Abstract
Body fat distribution is an important predictor of metabolic abnormalities in obese humans. Dysregulation of free fatty acid (FFA) release, especially from upper body subcutaneous adipose tissue, appears to contribute substantially to these metabolic disturbances. Why different individuals preferentially store fat in upper vs. lower body subcutaneous fat or subcutaneous vs. visceral fat is not completely understood. Current evidence suggests that defects in regional lipolysis are not the cause of net fat retention in larger fat depots. Regional variations in the storage of fatty acids, both meal derived and direct reuptake, and storage of circulating FFAs that may help to explain why some depots expand at the expense of others have been reported. We review the quantitative data on regional lipolysis, meal, and FFA storage in adults to provide an overview of fat balance differences in adults with different fat distribution patterns.
Keywords: adipose tissue, lipoprotein lipase, free fatty acids, dietary fat
in the context of obesity, the metabolic complications are much more common in those with an upper body/visceral fat distribution compared with those having lower body fat distribution. Although there is considerable interest in the contribution of adipokines to these comorbidities, we will focus on the role of fatty acids as they relate to obesity-associated complications and regional adiposity. The objective of this review is to describe recent findings that contribute to our knowledge of regional fat distribution and why it matters.
Consequences of High-Circulating Free Fatty Acids
The effect of free fatty acids (FFAs) on glucose metabolism in humans has been studied extensively; it is well established that obesity and increased plasma FFA concentrations are risk factors for the development of type 2 diabetes mellitus (T2DM) (20, 26). The ability to manipulate FFA concentrations has allowed scientists to show that the FFA/metabolic function associations are not merely related abnormalities but cause and effect. Studies using acipimox, an inhibitor of lipolysis, to lower FFA or lipid emulsion infusions to raise FFA have helped define the contribution of FFA to insulin action with respect to glucose, lipoprotein, and vascular regulation (32). Elevated plasma FFAs upregulate glucose production and impair muscle glucose uptake, oxidation, and storage (15, 32). FFAs also affect insulin secretion (25) as intracellular metabolites of fatty acids such as long-chain acyl-CoA and diacylglycerol trigger insulin release (29, 30) as well as β-cell dysfunction (31). The perturbations in glucose metabolism and insulin secretion that reflect increased systemic FFAs are implicated in the etiology of T2DM.
In addition to its effects on glucose metabolism and diabetes risk, elevated FFA concentrations have been shown to be risk markers for ischemic heart disease (28), perhaps indirectly through playing a part in the development of hypertension (36, 38) or directly via induction of vascular endothelial injury (27). Elevation of FFAs induced by lipid emulsion/heparin infusions has been shown to increase oxidative stress markers (37).
Contribution of Regional Body Fat Distribution to Circulating FFAs
Circulating FFAs appear to play a significant role in the development of T2DM and cardiovascular disease. Thus, understanding the origin of FFAs may help in developing therapies that might prevent disease. In the context of obesity, a predominantly upper body fat distribution, defined as a waist-to-hip circumference ratio of >0.85 in women and >0.95 in men, is associated with greater postabsorptive and postprandial plasma FFA concentrations. Often times an upper body fat distribution is associated with large omental and/or mesenteric fat stores, i.e., visceral fat. The importance of these two depots as visceral fat relates to the fact that the FFAs and adipokines they release enter into the portal vein rather than the systemic circulation. As such, visceral fat may have a disproportionate effect on hepatic metabolism.
Variations in systemic plasma FFA concentrations are in large part due to variations in lipolysis (23), although FFA clearance may play a role regulating FFA concentrations under some conditions. Visceral fat is a good predictor of insulin suppression of systemic FFA release in humans (2), but this does not mean that visceral fat is the source of excess FFAs (2, 24). Although there are some differences in the regional contribution to plasma FFAs according to fat distribution (19, 24), the majority of FFAs are released from upper body subcutaneous fat in both the postprandial (19, 24) and postabsorptive (2, 12) states.
The varying contribution of different regional depots to plasma FFAs emphasizes the importance of understanding interindividual differences in body fat accumulation. In order for one fat depot to expand at the expense of another, an imbalance must exist between in storage and release of fatty acids in that depot relative to other depots. The reason why people differ in body fat distribution has not been fully elucidated, but we will review what has been learned.
Differences in Lipolysis Do Not Explain Variations in Body Fat Distribution
If defects in lipolysis were a main cause of differences in regional fat deposition, lipolysis would vary in a manner that would be expected to result in retention of fat in certain depots. Specifically, decreases in regional lipolysis would be expected to lead to increases in regional fat accumulation. Evidence against this hypothesis comes from studies showing similar regional lipolysis patterns in normal-weight women and men with markedly different fat distributions. Upper body adipose tissue is more lipolytically active than lower body adipose tissue, regardless of sex (10). In addition, women with upper body obesity have greater FFA release from upper body subcutaneous fat per kilogram of fat than do lower body obese women under fasted (19) and fed (8) conditions. These data argue that selective regional defects in lipolysis are unlikely to explain differences in regional subcutaneous fat accumulation among men and women. The inability to measure FFA release directly into the portal vein in humans has left us with only indirect estimates of visceral adipose tissue lipolysis (24), and thus we cannot know whether defective visceral adipose tissue lipolysis contributes to visceral fat gain in predisposed individuals.
Fatty Acid Uptake Appears to Contribute to Differences in Regional Body Fat Accumulation
Meal fatty acid metabolism.
Adipocytes take up circulating fat via two pathways. The major and most well-understood pathway of fatty acid uptake is dependent upon lipoprotein lipase (LPL), and the other, less well-appreciated mechanism is direct uptake and storage of circulating FFA. LPL is responsible for the hydrolysis of meal-derived triglycerides in chylomicrons and VLDL-triglyceride (TG) at the capillary endothelium. The fatty acids thus released can be taken up by local cells or “spillover” into the systemic circulation (21, 34). Given that dietary fat intake, and thus chylomicron TG delivery to the circulation, is commonly ≥100 g/day and VLDL-TG secretion is ∼15–25 g/day, it is easy to understand why variations in LPL activity would be seen as an important issue for fatty acid storage.
Regional meal fat storage has been measured by providing volunteers with a meal containing a (3H or 14C) fatty acid tracer and then performing adipose tissue biopsies ∼24 h later. The storage of dietary fatty acids is traced by measuring the adipose tissue lipid-specific activity and relating that to the meal fatty acid-specific activity. The pioneering studies of Mårin and colleagues (17, 18) stimulated further studies that have shown that more meal fatty acids (per g adipose lipid) are stored in abdominal fat than in leg fat in both normal-weight men and women. However, following the consumption of high-fat, high-calorie meals, women store an increased proportion of dietary fat in leg fat compared with men (43), and this is associated with greater activity of LPL in femoral adipose tissue. For both normal-weight men and women, the storage of dietary fatty acids in upper and lower body subcutaneous fat was strongly correlated with postprandial, but not postabsorptive, adipose tissue LPL activity (42). Of note, we could not detect a similar relationship between adipose tissue LPL activity and meal fatty acid storage in a mixed group of normal-weight, overweight, and obese women despite measuring LPL in the fed state (43).
The effects of a high-fat vs. normal-fat meal on regional meal fatty acid storage, including visceral fat, have also been studied in women with a wide range of adiposity and body fat distribution (43). Following consumption of an isocaloric normal-fat (27% fat) meal, meal fatty acid storage (mg meal fat/g adipose lipid) was greater in women with more leg fat compared with those with less leg fat. This was in contrast to the patterns of meal fatty acid storage in upper body subcutaneous fat (no relationship) and visceral fat, where those with more stored less meal-derived fat per gram of adipose lipid than those with less visceral fat (43). This suggests to us that the different adipose depots may have different rate-limiting steps for meal fatty acid storage. If this is the case, then circumstances that alter these rate-limiting steps could alter regional fat storage. For example, following consumption of a high-fat meal, the pattern of meal fatty acid storage in leg, abdominal, and visceral fat storage changed remarkably (43). Specifically, within each regional depot, those with more fat stored less meal fat per gram of adipose lipid than those with less fat (43). This change suggests that consumption of high-fat, high-calorie meals might impact body fat distribution over time.
Direct uptake and storage of FFA.
During intravenous glucose infusions (5) and following meal ingestion (3), disappearance of circulating FFAs across abdominal subcutaneous adipose has been detected using the stomach vein catheterization techniques. Despite this, we had assumed that adipose tissue would not simultaneously take up and store circulating FFAs in the postabsorptive state because under these conditions adipose tissue actively exports FFAs. In the process of performing experiments to test this assumption, we made the surprising observation that subcutaneous adipose tissue does indeed store a detectable fraction of systemic FFAs in humans (16, 35). These FFAs, released from adipocytes into the systemic circulation, appear to be taken up and reesterified in distant adipocytes. The pattern of direct FFA storage is different in men and women, and these differences suggest that this LPL-independent pathway of fatty acid storage may be a significant process in the regulation or maintenance of body fat distribution (35).
In vivo detection of direct FFA storage can be measured through bolus intravenous administration of 3H- or 14C-labeled FFAs followed by carefully timed adipose tissue biopsies (35). Direct storage measurement in subcutaneous adipose tissue in lean and obese men and women showed greater overall direct FFA storage in women. This is consistent with in vitro data demonstrating that subcutaneous adipose tissue from women took up and esterified extracellular radiolabeled FFAs twice as well as comparable tissue from men (6). This greater subcutaneous storage in women is concordant with the fact that females have more subcutaneous body fat than males at any given body mass index (BMI). We also found that the efficiency of FFA storage per gram of fat was 30% greater in abdominal subcutaneous fat than in femoral subcutaneous fat in nonobese men, but there was no difference between these two depots in nonobese women (35). In obese women, the direct storage of FFAs was 40% more efficient in the femoral than in the abdominal region (35), and the efficiency of FFA storage increases as a function of leg fat mass in premenopausal women (16). In contrast, direct FFA storage into upper body subcutaneous and visceral fat does not follow this pattern. In women, upper and lower body subcutaneous fat store 6.7 and 4.9% circulating FFA, respectively, directly, whereas storage of FFAs in visceral adipose tissue was only ∼1.0% irrespective of visceral fat mass (16). The differences in regional efficiency of direct FFA uptake support sex-related variations in body fat distribution, where women tend to store more fat in the lower body and men in the upper body.
The reason(s) for the regional and sex differences in direct FFA storage is not known. Sex hormones may play a potential role, since variations in hormone levels are accompanied by changes in body fat distribution, such as is the case after menopause (7, 9). We did not find blood flow differences that could account for greater storage in women than men or between abdomen and thigh in men (16). We did find greater expression of mRNA encoding fatty acid transport proteins in women than men and in the abdominal vs. femoral subcutaneous adipose tissue in men (16). Thus, regional variation in membrane uptake of FFAs may affect regional body fat accumulation, but many other possible explanations need to be explored.
Integrating Regional Fatty Acid Storage and Release
Tracer studies have allowed us to follow the pathways through which fat is metabolized in the body and quantify the extent to which these pathways may modulate body fat distribution. A summary of the extent to which oxidation, storage, and lipolysis contribute to regional body fat accumulation in normal-weight men and women is depicted in Fig. 1. For this example, we used normal-weight men and women (BMI ∼22.5 kg/m2) with average body fat content (15% for men, 30% for women). Both men and women were assumed to consume a diet that provides 75 g/day of fat over three meals, and energy/macronutrient balance is maintained. We assume that 2 h of the day are spent doing physical activity at a level that will affect lipolysis and fat oxidation and that 8 h are spent in the postprandial state. We used data for meal fat storage, data for regional FFA release under fed, fasted, and exercise conditions, and direct FFA storage data to display what is known about the interchange of fatty acids between various sources.
Fig. 1.
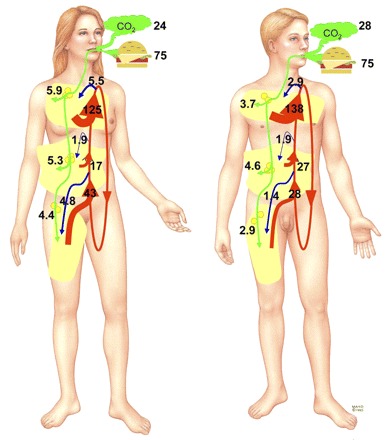
Fatty acid kinetics (in g) over 24 h in a normal-weight (body mass index ∼22.5 kg/m2) man with 15% body fat and a normal-weight woman with 30% body fat are depicted. To make the calculations, we assumed that 14, 8, and 2 h of the day were spent in the postprandial state, in the postabsorptive state, and doing physical activity at a level that would affect lipolysis and fat oxidation, respectively. Regional fatty acid metabolism is represented from legs to chest as lower body subcutaneous adipose tissue, visceral adipose tissue, and upper body subcutaneous adipose tissue. Green arrows represent the regional meal fat storage via lipoprotein lipase-mediated pathways and oxidation assuming a 75 g/day ingestion of meal fat. Blue arrows represent the direct storage of free fatty acids into regional depots from the circulating free fatty acid (FFA) pool. Red arrows indicate the regional flow of FFAs that enter the circulation via lipolysis. Size of arrow is proportional to the contributory flow of FFAs to each pool. Reprinted with permission from the Mayo Foundation for Medical Education and Research.
Data from tracer studies measuring meal fatty acid oxidation using were used to calculate 24-h meal fat oxidation as depicted by CO2 in Fig. 1, assuming 22 h of nonexercise (14, 33, 39, 42, 43) and 2 h of exercise (40). Average nonexercise oxidation is estimated to be ∼21 g/day in women and ∼25 g in men. Meal fatty acid oxidation with prior exercise was estimated to be 3.3 g (41). Thus, of the 75 g of fatty acids oxidized over 24 h, ∼25–30 g are from dietary fat and the remainder from endogenous fatty acids, most likely FFAs.
Meal fatty acid storage in visceral, upper body, and lower body subcutaneous depots was estimated using published data where normal-fat meals were employed (14, 33, 39, 42, 43). The storage in visceral and upper and lower body subcutaneous fat in women and men is provided as green arrows in Fig. 1.
Entry of FFAs from leg, visceral, and upper body subcutaneous adipose tissue lipolysis into the systemic circulation was estimated from regional catheterization studies performed under resting, overnight postabsorptive (8, 10, 12, 19, 22, 24), postprandial (8, 10, 22), and exercise (1, 4, 44). The FFA release values as shown by red arrows in Fig. 1 represent the integrated estimates from these studies and are time-weighted averages for 14 h in the postabsorptive state, 8 h postprandial, and 2 h of exercise.
As expected, postabsorptive lipolysis was a large contributor to overall daily lipolysis. Over 14 h, lipolysis in women is estimated to be 88, 27, and 11 g from subcutaneous upper body fat, subcutaneous lower body fat, and visceral adipose tissue, respectively. In men, the 14-h postabsorptive lipolysis is estimated to be 102, 16, and 17 g, respectively. The visceral data represent FFAs that enter the systemic circulation from the hepatic vein, which underestimates true visceral adipose tissue lipolysis.
For 8 h in the postprandial state, upper body lipolysis is ∼13 g in women and ∼18 g in men, lower body lipolysis is ∼5 g in women and ∼4 g in men, and visceral adipose tissue lipolysis was ∼4 g in women and ∼6 g in men.
Unfortunately, there seem to be little or no data on splanchnic lipolysis during exercise in women. To estimate this value, we used lipolysis data from an epinephrine infusion experiment (11) because catecholamines are significant regulators of lipolysis during exercise. However, we acknowledge that the metabolic response to exercise is more complicated, and thus the splanchnic values are less reliable for women.
Figure 1 shows that lipolysis is an unlikely contributor to regional fat distribution because release of FFAs from upper body subcutaneous fat, visceral adipose tissue, and lower body subcutaneous fat is greater with larger depots. For women, who tend to accrue lower body fat, lipolysis of lower body fat is greater than in men. Men tend to accumulate upper body fat and have greater FFA release from upper body subcutaneous and visceral fat.
Finally, we used the limited data on direct FFA storage (represented as blue arrows in Fig. 1) (16, 35) to calculate how much of the systemic FFA reenter the different fat depots. Unfortunately, we have data only from women for visceral FFA uptake and thus cannot know what the role of this process is in men. The extent to which postprandial and exercise alter direct FFA uptake is unknown.
Thus, in normal-weight individuals, variations in net regional fat storage are likely due to differences in fatty acid uptake, probably from a combination of meal-related and direct FFA storage. Whether this mechanism is similar in overweight individuals is under investigation.
Conclusion
We are making progress in understanding how differences in body shapes develop in humans. Preferential accumulation of body fat in specific regions is more likely due to preferential fat uptake than defective release. Recent evidence suggests that both LPL-mediated and LPL-independent fat storage may play a role in sex-related variations in fat distribution. The mechanisms that determine why some fat cells store more fatty acids than others, differences in delivery, plasma membrane transport, or intracellular processing remain to be clarified. The rate-controlling steps by which fatty acids are stored in adipocytes may vary according to depot (16, 43). Future research will hopefully provide a more complete picture of fatty acid trafficking into adipocytes in the hopes of understanding why some people are shaped differently than others. The answer to this question is important, because how fat is distributed in our bodies is related to disease risk.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-40484, DK-45343, and DK-50456 and the Mayo Foundation.
REFERENCES
- 1.Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest 53: 1080–1090, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basu A, Basu R, Shah P, Vella A, Rizza RA, Jensen MD. Systemic and regional free fatty acid metabolism in type 2 diabetes. Am J Physiol Endocrinol Metab 280: E1000–E1006, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ, Gilbert M, Karpe F, Frayn KN. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 56: 168–176, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Burguera B, Proctor DN, Dietz W, Guo Z, Joyner MJ, Jensen MD. Leg FFA kinetics during exercise in men and women. Am J Physiol Endocrinol Metab 278: E113–E117, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Coppack SW, Persson M, Judd RL, Miles JM. Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol Endocrinol Metab 276: E233–E240, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Edens NK, Fried SK, Kral JG, Hirsch J, Leibel RL. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am J Physiol Endocrinol Metab 265: E374–E379, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani AR. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab 82: 414–417, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Guo ZK, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 48: 1586–1592, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism 40: 1323–1326, 1991 [DOI] [PubMed] [Google Scholar]
- 10.Jensen MD. Gender differences in regional fatty acid metabolism before and after meal ingestion. J Clin Invest 96: 2297–2303, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen MD, Cryer PE, Johnson CM, Murray MJ. Effects of epinephrine on regional free fatty acid and energy metabolism in men and women. Am J Physiol Endocrinol Metab 270: E259–E264, 1996 [DOI] [PubMed] [Google Scholar]
- 12.Jensen MD, Johnson CM. Contribution of leg and splanchnic free fatty acid (FFA) kinetics to postabsorptive FFA flux in men and women. Metabolism 45: 662–666, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Jensen MD, Sarr MG, Dumesic DA, Southorn PA, Levine JA. Regional uptake of meal fatty acids in humans. Am J Physiol Endocrinol Metab 285: E1282–E1288, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Kelley DE, Mokan M, Simoneau JA, Mandarino LJ. Interaction between glucose and free fatty acid metabolism in human skeletal muscle. J Clin Invest 92: 91–98, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koutsari C, Dumesic DA, Patterson BW, Votruba SB, Jensen MD. Plasma free fatty acid storage in subcutaneous and visceral adipose tissue in postabsorptive women. Diabetes 57: 1186–1194, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mårin P, Odén B, Björntorp P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab 80: 239–243, 1995 [DOI] [PubMed] [Google Scholar]
- 18.Mårin P, Lönn L, Andersson B, Odén B, Olbe L, Bengtsson BA, Björntorp P. Assimilation of triglycerides in subcutaneous and intraabdominal adipose tissues in vivo in men: effects of testosterone. J Clin Endocrinol Metab 81: 1018–1022, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest 88: 609–613, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Nelson RH, Basu R, Johnson CM, Rizza RA, Miles JM. Splanchnic spillover of extracellular lipase-generated fatty acids in overweight and obese humans. Diabetes 56: 2878–2884, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TT, Mijares AH, Johnson CM, Jensen MD. Postprandial leg and splanchnic fatty acid metabolism in nonobese men and women. Am J Physiol Endocrinol Metab 271: E965–E972, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Nielsen S, Guo Z, Albu JB, Klein S, O'Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest 111: 981–988, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen S, Guo ZK, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 113: 1582–1588, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paolisso G, Gambardella A, Amato L, Tortoriello R, D'Amore A, Varricchio M, D'Onofrio F. Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia 38: 1295–1299, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Paolisso G, Tataranni PA, Foley JE, Bogardus C, Howard BV, Ravussin E. A high concentration of fasting plasma non-esterified fatty acids is a risk factor for the development of NIDDM. Diabetologia 38: 1213–1217, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Piro S, Spampinato D, Spadaro L, Oliveri CE, Purrello F, Rabuazzo AM. Direct apoptotic effects of free fatty acids on human endothelial cells. Nutr Metab Cardiovasc Dis 18: 96–104, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Pirro M, Mauriege P, Tchernof A, Cantin B, Dagenais GR, Despres JP, Lamarche B. Plasma free fatty acid levels and the risk of ischemic heart disease in men: prospective results from the Quebec Cardiovascular Study. Atherosclerosis 160: 377–384, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Prentki M. New insights into pancreatic beta-cell metabolic signaling in insulin secretion. Eur J Endocrinol 134: 272–286, 1996 [DOI] [PubMed] [Google Scholar]
- 30.Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in beta-cell adaptation and failure in the etiology of diabetes. Diabetes 51: S405–S413, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest 116: 1802–1812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab Rev 14: 263–283, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 279: E455–E462, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Roust LR, Jensen MD. Postprandial free fatty acid kinetics are abnormal in upper body obesity. Diabetes 42: 1567–1573, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes 56: 1369–1375, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 100: 1230–1239, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stojiljkovic MP, Lopes HF, Zhang D, Morrow JD, Goodfriend TL, Egan BM. Increasing plasma fatty acids elevates F2-isoprostanes in humans: implications for the cardiovascular risk factor cluster. J Hypertens 20: 1215–1221, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Stojiljkovic MP, Zhang D, Lopes HF, Lee CG, Goodfriend TL, Egan BM. Hemodynamic effects of lipids in humans. Am J Physiol Regul Integr Comp Physiol 280: R1674–R1679, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Uranga AP, Levine J, Jensen M. Isotope tracer measures of meal fatty acid metabolism: reproducibility and effects of the menstrual cycle. Am J Physiol Endocrinol Metab 288: E547–E555, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Votruba SB, Atkinson RL, Hirvonen MD, Schoeller DA. Prior exercise increases subsequent utilization of dietary fat. Med Sci Sports Exerc 34: 1757–1765, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Votruba SB, Atkinson RL, Schoeller DA. Sustained increase in dietary oleic acid oxidation following morning exercise. Int J Obes (Lond) 29: 100–107, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Votruba SB, Jensen MD. Sex-specific differences in leg fat uptake are revealed with a high-fat meal. Am J Physiol Endocrinol Metab 291: E1115–E1123, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Votruba SB, Mattison RS, Dumesic DA, Koutsari C, Jensen MD. Meal fatty acid uptake in visceral fat in women. Diabetes 56: 2589–2597, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Wahren J, Sato Y, Ostman J, Hagenfeldt L, Felig P. Turnover and splanchnic metabolism of free fatty acids and ketones in insulin-dependent diabetics at rest and in response to exercise. J Clin Invest 73: 1367–1376, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]


