypertension, and certainly any change was relatively minor compared with that found in controls. This led to a similar level of glucose uptake in control/ANG II, hLpL0, and hLpL0/ANG II hearts.
Fig. 2.
Effect of ANG II on cardiac glucose uptake and metabolic genes. A: plasma 2-d-[3H]glucose clearance in each group. Open symbols represent control mice, and filled symbols represent hLpL0 mice. ANG II-treated mice are shown in circles and nontreated mice in squares; n = 7–10. B: cardiac 2-d-[3H]glucose uptake on day 14 of the ANG II treatment; n = 7–10. C and D: mRNA levels of GLUT1 (C) and PDK4 (D) were measured by quantitative PCR. Open bars represent day 5, and filled bars represent day 14. Primers used for each gene were as follows: GLUT1, forward (F) CATCGTCGTTGGGATCCTTA/reverse (R) GAGCAGTAGAGGCCACAAGTCT; PDK4, (F) GACCGCTTAGTGAACAC/(R) GTAACGGGGTCCACTG. Sequences are listed 5′ to 3′. Data were normalized to control group; n = 5–11. *P < 0.05 and **P < 0.01 vs. corresponding control group. ††P < 0.01 vs. control group.
During 2-wk ANG II treatment, GLUT1 expression significantly increased in control but not in hLpL0 hearts (Fig. 2C). There were no significant changes in GLUT4 mRNA (control vs. control/ANG II 1.0 ± 0.1 vs. 1.1 ± 0.2, n = 5–11, NS; hLpL0 vs. hLpL0/ANG II 0.9 ± 0.1 vs. 0.7 ± 0.1, n = 5–11, NS). All data were normalized to the control group. Primer sequences were as described previously (4). We should note that in previous studies differences in GLUT4 mRNA between control and hLpL0 hearts were found in isolated cardiomyocytes but not in whole hearts (4), perhaps because cardiomyocytes do not form the majority cell type in the heart.
PDK4 suppresses glucose oxidation.
ANG II treatment reduced PDK4 expression in control hearts (Fig. 2D). This reduction in PDK4 resulted in levels that were equal to those in hLpL0 and hLpL0/ANG II mice.
ANG II treatment decreased FA uptake genes but preserved LpL activity in control hearts.
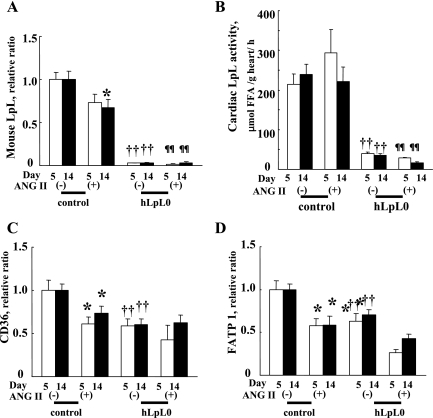
During 2 wk of ANG II treatment in control mice, LpL expression was decreased by 33% (Fig. 3A). However, LpL activity was not altered (Fig. 3B). Furthermore, ANG II treatment in control hearts reduced CD36 and FATP1 expression to levels found in hLpL0 and hLpL0/ANG II hearts (Fig. 3, C and D).
Fig. 3.
Cardiac fatty acid (FA) uptake, gene expression, and cardiac lipoprotein lipase (LpL) activity at day 5 and day 14. mRNA levels of mouse LpL (A), CD36 (C), and FATP1 (D) were examined at days 5 and 14. Open bars represent day 5, and filled bars represent day 14. Primers used for each gene were as follows: LpL, (F) TCTGTACGGCACAGTGG/(R) CCTCTCGATGACGAAGC; CD36, (F) ATTGGTCAAGCCAGCT/(R) TGTAGGCTCATCCACTAC; FATP1, (F) GGCTCCTGGAGCAGGAACA/(R) ACGGAAGTCCCAGAAACCAA. Sequences are listed 5′ to 3′. All data were normalized to the untreated control group; n = 5–11. B: cardiac LpL activity in heart homogenates was measured using hydrolysis of radiolabeled triolein as described as methods. Open bars represent day 5, and filled bars represent day 14; n = 5–6. *P < 0.05 and **P < 0.01 vs. corresponding control group. ††P < 0.01 vs. control group. ¶¶P < 0.01 vs. control/ANG II group.
ANG II treatment did not decrease mitochondrial FA oxidation gene expression in control hearts.
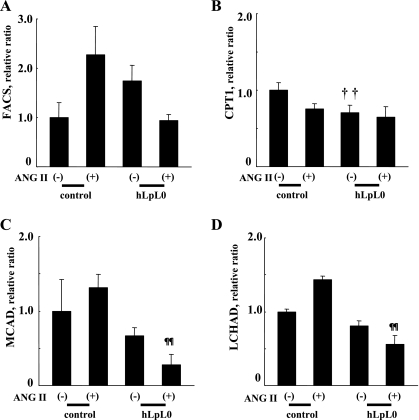
We next assessed genes regulating mitochondrial FA uptake and mitochondrial â-oxidation: fatty acyl-CoA synthase (FACS), carnitine palmitoyltransferase (CPT)1, medium-chain acyl-CoA dehydrogenase (MCAD), long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD). At day 14, FACS, CPT1, MCAD, and LCHAD were not altered by ANG II treatment of control hearts (Fig. 4, A–D). In contrast, MCAD and LCHAD mRNA levels were significantly reduced in hLpL0/ANG II hearts compared with control/ANG II hearts.
Fig. 4.
Expression of mitochondrial FA oxidation genes. mRNA expression of fatty acyl-CoA synthase (FACS, A), carnitine palmitoyltransferase (CPT1, B), medium-chain acyl-CoA dehydrogenase (MCAD, C), and long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD, D) was examined at day 14. Primers used for each gene were as follows: FACS, (F) GACCAGTCCCTGAACGAGAT/(R) AAACCCTTGATGTCTGGGAC; CPT1, (F) TCTATGAGGGCTCGCG/(R) CGTCAGGGTTGTAGCA; MCAD, (F) TGGCATATGGGTGTACAGGG/(R) CCAAATACTTCTTCTTCTGTTGATCA; LCHAD, (F) GAACAAATGCCAAAAGGTCTG/(R) TGACGGCCACTACGATCAC. Sequences are listed 5′ to 3′. All data were normalized to each control group; n = 5–11. ††P < 0.01 vs. corresponding control group. ¶¶P < 0.01, vs. control/ANG II group.
Acyl-CoA oxidase a marker for peroxisomal FA oxidation, was reduced by 2 wk of the ANG II treatment to 46 ± 9% of baseline in control hearts, equivalent to that found in hLpL0/ANG II hearts (untreated hLpL0 hearts were 70 ± 9% and hLpL0/ANG II hearts were 48 ± 8% of control).
ANG II treatment effects on heart triglyceride and glycogen content.
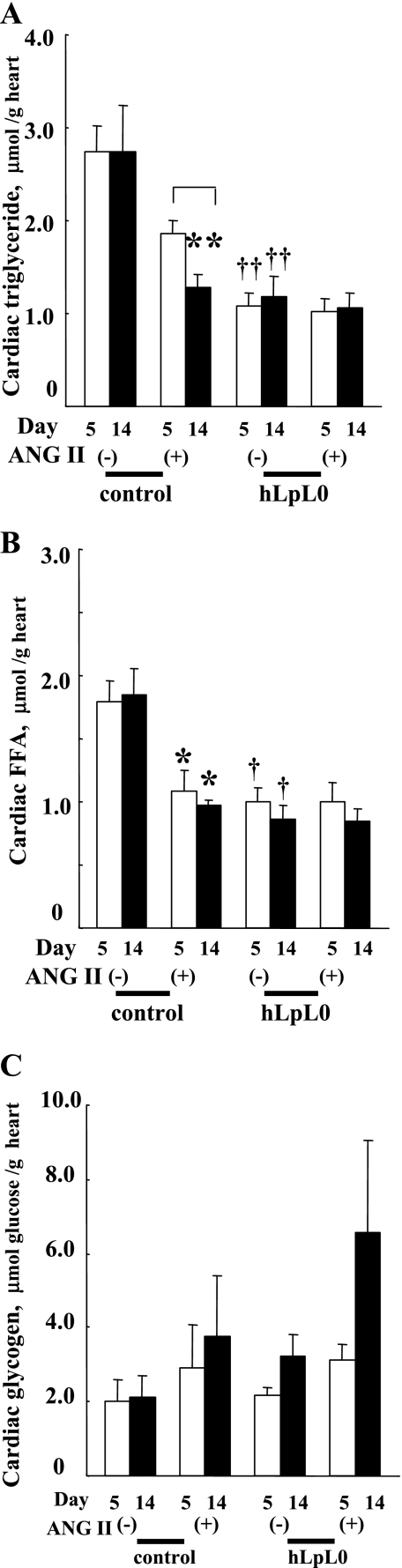
Stored triglycerides and glycogen are an alternative source of cardiac energy. ANG II treatment decreased cardiac triglyceride content in control mice; the reduction was 53% after 14 days of ANG II treatment (Fig. 5A). In contrast, hLpL0 mice had reduced levels of cardiac triglyceride, and ANG II treatment did not further decrease triglyceride content. ANG II treatment of control mice also decreased cardiac FFA content by ∼40% (Fig. 5B). hLpL0 mice had reduced levels of cardiac FFA, and ANG II treatment did not further change cardiac FFA.
Fig. 5.
Effect of ANG II on cardiac contents of lipids and glycogen. Cardiac triglyceride (A), FFA (B), and glycogen (C) at day 5 and 14 were measured as described in methods. Open bars represent day 5, and filled bars represent day 14; n = 5–7. *P < 0.05 and **P < 0.01 vs. corresponding control group. †P < 0.05 and ††P < 0.01 vs. corresponding control group. §P < 0.05, day 5 vs. day 14 within a group.
Glycogen content was preserved in control/ANG II hearts and, in fact, tended to increase in hLpL0/ANG II hearts during the treatment (Fig. 5C).
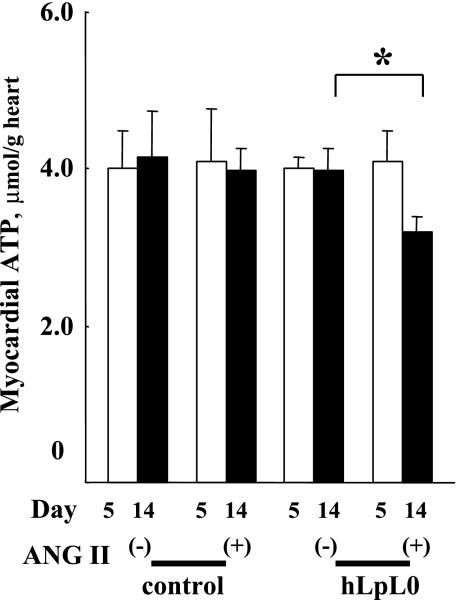
ANG II treatment reduced ATP content of hLpL0 hearts.
hLpL0 hearts have a defect in lipid uptake, exhibit reduced triglyceride stores, and do not increase their glucose uptake with ANG II. Therefore, we tested whether these hearts were energy deficient. ATP was significantly decreased in hLpL0/ANG II hearts but preserved in control/ANG II hearts (Fig. 6). Thus hLpL0/ANG II hearts developed energy deficiency during 2 wk of ANG II treatment.
Fig. 6.
Effect of ANG II on myocardial ATP. Cardiac intracellular ATP at day 5 and at day 14 was measured. Open bars represent day 5, and filled bars represent day 14; n = 5–7. *P < 0.05 vs. corresponding nontreated group. §P < 0.05, day 5 vs. day 14 within a group.
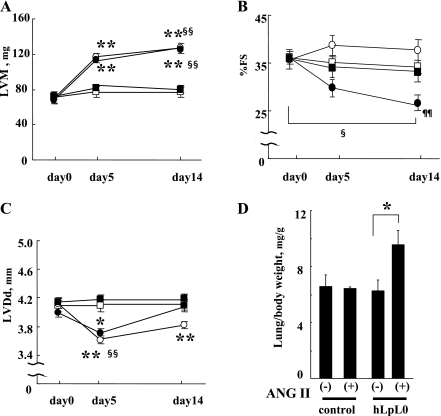
ANG II treatment induced heart failure only in hLpL0 mice.
Along with the decrease in energy stores, hLpL0/ANG II hearts developed signs of cardiac dysfunction. ANG II treatment increased LVM similarly in both control and hLpL0 mice by ∼50 mg (Fig. 7A). In control hearts, %FS was preserved and LVDd significantly decreased during the 2 wk of ANG II infusion (Fig. 7, B and C). These results demonstrated that control/ANG II hearts had compensated hypertrophy but did not develop heart failure at the end of 2 wk. Although hLpL0/ANG II hearts developed hypertrophy to the same extent as control/ANG II hearts, %FS was reduced and LVDd started to enlarge by day 14 (Fig. 7, B and C). Further evidence that hLpL0/ANG II mice developed heart failure was obtained by assessing pulmonary congestion; lung weight/BW ratio in hLpL0 mice increased by 50% (Fig. 7D).
Fig. 7.
Effect of ANG II treatment on echocardiographic parameters. A: left ventricular (LV) mass before (day 0) and after the ANG II treatment in each group. Open symbols represent control mice, and filled symbols represent heart-specific LpL knockout (hLpL0) mice. ANG II-treated mice are shown in circles and nontreated mice in squares; n = 7–9. B: %fractional shortening before and after the ANG II treatment in each group. Symbols are the same as in A; n = 7–9. C: left ventricular diastolic dimension (LVDd) before and after the ANG II treatment in each group. Symbols are the same as in A; n = 7–9. D: lung weight-to-body weight ratio at day 14; n = 5–7. *P < 0.05 and **P < 0.01 vs. corresponding nontreated group. §P < 0.05 and §§P < 0.01 vs. day 0 within a group. ¶¶P < 0.01 vs. corresponding control/ANG II group.
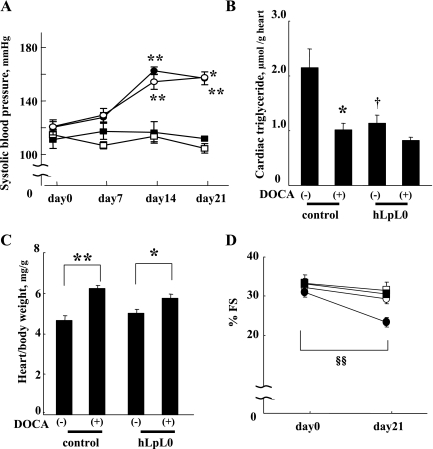
hLpL0 hearts developed systolic dysfunction with DOCA-salt induced hypertension.
We also tested whether loss of cardiac LpL affects cardiac function in a different model of hypertension, the DOCA-salt model. As shown in Fig. 8A, hypertension did not develop until more than 1 wk after surgery. Consequently, the study was extended to a 3-wk period to ensure that the treated mice had >1 wk of sustained hypertension. DOCA-salt treatment increased systolic blood pressure by ∼40 mmHg in both control and hLpL0 mice. In control mice, DOCA-salt hypertension significantly decreased cardiac triglyceride content (Fig. 8B) but did not alter FFA levels (control vs. control/DOCA 5.0 ± 0.6 vs. 5.0 ± 0.6 μmol/g heart, n = 5, NS). Hypertension also increased HW/BW ratio (Fig. 8C) but did not alter LVDd (from 3.77 ± 0.05 to 4.12 ± 0.09 mm, n = 5, NS). %FS was preserved (Fig. 8D). In hLpL0 mice, DOCA-salt treatment also significantly increased HW/BW ratio (Fig. 8C). LVDd was not altered by DOCA-salt treatment (from 3.86 ± 0.18 to 4.23 ± 0.14 mm, n = 4, NS). %FS was significantly reduced with DOCA-salt treatment in hLpL0 mice only (Fig. 8D).
Fig. 8.
Effect of deoxycorticosterone acetate (DOCA)-salt treatment on blood pressure, cardiac triglyceride, heart weight, and %fractional shortening. Systolic blood pressure before (day 0) and after the DOCA-salt treatment in each group. Open symbols represent control mice, and filled symbols represent hLpL0 mice. DOCA-treated mice are shown in circles and sham-operated mice in squares; n = 4–5. B: cardiac triglyceride at day 21; n = 4–5. C: heart weight-to-body weight ratio at day 21; n = 4–5. D: %fractional shortening at day 0 and day 21 in each group. Symbols are the same as in A; n = 4–5. *P < 0.05 and **P < 0.01 vs. corresponding nontreated group. †P < 0.05 vs. corresponding control group. §§P < 0.01 vs. day 0 within a group.
DISCUSSION
Methods to dissect the importance of two kinds of exogenous FAs, plasma FFAs and lipoprotein-derived FAs, were lacking until the creation of the hLpL0 mice. Our study examined the importance of LpL-derived FAs in the cardiac metabolic response to hypertension. Using hLpL0 mice, we observed the following: 1) ANG II treatment led to changes associated with increased glucose metabolism in control hearts. However, ANG II treatment did not appear to further change glucose metabolism in hLpL0 hearts. 2) In control hearts, ANG II treatment reduced FA uptake-related genes to levels found in hLpL0 hearts. ANG II treatment did not further change those genes in hLpL0 hearts. 3) LpL activity was preserved in control/ANG II hearts. 4) Mitochondrial FA oxidation-related genes were not reduced in control/ANG II hearts, although mRNA levels of those genes were lower in hLpL0/ANG II hearts than control/ANG II hearts. 5) Cardiac triglyceride stores and FFA levels were decreased by ANG II in control mouse hearts. Neither lipid was reduced below the depressed baseline levels in hLpL0 mice. 6) ANG II treatment decreased intracellular ATP content only in hLpL0 hearts. 7) Both ANG II and DOCA-salt induced hypertension caused heart failure only in hLpL0 mice.
It is widely known that pressure-overloaded and volume-overloaded hypertrophied hearts use more glucose (41). ANG II treatment (2 wk) increased basal 2-DG uptake by 4.3-fold in control/ANG II hearts; this led to uptake levels that were not significantly different from those of hLpL0 hearts. ANG II treatment (2 wk) did not increase 2-DG uptake in hLpL0 hearts. Corresponding to the glucose uptake, ANG II treatment decreased cardiac PDK4 mRNA, which suggested that control/ANG II hearts had increased glucose oxidation. PDK4 levels were not significantly different among control/ANG II, hLpL0, and hLpL0/ANG II hearts. Glycogen content did not decrease in either control/ANG II or hLpL0/ANG II hearts. Thus, although control/ANG II mouse hearts acquired and appeared to use additional calories from glucose, this did not happen in hLpL0 mice.
Why do hLpL0 hearts fail to show an increase in glucose uptake with ANG II treatment? The pathways responsible for glucose uptake in the heart are incompletely understood. Studies in genetically modified mice have suggested that there are several “backup” pathways or mechanisms that are still to be elucidated. Surprisingly, a knockout of the insulin receptor specifically in cardiomyocytes did not reduce heart glucose uptake (7). Similarly, GLUT4 knockout mice do not appear to have a heart defect in glucose uptake (1). These observations may be due to non-insulin-stimulated alternative transporters such as GLUT1 (14, 42).
Our data suggest that the heart has a maximum glucose acquisition, and this ceiling was reached in nonhypertensive hLpL0 hearts. hLpL0 hearts have greater glucose oxidation (4), but it is unlikely that glucose oxidation can be increased with ANG II treatment in the absence of additional substrate uptake. Recently, a line of transgenic mice that have augmented cardiac glucose uptake due to transgenic expression of GLUT1 was created. These mice have improved response to hypertension and ischemia (23, 24). Raising the limit on cardiac glucose uptake could potentially benefit the stressed hLpL0 hearts.
Hearts from control/ANG II mice had reduced expression of FA uptake genes, CD36 and FATP1. These reductions were similar to those of hLpL0 and hLpL0/ANG II hearts. This suggests that FA uptake via non-LpL pathways was similarly reduced in control/ANG II and hLpL0/ANG II hearts. In contrast, LpL activity was preserved in control/ANG II hearts, despite a small reduction in LpL expression. In spontaneous hypertensive rats, endothelial cell-associated LpL activity decreases (40). In contrast, LpL activity is unchanged in DOCA-salt hypertensive rats (25). Thus, unlike hLpL0 hearts, control/ANG II hearts retained the ability to acquire lipoprotein-derived FAs.
Previously, we demonstrated decreased FA oxidation in isolated perfused hLpL0 hearts (4). In the current study, we measured cardiac mRNA expression to infer the substrate usage pattern of ANG II-induced hypertrophied hearts. FA â-oxidation in animal cells occurs both in the mitochondria and in the peroxisome (21, 29). Peroxisomal FA oxidation contributes only 10–30% of total energy while mitochondrial FA oxidation is the main source of energy for the heart (37, 49). In control/ANG II hearts, mitochondrial FA oxidation-related genes such as FACS, CPT1, MCAD, and LCHAD were not significantly reduced, which suggested that control/ANG II hearts were able to obtain energy from FA oxidation while increasing glucose-derived energy during the 2 wk. Compared with control/ANG II hearts, hLpL0/ANG II hearts had significantly reduced MCAD and LCHAD expression, which suggested that hLpL0/ANG II hearts obtained less energy from FA oxidation than control/ANG II hearts.
In addition to an inability to increase glucose uptake and to acquire lipoprotein triglyceride, hLpL0 hearts are limited in a third source of energy, stored triglyceride. Endogenous triglyceride contribution to overall energy production changes depending on the concentration of exogenous fat; in isolated working rat hearts, it can range from 11% in hearts perfused with high fat to 59% in hearts perfused without fat (39). The reduced triglyceride stores of hLpL0 hearts despite normal FFA uptake reflect the reduced ability of these hearts to acquire energetic lipids in vivo. ANG II treatment of control mice decreased triglyceride stores to the same levels of hLpL0 hearts. Thus lipid uptake in control/ANG II hearts was insufficient to maintain stored triglyceride.
Although a standard approach to assessing cardiac fuel usage has been via use of isolated perfused hearts, these systems do not reproduce in vivo conditions related to usage of lipoprotein triglyceride because they typically use lipoprotein-deficient albumin-containing buffers and rely only on FFA oxidation to study fuel metabolism. Although in vivo studies can assess uptake of substrates either by using nonmetabolized tracers such as 2-DG or by assessing uptake of lipid tracers before their oxidation (5), these methods cannot directly determine substrate oxidation. Because the primary focus of our research was determination of the importance of lipoprotein substrates for the heart, lipid oxidation must be inferred from mRNA expressions.
The requirement of chemical energy in the form of ATP to support systolic work of the heart is absolute, and the hypothesis that the failing heart is energy starved is now widely believed (17, 20, 32, 33, 47). We obtained direct evidence that hLpL0/ANG II hearts were energy deficient. Intracellular ATP decreases slowly and progressively in failing hearts (44). In our study, control/ANG II hearts, which mimic compensated hypertrophied hearts, had preserved ATP content. On the other hand, hLpL0/ANG II hearts, which developed heart failure with hypertrophy, had decreased ATP content. These findings suggest that lack of cardiac LpL induced energy deficiency, thereby leading to cardiac dysfunction. However, a direct proof of this hypothesis will require a modification to restore ATP but not lipid uptake in these hearts. It is possible that other changes in cardiac metabolism cause the inability of hLpL0 hearts to adapt to hypertension.
The DOCA-salt treatment also induced hypertrophy in both control and hLpL0 mice and caused systolic dysfunction only in hLpL0 mice. These results demonstrated that ANG II-induced heart failure was not due to a drug effect of ANG II.
In summary, we demonstrated that cardiac LpL and, by inference, a continued supply of FAs derived from plasma lipoproteins are critical for normal cardiac response to hypertension. In part, this appears to result from the heart's inability to augment glucose uptake in the setting of reduced FA availability. Recently, it has been reported that dietary fat is beneficial for patients with heart failure (45). In rodent studies, high fat feeding did not worsen heart failure in a rat model of myocardial infarction and improved cardiac contractile function in hypertensive Dahl salt-sensitive rats (28, 31). Others have shown that hypertensive hypertrophied mice without signs of heart failure have preserved FA oxidation in isolated working hearts (36). FA oxidation was only slightly reduced in rat hearts with early heart failure induced by transverse aorta banding and isoproterenol stress (30). In agreement with these data showing continued FA oxidation during stress, our results demonstrate that loss of LpL activity leads to heart failure with hypertensive stress. Whether this is due to FA deficiency or some other action of LpL remains to be determined.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grants HL-73029, HL-45095, and HL-77113 (SCCOR). H. Yamashita was supported by a Mentor-Based Postdoctoral Fellowship from the American Diabetes Association. K. G. Bharadwaj was supported by a postdoctoral research grant from the American Heart Association, Founders Affiliate.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, Minnemann T, Boers ME, Hadro E, Oberste-Berghaus C, Quist W, Lowell BB, Ingwall JS, Kahn BB. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 104: 1703–1714, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA 103: 10086–10091, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustus A, Yagyu H, Haemmerle G, Bensadoun A, Vikramadithyan RK, Park SY, Kim JK, Zechner R, Goldberg IJ. Cardiac-specific knock-out of lipoprotein lipase alters plasma lipoprotein triglyceride metabolism and cardiac gene expression. J Biol Chem 279: 25050–25057, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Augustus AS, Buchanan J, Park TS, Hirata K, Noh HL, Sun J, Homma S, D'Armiento J, Abel ED, Goldberg IJ. Loss of lipoprotein lipase-derived fatty acids leads to increased cardiac glucose metabolism and heart dysfunction. J Biol Chem 281: 8716–8723, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Augustus AS, Kako Y, Yagyu H, Goldberg IJ. Routes of FA delivery to cardiac muscle: modulation of lipoprotein lipolysis alters uptake of TG-derived FA. Am J Physiol Endocrinol Metab 284: E331–E339, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Ballard FB, Danforth WH, Naegle S, Bing RJ. Myocardial metabolism of fatty acids. J Clin Invest 39: 717–723, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, Zhang D, Cooksey RC, McClain DA, Litwin SE, Taegtmeyer H, Severson D, Kahn CR, Abel ED. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest 109: 629–639, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bishop SP, Altschuld RA. Increased glycolytic metabolism in cardiac hypertrophy and congestive failure. Am J Physiol 218: 153–159, 1970. [DOI] [PubMed] [Google Scholar]
- 9.Chandler MP, Kerner J, Huang H, Vazquez E, Reszko A, Martini WZ, Hoppel CL, Imai M, Rastogi S, Sabbah HN, Stanley WC. Moderate severity heart failure does not involve a downregulation of myocardial fatty acid oxidation. Am J Physiol Heart Circ Physiol 287: H1538–H1543, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Christe ME, Rodgers RL. Altered glucose and fatty acid oxidation in hearts of the spontaneously hypertensive rat. J Mol Cell Cardiol 26: 1371–1375, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Crass MF, 3rd. Exogenous substrate effects on endogenous lipid metabolism in the working rat heart. Biochim Biophys Acta 280: 71–81, 1972. [DOI] [PubMed] [Google Scholar]
- 12.Crass MF, 3rd. Regulation of triglyceride metabolism in the isotopically prelabeled perfused heart. Fed Proc 36: 1995–1999, 1977. [PubMed] [Google Scholar]
- 13.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57: 450–458, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Doria-Medina CL, Lund DD, Pasley A, Sandra A, Sivitz WI. Immunolocalization of GLUT-1 glucose transporter in rat skeletal muscle and in normal and hypoxic cardiac tissue. Am J Physiol Endocrinol Metab 265: E454–E464, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226: 497–509, 1957. [PubMed] [Google Scholar]
- 16.Grynberg A, Demaison L. Fatty acid oxidation in the heart. J Cardiovasc Pharmacol 28, Suppl 1: S11–S17, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res 95: 135–145, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Johns C, Gavras I, Handy DE, Salomao A, Gavras H. Models of experimental hypertension in mice. Hypertension 28: 1064–1069, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Kammermeier H Microassay of free and total creatine from tissue extracts by combination of chromatographic and fluorometric methods. Anal Biochem 56: 341–345, 1973. [DOI] [PubMed] [Google Scholar]
- 20.Katz AM Is the failing heart energy depleted? Cardiol Clin 16: 633–644, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Lazarow PB, De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci USA 73: 2043–2046, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lerch RA, Bergmann SR, Ambos HD, Welch MJ, Ter-Pogossian MM, Sobel BE. Effect of flow-independent reduction of metabolism on regional myocardial clearance of 11C-palmitate. Circulation 65: 731–738, 1982. [DOI] [PubMed] [Google Scholar]
- 23.Liao R, Jain M, Cui L, D'Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation 106: 2125–2131, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Lin Z, Weinberg JM, Malhotra R, Merritt SE, Holzman LB, Brosius FC, 3rd. GLUT-1 reduces hypoxia-induced apoptosis and JNK pathway activation. Am J Physiol Endocrinol Metab 278: E958–E966, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Mallov S, Alousi AA. Effect of altered cardiac metabolism and work on lipoprotein lipase activity of heart. Am J Physiol 212: 1158–1164, 1967. [DOI] [PubMed] [Google Scholar]
- 26.Massie BM, Schaefer S, Garcia J, McKirnan MD, Schwartz GG, Wisneski JA, Weiner MW, White FC. Myocardial high-energy phosphate and substrate metabolism in swine with moderate left ventricular hypertrophy. Circulation 91: 1814–1823, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Merkel M, Eckel RH, Goldberg IJ. Lipoprotein lipase: genetics, lipid uptake, regulation. J Lipid Res 43: 1997–2006, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, Hoit BD, Stanley WC, Chandler MP. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol 290: H1899–H1904, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Norseth J, Thomassen MS. Stimulation of microperoxisomal beta-oxidation in rat heart by high-fat diets. Biochim Biophys Acta 751: 312–320, 1983. [DOI] [PubMed] [Google Scholar]
- 30.O'Donnell JM, Fields AD, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol 44: 315–322, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clin Exp Pharmacol Physiol 32: 825–831, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Olson RE Myocardial metabolism in congestive heart failure. J Chronic Dis 9: 442–464, 1959. [DOI] [PubMed] [Google Scholar]
- 33.Olson RE, Schwartz WB. Myocardial metabolism in congestive heart failure. Medicine 30: 21–41, 1951. [DOI] [PubMed] [Google Scholar]
- 34.Opie LH Metabolism of the heart in health and disease. I. Am Heart J 76: 685–698, 1968. [DOI] [PubMed] [Google Scholar]
- 35.Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurement in tissues. Anal Biochem 60: 405–412, 1974. [DOI] [PubMed] [Google Scholar]
- 36.Pellieux C, Aasum E, Larsen TS, Montessuit C, Papageorgiou I, Pedrazzini T, Lerch R. Overexpression of angiotensinogen in the myocardium induces downregulation of the fatty acid oxidation pathway. J Mol Cell Cardiol 41: 459–466, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Reubsaet FA, Veerkamp JH, Trijbels JM, Monnens LA. Total and peroxisomal oxidation of various saturated and unsaturated fatty acids in rat liver, heart and m. quadriceps. Lipids 24: 945–950, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Sack MN, Rader TA, Park S, Bastin J, McCune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94: 2837–2842, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem 266: 8162–8170, 1991. [PubMed] [Google Scholar]
- 40.Sambandam N, Chen X, Cam MC, Rodrigues B. Cardiac lipoprotein lipase in the spontaneously hypertensive rat. Cardiovasc Res 33: 460–468, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev 7: 161–173, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Santalucia T, Camps M, Castello A, Munoz P, Nuel A, Testar X, Palacin M, Zorzano A. Developmental regulation of GLUT-1 (erythroid/Hep G2) and GLUT-4 (muscle/fat) glucose transporter expression in rat heart, skeletal muscle, and brown adipose tissue. Endocrinology 130: 837–846, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Schoonderwoerd K, Broekhoven-Schokker S, Hulsmann WC, Stam H. Enhanced lipolysis of myocardial triglycerides during low-flow ischemia and anoxia in the isolated rat heart. Basic Res Cardiol 84: 165–173, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Shen W, Asai K, Uechi M, Mathier MA, Shannon RP, Vatner SF, Ingwall JS. Progressive loss of myocardial ATP due to a loss of total purines during the development of heart failure in dogs: a compensatory role for the parallel loss of creatine. Circulation 100: 2113–2118, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Taegtmeyer H, Ballal K. No low-fat diet for the failing heart? Circulation 114: 2092–2093, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension 11: 416–426, 1988. [DOI] [PubMed] [Google Scholar]
- 47.van Bilsen M, Smeets PJ, Gilde AJ, van der Vusse GJ. Metabolic remodelling of the failing heart: the cardiac burn-out syndrome? Cardiovasc Res 61: 218–226, 2004. [DOI] [PubMed] [Google Scholar]
- 48.van Bilsen M, van der Vusse GJ, Willemsen PH, Coumans WA, Roemen TH, Reneman RS. Lipid alterations in isolated, working rat hearts during ischemia and reperfusion: its relation to myocardial damage. Circ Res 64: 304–314, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Veerkamp JH, van Moerkerk HT. Peroxisomal fatty acid oxidation in rat and human tissues. Effect of nutritional state, clofibrate treatment and postnatal development in the rat. Biochim Biophys Acta 875: 301–310, 1986. [DOI] [PubMed] [Google Scholar]
- 50.Wick AN, Drury DR, Nakada HI, Wolfe JB. Localization of the primary metabolic block produced by 2-deoxyglucose. J Biol Chem 224: 963–969, 1957. [PubMed] [Google Scholar]
- 51.Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, Seo T, Hu Y, Lutz EP, Merkel M, Bensadoun A, Homma S, Goldberg IJ. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest 111: 419–426, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]