Abstract
It has previously been shown that insulin is secreted in discrete secretory bursts by sampling directly from the portal vein in the dog and humans. Deficient pulsatile insulin secretion is the basis for impaired insulin secretion in type 2 diabetes. However, while novel genetically modified disease models of diabetes are being developed in rodents, no validated method for quantifying pulsatile insulin secretion has been established for rodents. To address this we 1) developed a novel rat model with chronically implanted portal vein catheters, 2) established the parameters to permit deconvolution of portal vein insulin concentrations profiles to measure insulin secretion and resolve its pulsatile components, and 3) measured total and pulsatile insulin secretion compared with that in the dog, the species in which this sampling and deconvolution approach was validated for quantifying pulsatile insulin secretion. In rats, portal vein catheter patency and function were maintained for periods up to 2–3 wk with no postoperative complications such as catheter tract infection. Rat portal vein insulin concentration profiles in the fasting state revealed distinct insulin oscillations with a periodicity of ∼5 min and an amplitude of up to 600 pmol/l, which was remarkably similar to that in the dogs and in humans. Deconvolution analysis of portal vein insulin concentrations revealed that the majority of insulin (∼70%) in the rat is secreted in distinct insulin pulses occurring at ∼5-min intervals. This model therefore permits direct accurate measurments of pulsatile insulin secretion in a relatively inexpensive animal. With increased introduction of genetically modified rat models will be an important tool in elucidating the underlying mechanisms of impaired pulsatile insulin secretion in diabetes.
Keywords: deconvolution, pulse mass
glucose homeostasis depends on adequate insulin secretion. Insulin is secreted in coordinate discrete secretory bursts by the pancreatic islets of Langerhans (7, 12, 17). Although this pattern of insulin secretion results in relatively modest oscillations in insulin concentration in the systemic circulation, direct sampling from the portal vein in dogs and humans reveals large amplitude oscillations in insulin concentration with a periodicity of ∼4 min (23, 30). Therefore, to evaluate pulsatile insulin secretion, it is preferable to sample directly from the portal vein. This has been accomplished in humans under exceptional circumstances (30), but in general animal models are required to investigate pulsatile insulin secretion. Because of blood volume considerations, these studies (21, 24, 25) were for the most part initially carried out in large animals such as the dog.
Since anesthesia profoundly suppresses pulsatile insulin secretion (35), we developed a surgical approach that permitted chronic portal vein catheters to allow direct portal vein sampling in the conscious dog (34). By suppressing endogenous insulin secretion and infusing known insulin pulses upstream of the sampling catheter, we then evaluated the most favorable approach to quantify pulsatile insulin secretion from portal vein insulin concentration time series (23). We thereby validated a multiparameter deconvolution program that recovered the known insulin infusion rates and accurately resolved pusaltile from basal nonpulsatile insulin secretion from portal vein insulin concentrations time series (23). We further established the optimal sampling intensity and minimum duration required to reliably quantify insulin secretion from the portal vein (22). Subsequent studies (21, 24, 25) in the canine model provided insights into the regulation of pulsatile insulin secretion.
However, there is an increasing need to bring physiological methods to evaluate rodent genetic models of disturbed glucose metabolism. Recently, it has become feasible to consider similar studies in the rat given the much smaller blood samples required to measure insulin with ELISA methods. Given this development, we sought to extend the approach previously developed and validated in the dog to permit measurement of pulsatile insulin secretion in the conscious free living rat. Since the approach was initially validated in the dog, we simultaneously undertook studies in dogs to permit a direct comparison of the measured insulin secretion profile in the dog and rat.
RESEARCH DESIGN AND METHODS
Study design.
The studies were approved by the University of California Los Angeles Institutional Animal Care and Use Committee. First, to permit measurement of the magnitude and frequency of insulin secretory bursts into the portal vein of the rat, a surgical preparation was developed with chronic indwelling portal vein sampling catheters. Concurrently, we established a portal vein sampling protocol with sufficient sampling intensity and duration to permit quantification of pulsatile insulin secretion, while not compromising the rat blood volume. Second, we undertook studies to establish the insulin decay kinetics and insulin volume of distribution in the rat portal vein to permit deconvolution of the insulin concentration time series into insulin secretion and in particular the magnitude and frequency of its principal constituent component, insulin secretory bursts. Third, we applied these methods along with those previously available to quantify the fasting insulin secretion rate in Sprague-Dawley rats at age 3 mo. Rats were housed individually at University of California Los Angeles animal housing facility. Rats were maintained in a standard 12:12-h light-dark cycle and fed ad libitum Rodent Diet 8604 (72% carbohydrate, 24% protein, and 4% fat; Harlan Teklad, Madison, WI). In total, 15 Sprague-Dawley male rats were studied.
In addition, seven mixed-bred mongrel dogs ∼1- to 3-yr-old and weighing 20–24 kg were used in the present study. Dogs were housed in individual kennels and fed standard canine chow. Additionally, all dogs were conditioned for at least 3 wk to adapt to a laboratory sling and laboratory personnel. After the conditioning period, complete blood counts were obtained for all dogs to ensure that subjects were within physiological limits. All animal use procedures incorporated in the reported studies were conducted in accordance with an animal use protocol approved by the Institutional Animal Care and Use Committee at the Mayo Clinic. Fasting portal insulin concentration data from these dogs were used in previous study (15).
Surgical implantation of catheters.
Rats were anesthetized by isoflourane (2.5%) inhalation (Isoflourane Vapor 19.1, Summit Anesthesia, Portland, OR). Under aseptic conditions, an approximate 3-cm midline laparotomy was performed to expose the portal and mesenteric veins. with the use of blunt forceps, the portal vein was separated from the underlying connective tissue and an area of ∼1 cm of the vein was clamped by two smooth small clamps (Fine Science, Foster City, CA) for no longer than 3 min. Thereafter, one stitch was made in the superficial wall of the vein using sterile 7–0 cardiovascular silk thread suture (Western Medical, Arcadia, CA). A 26-gauge needle was then used to penetrate the full thickness of the ventral exposed wall of the portal vein caudal to the anchoring suture. The tip of the portal vein sampling catheter (silastic tubing, 0.051-cm ID cannula with 0.03-cm ID tip) was cut at an angle of ∼45 degrees (beveled) and then advanced through the hole made by the needle into the lumen of the portal vein and then advanced (∼1.5 cm) until the catheter tip was placed at the intrahepatic bifurcation of the portal vein. The catheter was then secured to the vessel with the suture, and the clamps were gently removed. In a subset of animals undergoing validation studies (n = 8), an additional mesenteric vein catheter was inserted. The above-described technique was repeated with the exception that the catheter was placed distal from the liver into the inferior mesenteric vein. The catheters were secured to the abdominal wall by suture, and then the peritoneum and an abdominal muscles were closed with 4-0 Vicryl suture (Western Medical) and the skin in layers with 4.0 nonabsorbable nylon suture (Western Medical). The portal vein and mesenteric vein catheters were then channeled subcutaneously to the back of the neck for encasement into an infusion harness. Additionally, an indwelling catheter was inserted into the left carotid artery (polyethylene tubing, PE-50, Clay Adams 0.058 ID). In short, under aseptic conditions an ∼2.5-cm neck incision was performed, the underlying connective tissue layer was teased out, and the left carotid artery was exposed. The artery was subsequently ligated above the clavicle, and PE-50 tubing was introduced anterogradely and attached to the artery and underlying tissue by silk suture. All catheters were filled with 100 U/ml of heparin/saline, exteriorized to the back of the neck and encased in the infusion harness (Instech). Catheters were flushed daily with 100 U/ml heparin/saline solution except on the day of the experiment when catheters were aspirated and then flushed with saline only to avoid heparin “spill over” into circulation. Immediately postoperatively, and for the next 3, days all animals received preventative antibiotic treatment (sulfamethoxazole, Hi-Tech Pharmaceutical, Amityville, NY; 200 mg in drinking water). Rats were studied 5–7 days after surgical implantation of catheters. The rats maintained their preoperative body weight and had normal food intake and normal hematocrit (42 ± 2%) at the time of subsequent studies.
The surgical implantation of the portal vein and arterial sampling catheters in the dog has been previously described in detail (23). In brief, after an overnight fast, the dogs were anesthetized using thiobarbiturate. Once anesthesia was achieved, it was subsequently maintained with 1.5–2.5% halothane in 3 liters of oxygen and 2 liters of nitric oxide/min. After exposure through a midline incision, the portal vein was dissected free for a distance of 5–6 cm and the portal vein sampling catheter was placed into the portal vein at the bifurcation of the portal vein in the liver. An arterial sampling catheter was also placed into the carotid artery and similarly tunneled into the subcutaneous pocket. All dogs were studied 2 wk after the surgery week and all recovered their preoperative body weight and had a normal hematocrit (40%), hemoglobin (14 g/dl), and white blood cell count before the study.
Volume of distribution and decay kinetics for insulin in the rat portal vein.
Deconvolution of portal vein insulin concentration time series to quantify the corresponding insulin secretion rate requires knowledge of the corresponding volume of distribution and decay constants (i.e., monoexponential vs. biexponential half lives). The model comprises insulin delivered by the pancreas into a tributary of the portal vein and measured at a downstream portal vein sampling catheter. This was accomplished by administration of 1.0, 5.0, or 15 mU of insulin (Novolin, NovoNordisk; in 0.3 ml of saline in 0.25% albumin) via the mesenteric vein catheter over 10 s during suppression of endogenous insulin secretion by somatostatin (Bachem, Torrance, CA) at 10 μg·kg−1·min−1 via the jugular vein catheter. After bolus injection of insulin, blood was sampled from both the portal vein and carotid artery at 15-s intervals for 2 min and thereafter every minute for the next 13 min for measurement of insulin concentrations. The blood glucose concentration was measured at 5-min intervals, and a variable glucose infusion was given via the jugular vein catheter to prevent hypoglycemia. The fitted decay of injected insulin was allowed to proceed to toward postinjection baseline.
Insulin secretion.
On the morning of the study, rats were weighed and sampling extensions were placed on the portal vein and carotid artery sampling catheters. The rats were then placed in a cage outfitted with a counter-weighted-swivel mount (Instech) and allowed to rest for at least 60 min (−90 to −30 min). After a 30-min equilibration period (−30 to 0 min), portal vein blood (∼80 μl) was sampled every minute for 35 min and arterial samples (∼100 μl) were sampled every 10 min. Blood samples were collected in prechilled microcentrifuge tubes containing protease inhibitor cocktail solution (Sigma, St. Louis, MO) and EDTA (0.15 mg/0.1 ml of blood) and immediately centrifuged. Plasma was immediately stored at −80°C or subsequent analysis. To minimize the effects of frequent blood sampling, blood from a donor animal was quantitatively replaced throughout the study period by administering ∼1 ml blood every 10 min into the carotid artery catheter. This blood replacement protocol has been shown to have a negligible effect on both systemic and portal vein insulin concentrations.
After an overnight fast (16 h), dogs were placed in a laboratory sling. Portal vein and arterial sampling catheters were exteriorized from the subcutaneous pocket after the use of a local anesthetic. Normal saline was infused through the foreleg infusion catheter at 30 ml/h throughout the study (0–150 min). Blood (∼1 ml) was then sampled from the portal vein catheter at 1-min intervals for measurement of the plasma insulin concentrations for 60 min. Additionally, blood samples (∼1 ml) were also obtained at 10-min intervals from the arterial catheter for corresponding measurements of plasma insulin concentrations. All samples for plasma insulin were taken into ice-cold glass tubes containing EDTA, immediately cold centrifuged, and stored at −20°C until analyzed. Plasma glucose levels were measured in additional 0.5-ml blood samples using the glucose oxidase method (Beckman Instruments, Fullerton, CA) collected at 10-min intervals from the arterial catheter during the entire study (0–150 min).
Analytical procedures.
Insulin concentrations in rat blood were measured in duplicate by an insulin ELISA (ALPCO Diagnostics, Salem, NH). The primary antibody is specific to rat insulin. The sensitivity range of the assay is 25–931 pmol/l, and all samples >931 pmol/l were diluted to achieve binding in the linear part of the standard curve. Intra- and interassay variation was at 3% or less. Insulin concentrations in dog plasma were measured in triplicate by radioimmunoassay (23). The operating range of the assay is 15 to 968 pmol/l, using 50-μl plasma samples. The intraassay and interassay coefficients of variation were 5 and 15%. Human insulin was measured in duplicate with an in-house two-site immunospecific ELISA. There is no cross-reactivity with proinsulin and split 32,33 and des-31,32 proinsulins. The lower detection limit for this assay is 4 pmol/l, and the assay range is 5–2,000 pmol/l. The intraassay and interassay coefficients of variation were ∼3 and 5%. Glucose concentrations were measured by the glucose oxidase method (Beckman Glucose Analyzer 2).
Calculations.
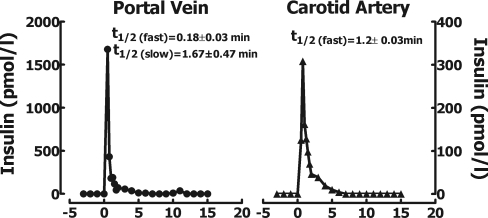
The portal vein plasma insulin concentrations were subjected to the multiparameter deconvolution analysis as previously described in detail (23). Briefly, multiparameter deconvolution technique used in this study assumes that time varying insulin concentrations can be decomposed mathematically into the following: 1) a finite number of discrete insulin secretory bursts occurring at specific times having 2) individual amplitudes and 3) a common half-duration, wherein bursts are superimposed upon 4) a basal time-invariant insulin secretory rate and 5) insulin disappearance in the portal vein is modeled via rapid and slow half-lives of 0.18 and 1.67 min and fractional slow-component amplitude of 0.065 in the rat (as obtained from this study; Fig. 1) and consisting of half-lives of 0.2 and 3 min in dogs (as obtained previously in Ref. 23). Insulin half-lives were estimated by fitting a biexponential or monoexponential function to the observed insulin concentration series (33). The optimal model was selected by the Akaike information coefficient (1).
Fig. 1.
Plasma insulin concentrations in rats at the portal vein (left) and carotid artery (right) after a bolus of insulin (5 mU) was injected into the inferior mesenteric vein at the time (0 min) during somatostatin (10 μg·kg−1·min−1) infusion to ablate endogenous insulin secretion. The decay curve of insulin concentrations was biexponential at the portal vein and monoexponential at the arterial sampling site. The mean calculated mono- and biexponential half-lives are shown on the graph.
Calculations of insulin secretion parameters.
Deconvolved insulin secretion rates (see Fig. 3) were expressed as mass units of insulin (pmol) released per unit of portal vein volume of distribution per unit of time (min), corrected for body weight (kg). Insulin burst mass (time integral of calculated burst mass) was computed as mass units of insulin (pmol) released per unit of portal vein volume of distribution per body weight (kg). Mean insulin burst mass (see Fig. 4D) was derived from a median insulin burst mass obtained during the sampling time interval (0–35 min). Mean insulin secretion (see Fig. 4C) was calculated as a sum of the nonpulsatile (basal) component of insulin secretion and insulin secretion derived from insulin pulses expressed as mass units of insulin (pmol) released per unit of portal vein volume of distribution per unit of time (min) and corrected for body weight (kg). The percentage of insulin derived from secretory burst (see Fig. 4A) was calculated as a ratio of insulin secretion derived from insulin pulses to total insulin secretion. Interpulse interval (see Fig. 4B) was derived as a median time interval (min) between consecutive insulin peaks. The mean rate of clearance of endogenously secreted insulin was calculated using the following equation: C (ml·min−1·kg body wt−1) = S (pmol/min)/I (pmol/l)/animal body wt (kg), where C is insulin clearance rate, S is insulin secretion rate (by deconvolution), and I is arterial plasma insulin concentration.
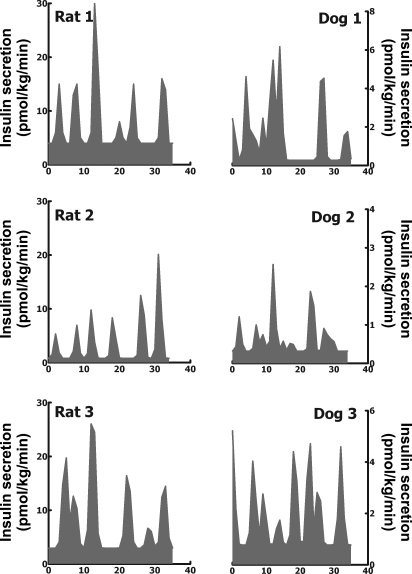
Fig. 3.
Insulin secretion rates calculated (by deconvolution analysis of portal vein insulin concentrations) at fasting in 3 representative adult Sprague-Dawley rats (left) and 3 representative adult Mongrel dogs (right).
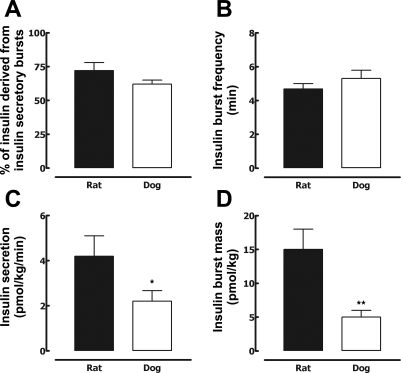
Fig. 4.
Mean percentage of insulin derived from insulin secretory bursts (A), mean insulin interpulse interval (B), mean deconvolved insulin secretion (C), and insulin burst mass (D) in 7 adult Sprague-Dawley rats and 7 adult Mongrel dogs at fasting. *P < 0.05, **P < 0.01 for dog vs. rat.
Statistical analysis.
Statistical analysis was performed using standard one-way ANOVA analysis (Statsoft, Tulsa, OK). Data are means ± SE. Findings were taken to be statistically significant at P < 0.05.
RESULTS
Parameters for detection of pulsatile insulin secretion in the rat.
Plasma insulin concentration decay curves in the rat portal vein and carotid artery after mesenteric vein insulin injection are shown in Fig. 1. The insulin concentration decay curve was biexponential in the portal vein with calculated half-lives of 0.2 and 1.7 min and monoexponential in the systemic circulation with a half-life of 1.2 min. The calculated volume of distribution for insulin in the rat portal vein after the bolus injection into the mesenteric vein was 15 ml/kg of body weight or on average ∼5 ml. In comparison the volume of distribution in the dog portal vein was 6 ml/kg body wt or on average ∼140 ml.
Insulin concentrations.
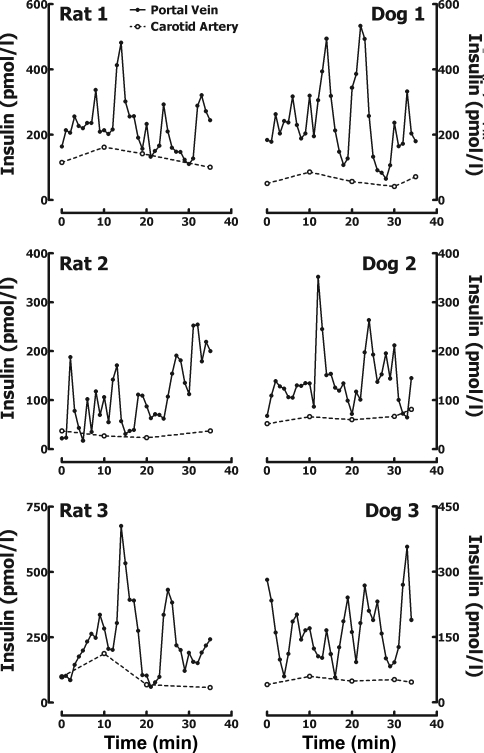
Systemic plasma glucose (96 ± 4 vs. 102 ± 2 mg/dl; P > 0.05; Fig. 2) and insulin concentrations (77 ± 15 vs. 70 ± 9 pmol/l, P > 0.05; Fig. 2) were comparable in both rats and dogs and remained relatively constant during the sampling protocol. Visual examination of portal vein insulin concentration profiles in the fasting state (representative examples shown in Fig. 2) revealed distinct insulin oscillations in both species with a periodicity of ∼5 min and an average peak amplitude of 310 ± 30 pmol/l.
Fig. 2.
Minute-by-minute sampled insulin concentration profiles in the portal vein (•) and in the carotid artery (○) in 3 representative adult Sprague-Dawley rats (left) and 3 representative adult Mongrel dogs (right).
Total and puslatile insulin secretion and insulin clearance.
The insulin secretion rate (normalized to body weight; Fig. 3) measured by direct minute-by-minute sampling from the hepatic portal vein (4.2 ± 0.9 vs. 2.2 ± 0.5 pmol·kg−1·min−1; P < 0.05; Fig. 4) as well as mean insulin burst mass (per body weight; 15 ± 3 vs. 5 ± 1 pmol/kg; P < 0.01; Fig. 4) was approximately two- to threeefold greater in the rat than the dog. However, the pattern of the insulin secretion profiles from the portal vein in the rat and dog revealed a remarkably similar pattern. Striking discrete insulin secretory bursts were superimposed on a relatively modest basal rate of nonpulsatile insulin secretion (Figs. 2 and 3). The measured percentage of insulin secretion adjudged to have been derived from insulin bursts was comparable in the rat and dog (72 ± 6 and 62 ± 4%; P > 0.05; Fig. 4). Also, the insulin pulse interval was comparable in the rat and dog (4.7 ± 0.3 and 4.7 ± 0.5 min; P > 0.05; Fig. 4). The clearance of endogenously secreted insulin remained unchanged during the study period (0–35 min), consistent with steady-state fasting conditions, and the relative rates of insulin clearance demonstrated a tendency to be higher, but just failed to reach statistical significance, in rats vs. dogs (54 ± 8 vs. 33 ± 6 ml·kg−1·min−1; P = 0.06).
DISCUSSION
In the present studies, we established the methods required to measure pulsatile insulin secretion in vivo directly from the portal vein of unrestrained conscious rats. Consistent with canine and human studies (23, 30), we report that 1) most insulin in rats is secreted in a high-frequency pulsatile manner (pulse interval of ∼4–5 min); and 2) as a consequence of this mode of insulin secretion the liver is exposed to insulin oscillations of ∼400–600 pmol/l in the fasting state.
It is now well established that in health insulin secretion is predominatly derived from discrete insulin secretory bursts that occur at ∼4-min intervals as a consequence of synchronous discharge of docked insulin secretory vesicles by pancreatic β-cells (17, 18). Since isolated perifused islets secrete insulin in 4-minute pulses (26), the pacemaker dictating the insulin pulse interval is presumably embodied in each islet, with metabolic pacemakers and electrical pacemakers postulated (3). The mechanisms that synchronize the coordinate secretion of pulses by dispersed islets within the pancreas are believed to involve the intrinsic neural network within the pancreas (31). When pulsatile insulin secretion is quantified by a validated approach using insulin measured by ELISA, the pulse interval is not altered in type 2 diabetes, but the magnitude of insulin secretory bursts is deficient (11). Since the latter constitutes the majority of insulin secretion, it is not surprising that insulin secretion is therefore deficient in type 2 diabetes mellitus (20, 29, 32) and is restored to normal if the insulin secretory burst mass is restored (11).
Given these observations, there is interest in understanding the basis of the deficit in insulin pulse mass in type 2 diabetes mellitus. A selective deficit in insulin pulse mass has been reproduced by creating a comparable loss of β-cell mass in a porcine model of type 2 diabetes mellitus (9, 13) and a canine model of impaired fasting glucose (15), implying a potentially important role of β-cell mass. Novel molecular genetic insights into the pathophysiology of type 2 diabetes mellitus are increasingly dependent on genetic manipulation of rodents. However, to date there has been no validated method developed to quantify pulsatile insulin secretion in rodents by direct sampling from the portal vein. Given that we have previously validated a direct portal vein catheterization approach and deconvolution method for measurement of pulsatile insulin secretion in vivo in dogs (23), we elected to extend this approach to the rat once the ELISA methods for measurement of rat insulin concentrations became available. This recent development permits measurement of insulin in a sufficiently small sample size that the multiple sampling required to quantify pulsatile insulin secretion was feasible in rodents.
Evaluation of insulin secretion by use of insulin concentrations measured in the systemic circulation, while technically feasible in humans and animal models, presents a number of significant limitations. It has been shown that hepatic insulin clearance is directly related to the amplitude of insulin pulses in the portal vein (16) and ∼80% of insulin is cleared in the first pass through the liver (16). Therefore, systemic insulin concentrations may underestimate changes in insulin secretion and pulsatile insulin secretion in particular. Furthermore, hepatic insulin clearance is altered with a decrease in β-cell mass in diabetes (4, 27) and obesity (6) and with aging (2).
The deconvolution approach used here assumes that insulin clearance is constant at the sampling site. To the extent that the insulin clearance varies from minute to minute at the sampling site, the deconvolved insulin secretory rate may be in error. We previously reported that first pass hepatic insulin extraction of endogenous insulin secretion increases in phase with each secretory burst (16) and is proportionate to the magnitude of the insulin burst mass (9, 15, 16). The resulting error in the deconvolved insulin secretion rate may therefore be substantial when quantifying endogenous pulsatile insulin secretion in the systemic sampling site. We originally validated this deconvolution approach for measurement of pulsatile insulin secretion by use of portal vein sampling (23). We compared the deconvolution approach (from portal vein insulin sampling) to direct measurement of the increment in insulin concentration in the portal vein across the pancreas multiplied by portal vein plasma flow (23) over a threefold range of insulin secretion. Reassuringly with portal vein blood sampling the two approaches provided comparable results (23), implying that variance in first pass hepatic insulin extraction (and therefore insulin clearance) at the portal vein sampling site has a negligible effect on the dynamics of insulin concentration compared with the rate of insulin secretion. This is likely reflected in the biexponential decay of insulin decay in the portal vein with a dominant first half life determined predominantly by the portal vein blood flow. These observations further emphasize the importance of developing techniques of measuring pulsatile insulin secretion by direct prehepatic sampling of insulin concentrations from the portal vein.
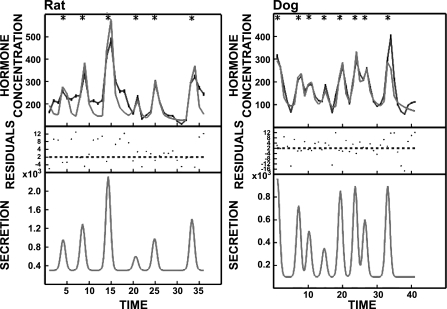
Perhaps the most striking finding is that the portal vein insulin concentration profile (Fig. 5) in the rat is remarkably similar to that in the dogs (23) and indeed humans (30). These data imply that an insulin concentration oscillatory profile of ∼400–600 pmol/l is optimal for hepatic insulin signaling in all three species and suggests that the high amplitude portal vein insulin oscillations do have physiological importance. Prior studies (5, 10, 14, 19, 28, 36) have revealed an apparent enhanced action of pulsatile vs. nonpulsatile insulin delivery for suppression of hepatic glucose release, but these studies used much smaller insulin oscillations than those present in the portal vein delivered into the systemic circulation. One negative study (8) reproduced the portal vein pulse amplitude observed in vivo in the dog but at a frequency less than half of that in vivo and to quantify hepatic glucose uptake rather than suppression of hepatic glucose release .
Fig. 5.
Representative portal vein insulin concentrations profiles (black) in a rat (top left) and a dog (top right) together with deconvolution defined best-fit curves (gray) to quantify endogenous insulin secretion shown at bottom. Insulin concentrations are expressed in pmol/l ,and insulin secretion rates are expressed in nmol·l−1·min−1. *P < 0.05, denotes insulin peaks tested for statistical significance.
In conclusion, we have developed the methods required to directly quantify insulin secretion and to resolve its pulsatile components by deconvolving time series insulin concentrations directly from the portal vein. These data reveal that the rat, in common with humans and dogs, secretes the majority of insulin in the form of discrete insulin secretory bursts at an interval of ∼4 min. Moreover the resulting insulin concentration profile in the portal vein in all three species consists of distinct insulin oscillations with an amplitude of ∼400–600 pmol/l and periodicity of 4 min. It remains to be resolved to what extent this high conserved insulin concentration wave front delivered to hepatocytes via hepatic sinusoids is important in insulin signaling. To this end, future studies in rodent models of diabetes with perturbations in insulin secretion will now be possible in which pulsatile insulin secretion is directly measured followed by examination of liver tissue to evaluate hepatic insulin signaling.
GRANTS
We acknowledge funding from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-059579) and the Larry Hillblom Foundation to P. C. Butler. A. V. Matveyenko is supported by the National Institutes of Health Ruth L. Kirschstein National Research Service Award.
Acknowledgments
We acknowledge the support and excellent suggestions of our colleagues at the Larry Hillblom Islet Research Center at University of California Los Angeles, Dr. A. Bhushan, Dr. A. Butler, Dr. T. Gurlo, and Dr. L. Haataja.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Akaike H An information criterion (AIC). Math Sci 14: 5–9, 1976. [Google Scholar]
- 2.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 52: 1738–1748, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bertram R, Sherman A, Satin LS. Metabolic and electrical oscillations: partners in controlling pulsatile insulin secretion. Am J Physiol Endocrinol Metab 293: E890–E900, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism 32: 438–446, 1983. [DOI] [PubMed] [Google Scholar]
- 5.Bratusch-Marrain PR, Komjati M, Waldhausl WK. Efficacy of pulsatile vs. continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes 35: 922–926, 1986. [DOI] [PubMed] [Google Scholar]
- 6.Faber OK, Christensen K, Kehlet H, Madsbad S, Binder C. Decreased insulin removal contributes to hyperinsulinemia in obesity. J Clin Endocrinol Metab 53: 618–621, 1981. [DOI] [PubMed] [Google Scholar]
- 7.Goodner CJ, Walike BC, Koerker DJ, Ensinck JW, Brown AC, Chideckel EW, Palmer J, Kalnasy L. Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science 195: 177–179, 1977. [DOI] [PubMed] [Google Scholar]
- 8.Grubert JM, Lautz M, Lacy DB, Moore MC, Farmer B, Penaloza A, Cherrington AD, McGuinness OP. Impact of continuous and pulsatile insulin delivery on net hepatic glucose uptake. Am J Physiol Endocrinol Metab 289: E232–E240, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Kjems LL, Kirby BM, Welsh EM, Veldhuis JD, Straume M, McIntyre SS, Yang D, Lefebvre P, Butler PC. Decrease in beta-cell mass leads to impaired pulsatile insulin secretion, reduced postprandial hepatic insulin clearance, and relative hyperglucagonemia in the minipig. Diabetes 50: 2001–2012, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Komjati M, Bratusch-Marrain P, Waldhausl W. Superior efficacy of pulsatile versus continuous hormone exposure on hepatic glucose production in vitro. Endocrinology 118: 312–319, 1986. [DOI] [PubMed] [Google Scholar]
- 11.Laedtke T, Kjems L, Porksen N, Schmitz O, Veldhuis J, Kao PC, Butler PC. Overnight inhibition of insulin secretion restores pulsatility and proinsulin/insulin ratio in type 2 diabetes. Am J Physiol Endocrinol Metab 279: E520–E528, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Lang DA, Matthews DR, Peto J, Turner RC. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med 301: 1023–1027, 1979. [DOI] [PubMed] [Google Scholar]
- 13.Larsen MO, Gotfredsen CF, Wilken M, Carr RD, Porksen N, Rolin B. Loss of beta-cell mass leads to a reduction of pulse mass with normal periodicity, regularity and entrainment of pulsatile insulin secretion in Gottingen minipigs. Diabetologia 46: 195–202, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes 32: 617–621, 1983. [DOI] [PubMed] [Google Scholar]
- 15.Matveyenko AV, Veldhuis JD, Butler PC. Mechanisms of impaired fasting glucose and glucose intolerance induced by ∼50% pancreatectomy. Diabetes: 2347–2356, 2006. [DOI] [PubMed]
- 16.Meier JJ, Veldhuis JD, Butler PC. Pulsatile insulin secretion dictates systemic insulin delivery by regulating hepatic insulin extraction in humans. Diabetes 54: 1649–1656, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Meier JJ, Butler PC. Insulin secretion. In: Endocrinology (5th ed.), edited by DeGroot LJ. Philadelphia, PA: Elsevier Saunders, 2005, p. 961–973.
- 18.Michael DJ, Xiong W, Geng X, Drain P, Chow RH. Human insulin vesicle dynamics during pulsatile secretion. Diabetes 56: 1277–1288, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Paolisso G, Sgambato S, Gentile S, Memoli P, Giugliano D, Varricchio M, D'Onofrio F. Advantageous metabolic effects of pulsatile insulin delivery in noninsulin-dependent diabetic patients. J Clin Endocrinol Metab 67: 1005–1010, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest 46: 1954–1962, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porksen N, Munn S, Steers J, Veldhuis JD, Butler PC. Effects of glucose ingestion versus infusion on pulsatile insulin secretion. The incretin effect is achieved by amplification of insulin secretory burst mass. Diabetes 45: 1317–1323, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Porksen N, Munn S, Steers J, Veldhuis JD, Butler PC. Impact of sampling technique on appraisal of pulsatile insulin secretion by deconvolution and cluster analysis. Am J Physiol Endocrinol Metab 269: E1106–E1114, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Porksen N, Munn S, Steers J, Vore S, Veldhuis J, Butler P. Pulsatile insulin secretion accounts for 70% of total insulin secretion during fasting. Am J Physiol Endocrinol Metab 269: E478–E488, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Porksen N, Munn SR, Steers JL, Veldhuis JD, Butler PC. Effects of somatostatin on pulsatile insulin secretion: elective inhibition of insulin burst mass. Am J Physiol Endocrinol Metab 270: E1043–E1049, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Porksen NK, Munn SR, Steers JL, Schmitz O, Veldhuis JD, Butler PC. Mechanisms of sulfonylurea's stimulation of insulin secretion in vivo: selective amplification of insulin secretory burst mass. Diabetes 45: 1792–1797, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Ritzel RA, Veldhuis JD, Butler PC. Glucose stimulates pulsatile insulin secretion from human pancreatic islets by increasing secretory burst mass: dose-response relationships. J Clin Endocrinol Metab 88: 742–747, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Sando H, Lee YS, Iwamoto Y, Ikeuchi M, Kosaka K. Isoproterenol-stimulated C-peptide and insulin secretion in diabetic and nonobese normal subjects: decreased hepatic extraction of endogenous insulin in diabetes. J Clin Endocrinol Metab 51: 1143–1149, 1980. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz O, Arnfred J, Nielsen OH, Beck-Nielsen H, Orskov H. Glucose uptake and pulsatile insulin infusion: euglycaemic clamp and [3–3H]glucose studies in healthy subjects. Acta Endocrinol 113: 559–563, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Seltzer HS, Allen EW, Herron AL Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 46: 323–335, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song SH, McIntyre SS, Shah H, Veldhuis JD, Hayes PC, Butler PC. Direct measurement of pulsatile insulin secretion from the portal vein in human subjects. J Clin Endocrinol Metab 85: 4491–4499, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Stagner JI, Samols E. Role of intrapancreatic ganglia in regulation of periodic insular secretions. Am J Physiol Endocrinol Metab 248: E522–E530, 1985. [DOI] [PubMed] [Google Scholar]
- 32.Temple RC, Carrington CA, Luzio SD, Owens DR, Schneider AE, Sobey WJ, Hales CN. Insulin deficiency in non-insulin-dependent diabetes. Lancet 1: 293–295, 1989. [DOI] [PubMed] [Google Scholar]
- 33.Veldhuis JD, Fraioli F, Rogol AD, Dufau ML. Metabolic clearance of biologically active luteinizing hormone in man. J Clin Invest 77: 1122–1128, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vore SJ, Aycock ED, Butler PC. Measurement of insulin pulsatility by sampling directly from the portal vein: a surgical model for placement of long-term prehepatic vascular sampling catheters. Lab Anim Sci 46: 202–205, 1996. [PubMed] [Google Scholar]
- 35.Vore SJ, Aycock ED, Veldhuis JD, Butler PC. Anesthesia rapidly suppresses insulin pulse mass but enhances the orderliness of insulin secretory process. Am J Physiol Endocrinol Metab 281: E93–E99, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Ward GM, Walters JM, Aitken PM, Best JD, Alford FP. Effects of prolonged pulsatile hyperinsulinemia in humans. Enhancement of insulin sensitivity. Diabetes 39: 501–507, 1990. [DOI] [PubMed] [Google Scholar]