Abstract
The effects of oral carbohydrate on modulating counterregulatory responses in humans remain undecided. This study's specific aim was to determine the effects of oral carbohydrate on autonomic nervous system (ANS) and neuroendocrine responses during hyperinsulinemic hypoglycemia and euglycemia. Nineteen healthy volunteers were studied during paired, single blind experiments. Nine subjects underwent two-step glucose clamps consisting of 60 min of euglycemia (5.0 mmol/l) followed by either 15 g of oral carbohydrate (cal) as orange juice or a noncaloric control (nocal) and subsequent 90 min of clamped hypoglycemia (2.9 mmol/l). Ten other subjects underwent two randomized 150-min hyperinsulinemic-euglycemic clamps with cal or nocal control administered at 60 min. Oral carbohydrate initially blunted (P < 0.05) epinephrine, norepinephrine, cortisol, glucagon, pancreatic polypeptide, muscle sympathetic nerve activity (MSNA), symptom, and systolic blood pressure responses during hypoglycemia. However, by the end of 90 min of hypoglycemia, plasma epinephrine and norepinephrine responses had rebounded and were increased (P < 0.05) compared with control. MSNA and cortisol levels remained suppressed during hypoglycemia (P < 0.05) after cal, whereas pancreatic polypeptide, glucagon, symptom, and blood pressure responses increased similar to control following initial suppression. Oral carbohydrate had no effects on neuroendocrine or ANS responses during hyperinsulinemic euglycemia. These results demonstrate that oral carbohydrate can have differential effects on the time course of ANS and neuroendocrine responses during hypoglycemia. We conclude that gastro-splanchnic-portal sensing of an amount of carbohydrate recommended for use in clinical practice for correction of hypoglycemia can have widespread and significant effects on central nervous system mediated counterregulatory responses in healthy humans.
Keywords: hypoglycemia, euglycemia, hyperinsulinemia, humans, oral carbohydrate
there are conflicting data regarding the effects of enteral carbohydrate (CHO) administration on autonomic nervous system (ANS) and neuroendocrine responses during hypoglycemia. In a series of elegant studies, Donovan and colleagues (15, 17, 22, 23, 27) have demonstrated that portal vein glucose sensing in rats and dogs can reduce sympathoadrenal responses during hypoglycemia. Burcelin et al. (6) have reported that portal vein glucose infusions at a rate similar to endogenous glucose production in mice can produce hypoglycemia. This, however, was not observed during portal glucose infusions in conscious dogs (30) or enteral glucose infusions in humans (38). In fact, previous work delivering oral CHO in humans by Berne et al. (4) had demonstrated an increase in sympathetic nervous system activity and hyperglycemia. Thus, from the above, it can be seen that the route of glucose delivery (i.e., oral, enteral, or intraportal) and species can have a profound effect on ANS responses.
The use of oral CHO has been used as a resuscitive measure for hypoglycemia in clinical practice for nearly a century. However, the effects of oral CHO on ANS and neuroendocrine counterregulatory responses during hypoglycemia in humans are largely unexplored. Only two studies appear to have addressed this question and have reported differing results. Heptulla et al. (20), using the glucose clamp technique to maintain constant peripheral and central nervous system (CNS) hypoglycemia, reported that 25 g of oral CHO during hypoglycemia significantly increased epinephrine, glucagon, and growth hormone responses (20). On the other hand, Smith et al. (35), also using the hypoglycemic clamp technique, reported that 20 g of oral glucose administered 20 min before hypoglycemia blunted epinephrine and symptomatic counterregulatory responses during the stress. However, there have been no data available regarding the effects of oral CHO on direct sympathetic nervous system activity during hypoglycemia and the effects of administering oral CHO at the start of the transition from euglycemia to hypoglycemia. Furthermore, the effects of the clinically recommended resuscitative dose of 15 g of CHO on ANS and neuroendocrine responses during hypoglycemia have not been determined. In this present study, the glucose clamp technique was used so that the effects of oral CHO could be sensed independent of changes of hyperinsulinemic hypoglycemia or euglycemia in the brain.
RESEARCH DESIGN AND METHODS
General Design
Four 150-min hyperinsulinemic glucose clamps were performed in 19 healthy volunteers (12 men/7 women), age 25 ± 1 yr, body mass index 24.3 ± 0.8 kg/m, and hemoglobin A1c 5.3 ± 0.1% (normal range 4–6.5%). All volunteers gave informed consent. The study was approved by Vanderbilt University Medical Center's Institutional Review Board. One protocol consisted of an initial 60-min hyperinsulinemic (2.0 mU·kg−1·min−1) euglycemic (5.0 mmol/l) clamp with oral ingestion of a 177-ml beverage containing either 15 g of CHO calories as orange juice (hypocal, n = 9) or a similar orange-colored noncaloric beverage (hyponocal, n = 9) followed by a 90-min hyperinsulinemic hypoglycemic clamp (2.9 mmol/l). An additional series of control glucose clamps was performed to determine the effects of oral CHO on ANS and neuroendocrine activity during 150 min of hyperinsulinemic (2.0 mU·kg−1·min−1) euglycemia (euglycal, n = 10 and euglynocal, n = 10).
Glucose Clamp Experiments
Volunteers were asked to avoid any exercise and consume their usual weight-maintaining diet for three days before each study. Each subject was admitted to the Vanderbilt General Clinical Research Center at 5:00 P.M. on the evening before an experiment. All subjects were studied after an overnight 10-h fast.
On the morning of each study, two intravenous cannulas were inserted under 1% lidocaine local anesthesia. One cannula was placed in a retrograde fashion in a vein on the back of the hand. This hand was placed in a heated box (55–60°C) so that arterialized blood could be obtained (1). The other cannula was placed in a large vein in the contralateral arm so that 20% dextrose could be infused via a variable-rate volumetric infusion pump (I-med, San Diego, CA).
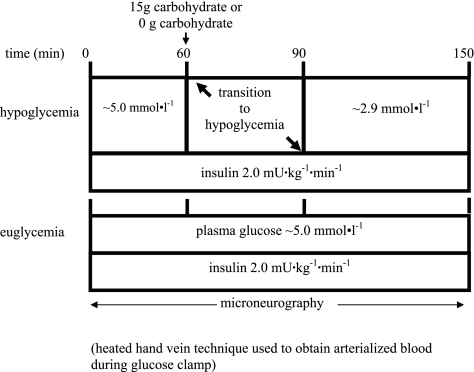
After insertion of venous cannulas, at 0 min, an insulin infusion solution was prepared with normal saline containing 3% (vol/vol) of the subject's own plasma. The infusion was started as a primed constant (2.0 mU·kg−1·min−1) infusion of insulin (Eli Lilly, Indianapolis, IN) via a precalibrated infusion pump and continued until 150 min. In the hypoglycemia experiments, the rate of fall of glucose (0.12 mmol/l) was controlled so that the hypoglycemic plateau was achieved at time 90 min using a modification of the glucose clamp technique (14). During the clamp period, plasma glucose was measured every 5 min, and the 20% dextrose infusion was adjusted so that plasma glucose levels were held constant at 2.9 ± 0.1 mmol/l (3). In the euglycemic control studies, plasma glucose was maintained at 5.0 mmol/l as described by DeFronzo et al. (14). In all clamp studies, potassium chloride (20 mmol/l) was infused during the clamp to reduce insulin-induced hypokalemia. After completion of the 150-min test period, all infusions were discontinued, with the exception of 20% dextrose. The plasma glucose was either rapidly restored to, or maintained at, euglycemia with 20% dextrose. The protocol time course is illustrated in Fig. 1.
Fig. 1.
Diagram of 4 protocols. Studies begin with 60 min of hyperinsulinemic (2.0 mU·kg−1·min−1) euglycemia (5.0 mmol/l) for 0–60 min followed by oral ingestion of a 177-ml beverage containing either 15 g carbohydrate as orange juice or 0 g carbohydrate at time 60 min with an additional 90 min of either hypoglycemia at 2.9 mmol/l (hypocal and hyponocal, respectively) or a continued hyperinsulinemic euglycemia (euglycal and euglynocal, respectively).
Humoral Analytical Analyses
Collection and processing of blood samples have been described previously (8). Blood samples for hormones were drawn every 15 min during the experiments. Catecholamines were determined by HPLC (7) with an interassay coefficient of variation (ICV) of 12% for epinephrine and 8% for norepinephrine. There were two modifications in the procedure for catecholamine determination; a five-point standard calibration curve was used, and initial and final samples of plasma were spiked with known amounts of epinephrine and norepinephrine to accurately identify relevant catecholamine peaks. Glucagon was measured using the method of Aguilar-Parada et al. (2) with an ICV of 15%. Insulin was measured [as described by Wide and Porath (37)] with an ICV of 11%. Radioimmunoassay techniques were used to measure cortisol (ICV of 6%; Clinical Assays Gamma Coat Radioimmunoassay Kit) and pancreatic polypeptide (ICV = 8%; see Ref. 19).
Measurement of Muscle Sympathetic Nerve Activity
Microneurography in the peroneal nerve between the fibular head and the popliteal fossa was used to directly measure muscle sympathetic nerve activity (MSNA). MSNA has been demonstrated to increase significantly during hyperinsulinemic euglycemic and hypoglycemic clamps (11–13, 16). In this study, nerve activity and electrocardiogram (ECG) were recorded on a PC-based Windaq data acquisition system at 1,000 Hz/channel (DATAQ Instruments, Akron, OH). Windaq files (5 min) were analyzed with a MatLab GUIDE interface (to adjust for an individual's ∼1.3-s nerve burst delay from a once-removed R-R interval, and automatically detected by pulse synchronicity, a 2:1 signal-to-noise ratio, and waveform shape). All counts were edited and verified through visual inspection by experienced personnel. Further criterion for acceptable MSNA recordings were: spontaneous burst appearance, increase in activity associated with apnea or during phase IV of a Valsalva maneuver, and nonresponsiveness to noise stimuli; tapping the nerve-associated muscle belly evoked afferent signals, whereas cutaneous stroking of the skin did not elicit a response.
Hypoglycemic symptoms were quantified using a previously validated semiquantitative questionnaire (9). Each individual was asked to rate his/her experience of the symptoms two times during the control period and every 15 min during experimental periods. Symptoms measured included sweaty, tremor/shaky, hot, thirsty/dry mouth, agitation/irritability, palpitations, tired/fatigued, confusion, dizzy, difficulty thinking, blurriness of vision, and sleepy. The ratings of the first six symptoms were summed to obtain an autonomic score, whereas the ratings from the last six symptoms provide a neuroglycopenic symptom score.
Cardiovascular Data
Heart rate was monitored continuously, and R-R intervals were collected with a 5-lead ECG. Systolic, diastolic, and mean arterial pressures were measured noninvasively every 10 min by oscillometry and recorded with a Dinamap blood pressure monitor (Critikon, Tampa, FL) during insulin infusions.
Statistical Analyses
Data were analyzed using an SPSS general linear models procedure for subjects in paired trials of hypocal and hyponocal or euglycal and euglynocal and expressed as means ± SE. The null hypothesis for statistical significance was accepted at P < 0.05. When interactions between treatments and time were significant, mean differences by post hoc analysis are reported.
RESULTS
Insulin and Glucose Levels
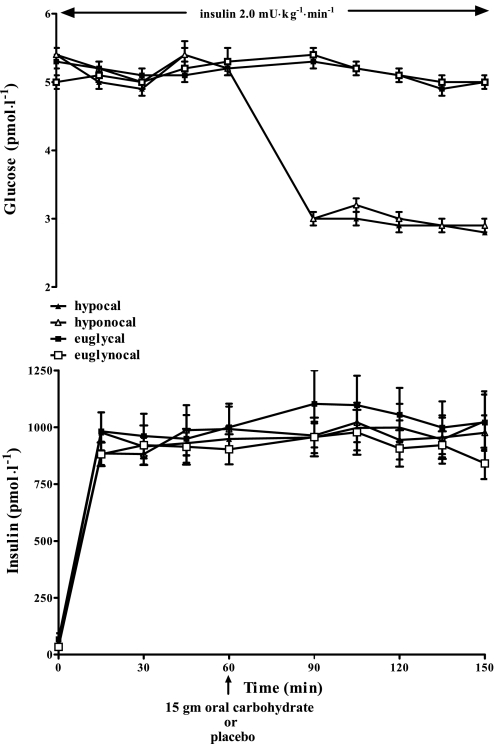
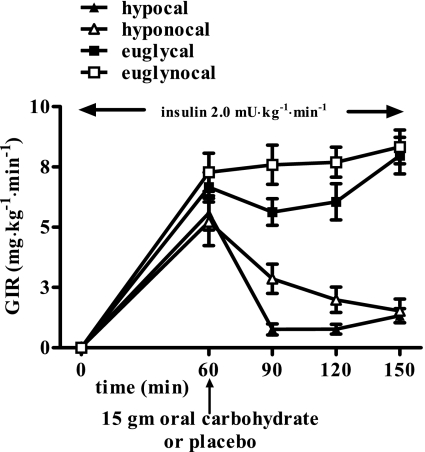
Figure 2 illustrates the insulin and glucose values obtained during the four study protocols. Mean plasma insulin levels were similar in all groups (978 ± 94, 918 ± 77, 1,046 ± 123, and 938 ± 76 pmol/l, for hypocal and hyponocal or euglycal and euglynocal, respectively). Glucose levels at 60 min were equivalent in all groups (5.2 ± 0.1 mmol/l); plasma glucose was equivalent at 2.9 ± 0.1 mmol/l during the hypoglycemic protocols and was 5.0 mmol/l during the euglycemic studies.
Fig. 2.
Plasma glucose (mmol/l) and insulin levels (pmol/l) in 19 overnight-fasted healthy humans receiving 15 g oral carbohydrate or placebo at time 60 min during hyperinsulinemia hypoglycemic and euglycemic conditions.
Epinephrine
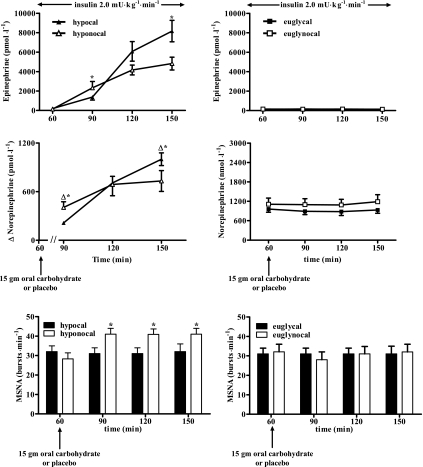
Epinephrine levels (Fig. 3) were reduced significantly (P < 0.05) at 90 min and 1,370 ± 298 and 2,322 ± 659 pmol/l during hypocal vs. hyponocal, respectively. However, by the end of hypoglycemia at time 150 min, epinephrine levels were increased (P < 0.05) following oral CHO vs. nocal placebo (8,160 ± 1,099 and 4,821 ± 662 pmol/l, respectively). Epinephrine levels were similar during both euglycemic clamp protocols.
Fig. 3.
Epinephrine (pmol/l), norepinephrine (pmol/l), and muscle sympathetic nerve activity (MSNA, in bursts/min) responses following 15 g carbohydrate in 19 overnight-fasted healthy humans or placebo during conditions of hyperinsulinemic hypoglycemia (hypocal and hyponocal, respectively) and hyperinsulinemic euglycemia (euglycal, euglynocal). *P < 0.05, value significantly different among hypocal and hyponocal at a given time point.
Norepinephrine
Norepinephrine responses from time 60 to 90 min (Fig. 3) were significantly reduced (P < 0.05) during hypocal vs. hyponocal (214 ± 12 and 405 ± 69 pmol/l, respectively). By the end of hypoglycemia at time 150 min, norepinephrine responses were increased (P < 0.05) during hypocal vs. hyponocal (1,000 ± 79 vs. 731 ± 128 pmol/l). Norepinephrine responses were not different during the two hyperinsulinemic euglycemic clamp studies.
MSNA
MSNA (Fig. 3) was significantly reduced (P < 0.05) during hypocal vs. hyponocal during hypoglycemia (90 min, 31 ± 3 and 41 ± 3, 120 min, 32 ± 4 and 41 ± 3, and 150 min, 32 ± 4 and 41 ± 3 bursts/min). MSNA did not change during euglycemic clamp studies following CHO or no CHO.
Neuroendocrine Responses
Pancreatic polypeptide, glucagon, and cortisol.
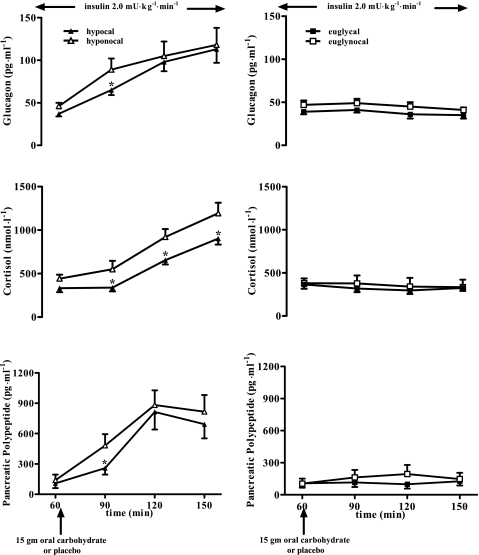
Pancreatic polypeptide levels (Fig. 4) were initially suppressed (P < 0.05) during hypoglycemia following oral CHO compared with no CHO (204 ± 60 vs. 482 ± 112 pmol/l). However, by the end of hypoglycemia, pancreatic polypeptide results were similar in both the cal and nocal groups (697 ± 132 and 926 ± 230 pmol/l, respectively). The time course of pancreatic polypeptide levels was similar in the hyperinsulinemic euglycemic clamps following CHO or no CHO. Glucagon levels (Fig. 4) following CHO were initially suppressed during hypoglycemia (65 ± 6 and 89 ± 13 ng/l, P < 0.05). However, by the end of hypoglycemia, there was no difference in the calorie or no calorie groups. Cortisol levels were suppressed (P < 0.05) during the entire time course of hypoglycemia following oral CHO (Fig. 4). There were no differences in the response of glucagon or cortisol following oral CHO or no CHO during the hyperinsulinemic euglycemic clamps.
Fig. 4.
Glucagon (ng/ml), cortisol (nmol/l), and pancreatic polypeptide levels in 19 overnight-fasted healthy humans receiving 15 g oral carbohydrate or placebo during hyperinsulinemic hypoglycemia in the 3 left graphs (hypocal and hyponocal, respectively) and the hyperinsulinemic euglycemia in the three right graphs (euglycal and euglynocal, respectively) at 60, 90, 120, and 150 min. *P < 0.05, value significantly lower in hypocal compared with hyponocal at a given time point.
Glucose infusion rate.
The amount of glucose required to maintain the desired glycemia is shown in Fig. 5. As expected, significantly less glucose was required following oral CHO. Glucose infusion rates, however, were equivalent at the end of each of each 150-min glucose clamp procedure.
Fig. 5.
Glucose infusion rate (mg·kg−1·min−1) in 15 and 0 g carbohydrate hypoglycemia protocols (hypocal and hyponocal) at 60, 90, 120, and 150 min and 15 and 0 g carbohydrate euglycemia protocols (euglycal and euglynocal, respectively) at 60, 90, 120, and 150 min.
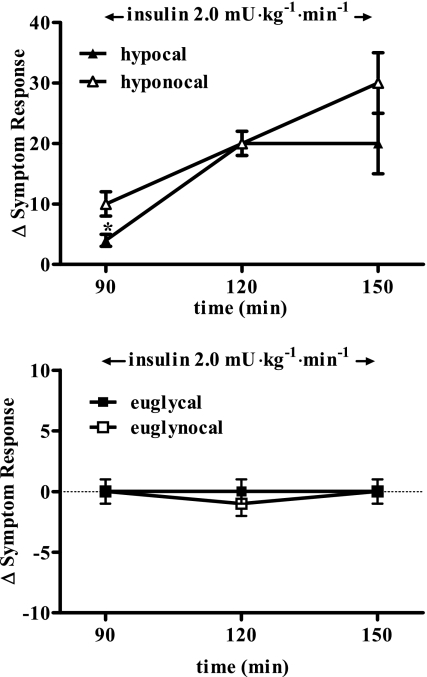
Symptom response.
Symptoms were equivalent at the start of the hypoglycemic clamps in both groups (14 ± 2). The increase in symptom responses (Fig. 6) was initially reduced (P < 0.05) at 90 min during hypoglycemia in the cal group vs. nocal group (5 ± 1 and 10 ± 4, respectively). The increase in autonomic symptom scores was primarily blunted by cal (+2 ± 1 vs. +6 ± 1). However, by the end of the 150-min study, there were no differences in the nocal and cal groups. Total symptom response was not different following oral calories or no calories during the euglycemic control clamps: 90 min, 1 ± 1 and 0 ± 1; 120 min, 2 ± 1 and −1 ± 1; and 150 min, 1 ± 1 and 0 ± 1, respectively.
Fig. 6.
Total symptom response (difference from time 60 min from a given time) in 15 and 0 g carbohydrate hypoglycemia protocols (left; hypocal and hyponocal) at 90, 120, and 150 min and 15 and 0 g carbohydrate euglycemia protocols (right; euglycal and euglynocal, respectively) at 90, 120, and 150 min. SBP, systolic blood pressure (mmHg); DBP, diastolic blood pressure (mmHg); MAP, mean arterial pressure (mmHg). *P < 0.05, significant difference from basal value.
Cardiovascular parameters.
Heart rate, systolic blood pressure, diastolic pressure, and mean arterial pressure are shown in Table 1. Systolic blood pressure was initially suppressed (90 min) during hypoglycemia following oral CHO. However, by the end of the 150-min study, systolic blood pressure was equivalent in both hypocal and hyponocal groups. Cardiovascular responses were unaffected by oral calories or no calories during the hyperinsulinemic euglycemic clamps.
Table 1.
Effects of 15 g carbohydrate or placebo on cardiovascular parameters during hypocal and hyponocal and paired euglycemia (5.0 mmol/l) protocols at 60 min prebeverage and then 90, 120, and 150 min
|
Time. min |
||||
|---|---|---|---|---|
| 60 | 90 | 120 | 150 | |
| Heart rate, beats/min | ||||
| Hypocal | 67±3 | 75±2* | 74±3* | 76±2* |
| Hyponocal | 63±4 | 75±3* | 76±3* | 74±3* |
| Euglycal | 63±4 | 63±4 | 65±4 | 69±4 |
| Euglynocal | 65±5 | 65±5 | 68±5 | 62±5 |
| SBP, mmHg | ||||
| Hypocal | 118±5 | 118±4 | 123±6* | 127±6* |
| Hyponocal | 117±5 | 128±6* | 126±5* | 127±6* |
| Euglycal | 116±3 | 120±5 | 121±5 | 122±6 |
| Euglynocal | 115±3 | 120±4 | 123±5 | 121±4 |
| DBP, mmHg | ||||
| Hypocal | 67±2 | 64±1 | 60±2* | 61±2* |
| Hyponocal | 68±2 | 67±2 | 61±2* | 62±1* |
| Euglycal | 69±2 | 68±1 | 69±2 | 67±3 |
| Euglynocal | 68±2 | 69±1 | 69±2 | 68±2 |
| MAP, mmHg | ||||
| Hypocal | 83±2 | 81±2 | 80±3 | 83±3 |
| Hyponocal | 84±3 | 87±3 | 83±3 | 84±2 |
| Euglycal | 85±2 | 86±2 | 86±3 | 84±4 |
| Euglynocal | 84±2 | 86±2 | 87±3 | 86±2 |
Values are means ± SE. Hypocal, hypocaloric; hyponocal, nonhypocaloric; eugycal, hyperinsulinemic euglycemia; euglynocal, nonhyperinsulinemic euglycemia; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure.
Significant difference from basal value (P < 0.05).
DISCUSSION
This study has investigated the effects of 15 g oral CHO (177 ml of orange juice) on ANS and neuroendocrine responses during clamped hyperinsulinemic hypoglycemia and euglycemia in healthy humans. The results demonstrate that, during moderate peripheral and CNS hypoglycemia, oral CHO ingestion is sensed and produces complex and significant effects on counterregulatory responses during hypoglycemia. Initially, there were widespread effects to suppress ANS (epinephrine, norepinephrine, pancreatic polypeptide, systolic blood pressure, symptoms, and MSNA) and neuroendocrine (glucagon, cortisol) responses. However, by the end of 90 min of hypoglycemia, there were three differential responses. Plasma cortisol and MSNA remained suppressed; glucagon, systolic blood pressure, symptoms, and pancreatic polypeptide increased similar to placebo control values, but epinephrine and norepinephrine demonstrated amplified responses and were elevated relative to placebo studies. A further series of studies was conducted to determine the effects of oral CHO on ANS and neuroendocrine responses during hyperinsulinemic euglycemia. Under these conditions, where there were smaller and specific effects of insulin to activate sympathetic neural activity, it was found that oral CHO had no effect on modulating ANS or neuroendocrine responses.
Orange juice is commonly used in clinical practice as a resuscitative measure for falling plasma glucose (10). However, the effects of the typical recommended dose of 15 g of oral CHO on glucose sensing and consequent ANS and neuroendocrine responses during hypoglycemia do not appear to have been investigated. Previous studies in animals (15, 21–23, 27, 28, 30, 36) have infused glucose directly in the portal vein to determine the importance of the site for glucose sensing. During hypoglycemia, several reports in dogs and rats have demonstrated that portal glucose sensing can attenuate catecholamine responses (15, 21–23, 27, 28). In mice, portal vein glucose infusion initiated during normoglycemic conditions can result in hypoglycemia (implying reduced ANS and neuroendocrine activity), although subsequent studies in dogs (24, 30) and humans (38) did not reproduce these findings.
To date, there are limited data in humans addressing the effects of oral CHO on ANS and neuroendocrine responses during hypoglycemia. Only two studies appear to have addressed this question with somewhat contradictory results (20, 35). In this present study, 15 g of CHO in the form of orange juice was administered at the induction of hypoglycemia following 60 min of hyperinsulinemic euglycemia. Oral CHO produced a complex array of neuroendocrine and ANS responses during hypoglycemia. At the start of the hypoglycemic clamp plateau (i.e., 30 min after administration of the oral calories and the transition from euglycemia), there was a widespread blunting of key neuroendocrine, ANS, and systolic blood pressure responses. This indicates that gastro-splanchnic-portal sensing of CHO can rapidly blunt hypothalamo-pituitary-adrenal (cortisol), pancreatic (glucagon) sympathoadrenal (epinephrine/norepinephrine), parasympathetic (the partially regulated pancreatic polypeptide), and sympathetic neural (norepinephrine, MSNA) responses. Additionally, the reduced sympathetic nervous system activity produced blunted systolic blood pressure and symptomatic responses to hypoglycemia. This initial suppression of ANS responses by gastro-splanchnic-portal sensing of glucose has been recognized in previous studies in animals (15, 21–23) and humans (35). Heptulla et al. (20), on the other hand, reported either no difference or an increase in neuroendocrine responses during the initial 30 min of hypoglycemia in humans following oral CHO. However, in this present study, following the initial suppression of ANS and neurohumeral responses, there was a series of differential responses. Epinephrine and norepinephrine responses were initially supressed following oral CHO but then rebounded during hypoglycermia to be increased by the end of the experiments relative to the nocal control studies. These findings are conceptually similar to Heptulla et al. (20) whom also demonstrated increased epinephrine levels during hypoglycemia following oral CHO. Smith et al. (35) also observed a numerical but nonsignificant rebound increase in catecholamines following oral CHO at the end of their shorter-duration hypoglycemia studies. The timing of oral CHO ingestion in relation to hypoglycemia and the duration of hypoglycemia differed in the studies of Heptulla et al., Smith et al., and our present study and may explain the differing results obtained in the three reports. In the present study, oral CHO was given at the start of the induction of hypoglycemia, and the plateau nadir was reached 30 min later. This was a different design compared with that of Heptulla et al. (20) in which oral CHO was administered at the start of the hypoglycemic plateau and of Smith et al. (35) in which the CHO was administered 20 min before the induction of hypoglycemia. In addition, differing amounts of CHO were studied in the differing experiments. These CHO “doses” ranged from 15 g in the present studies to 25 g in the studies of Heptulla et al. (20). Of note, the smallest dose of CHO that was used in the present study and is also the recommended amount for the initial reversal of hypoglycemia in clinical practice produced widespread changes in physiological responses. This indicates that gastro-splanchnic-portal sensing of ingested CHO can be activated by the relatively small amounts of CHO used in clinical practice. CHO (15 g) in the present study produced an initial widespread reduction in the various ANS physiological responses to hypoglycemia. The comprehensive reduction in ANS responses indicates that either the gastro-portal-signal is transmitted and sensed in multiple brain areas (i.e., both fore- and hindbrain nuclei) or that the signal is sensed in one critical gatekeeping center that subsequently regulates multiple branches of the ANS (5, 29). This present study cannot specifically provide data addressing the areas of CNS glucose sensing, but this topic has been recently elegantly reviewed by Levin (26) and Routh et al. (33). Perhaps reinforcing the hypothesis that multiple brain regions and neurons are involved in the sensing of oral CHO during hypoglycemia are our present data demonstrating that cortisol (hypothalamo-pituitary-adrenal), ANS, and glucagon responses were all initially blunted during hypoglycemia by oral CHO. The regulation of glucagon secretion during hypoglycemia is complex. There are data demonstrating that glucagon secretion during hypoglycemia is in part mediated via ANS mechanisms (31). However, there are also data in humans indicating that additional pathways exist for regulating the response of the hormone during hypoglycemia (18). Additionally, the afferent sensing pathway from the gut to the CNS has been a topic of considerable interest.
Recent elegant work in animals has determined that the portal vein, rather than hepatic artery or liver afferents, is an important site for hepatoportal glucose sensing during hypoglycemia (15, 21–23, 27, 28, 30, 36). Additionally, it has been demonstrated that afferent hepatoportal sensing of CHO during hypoglycemia is transmitted by spinal rather than vagal glucose-sensitive afferents (17). More recent work has demonstrated that sensing of hypoglycemia in the portal-mesenteric vein region is also modulated by the rate of fall of glucose (34). Saberi et al. (34) have reported that portal vein glucose sensing plays a larger role in suppressing sympathoadrenal responses during slow- rather than fast-onset hypoglycemia. Without the ability to infuse glucose in the portal vein, determining the precise site and pathway of afferent CHO sensing is much more complex in humans. Recent work has demonstrated the importance of gastric (ghrelin) and duoderal-intestinal afferent sensing mechanisms in releasing glucoregulatory and appetite-modulating hormones (glucagon-like peptide-1, cholecystokinin, peptide YY) (32). However, what is clear from the present study is that hypoglycemia is required for oral CHO to have major effects on regulating ANS and neuroendocrine counterregulatory responses. Oral CHO (15 g) administration during hyperinsulinemic euglycemic studies had little or no effects on ANS or cardiovascular or neuroendocrine responses. This demonstrates that insulin, per se, is not the mechanism or the signal responsible for oral CHO effects. The other notable finding from this study was the differential effect of time on physiological responses following oral CHO during hypoglycemia. Plasma cortisol and MSNA were suppressed throughout the entire 90-min study, whereas hypoglycemic symptoms, systolic blood pressure, pancreatic polypeptide, and glucagon responses were initially suppressed but then returned, by the end of hypoglycemia, to levels similar to placebo administration. On the other hand, epinephrine and norepinephrine responses following CHO rebounded higher than those observed following placebo. The reasons for the above differential responses are not apparent from our study and are interesting. Previous studies have determined that the absorption of oral CHO in healthy volunteers is not complete at 2 h (25). Furthermore, the glucose infusion rate data from the present study indicate that CHO absorption was occurring for at least 90 min. Therefore, the simple explanation that all of the ingested CHO was cleared from the splanchnic region during our study and thus the stimulus for blunted responses was removed is clearly not the case. Thus it is not clear why some responses were persistently suppressed (cortisol and MSNA), whereas other key responses (epinephrine, norepinephrine) were in fact amplified by the end of the study.
In summary, 15 g of oral CHO produced a complex array of ANS and neuroendocrine responses during hypoglycemia that comprised of an initial widespread blunting of responses followed by either amplified responses of epinephrine and norepinephrine or continued blunting of cortisol and MSNA or a return to normal response of glucagon, pancreatic polypeptide, symptom responses, and systolic blood pressure. Administration of 15 g of oral CHO during hyperinsulinemic euglycemia had no effects on physiological responses. In conclusion, these data demonstrate that an amount of oral CHO (15 g) commonly used in clinical practice to reverse hypoglycemia can be sensed and markedly influence central ANS, cardiovascular, and neuroendocrine physiological responses during subsequent hypoglycemia in healthy humans.
GRANTS
The work in this study was supported by National Institutes of Health Grants R01-DK-069803, MO1-RR-000095, P01-HL-056693, and P60-DK-020593.
Acknowledgments
We are grateful for the assistance and care provided by the nurses and staff of Vanderbilt's General Clinical Research Center and the volunteers' participation. We thank Eric Allen, Pam Venson, and Wanda Snead for expert technical assistance in analyzing blood samples, Dr. Paul Harris of the Vanderbilt Clinical Research Center for assistance in developing a MatLab analysis interface for MSNA analysis, and Daniel W. Byrne of the Vanderbilt Clinical Research Center for guidance in statistical analyses.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abumrad NN, Rabin D, Diamond MC, Lacy WW. Use of a heated superficial hand vein as an alternative site for measurement of amino acid concentration and for the study of glucose and alanine kinetics in man. Metabolism 30: 936–940, 1981. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar-Parada E, Eisentraut AM, Unger R. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci 257: 415–419, 1969. [DOI] [PubMed] [Google Scholar]
- 3.Amiel SA, Sherwin RS, Simonson DC, Tamborlane WV. Effect of intensive insulin therapy on glycemic thresholds for counterregulatory hormone release. Diabetes 37: 901–917, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Berne C, Fagius J, Niklasson F. Sympathetic response to oral carbohydrate administration. Evidence from microelectrode nerve recordings. J Clin Invest 84: 1403–1409, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI. Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 44: 180–184, 1995. [DOI] [PubMed] [Google Scholar]
- 6.Burcelin R, Dolci W, Thorens B. Portal glucose infusion in the mouse induces hypoglycemia evidence that the hepatoportal glucose sensor stimulates glucose utilization. Diabetes 49: 1635–1642, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Causon R, Caruthers M, Rodnight R. Assay of plasma catecholamines by liquid chromatography with electrical detection. Annal Biochem 116: 223–226, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Cherrington AD, Lacy WW, Chiasson JL. Effect of glucagon on glucose production during insulin deficiency in the dog. J Clin Invest 62: 664–667, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox D, Cryer PE, Gonder-Frederick L, Clarke WL, Antain B. Perceived symptoms in the recognition of hypoglycemia. Diabetes Care 16: 519–527, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Cryer PE Glucose counterregulation: prevention and correction of hypoglycemia in humans. Am J Physiol Endocrinol Metab 264: E149–E155, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Davis SN, Shavers C, Mosqueda-Garcia R, Costa F. Effects of differing antecedent hypoglycemia on subsequent counterregulation in normal humans. Diabetes 46: 1328–1335, 1997. [DOI] [PubMed] [Google Scholar]
- 12.Davis SN, Shavers C, Costa F. Differential gender responses to hypoglycemia are due to alterations in CNS drive and not glycemic thresholds. Am J Physiol Endocrinol Metab 279: E1054–E1063, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Davis SN, Mann S, Galassetti P, Neill RA, Tate D, Ertl AC, Costa F. Effects of differing durations of antecedent hypoglycemia on counterregulatory responses to subsequent hypoglycemia in normal humans. Diabetes 49: 1897–1903, 2000b. [DOI] [PubMed] [Google Scholar]
- 14.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol 237: E214–E223, 1979. [DOI] [PubMed] [Google Scholar]
- 15.Donovan CM, Hamilton-Wessler M, Halter JB, Bergman RN. Primacy of liver glucosensors in the sympathetic response to progressive hypoglycemia. Proc Natl Acad Sci Physiology 91: 2863–2867, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagius J, Berne C. The increase in sympathetic nerve activity after glucose ingestion is reduced in type I diabetes. Clin Sci (Lond) 98: 627–632, 2000. [PubMed] [Google Scholar]
- 17.Fujita S, Donovan CM. Celiac-superior mesenteric ganglionectomy, but not vagotomy, suppresses the sympathoadrenal response to insulin-induced hypoglycemia. Diabetes 54: 3258–3264, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Gosmanov NR, Szoke E, Israelian Z, Smith T, Cryer PE, Gerich JE, Meyer C. Role of the decrement in intraislet insulin for the glucagon response to hypoglycemia in humans. Diabetes Care 28: 1124–1131, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Hagopian W, Lever E, Cen D, Emmounoud D, Polonsky K, Pugh W, Moosa A, Jaspan JB. Predominance of renal and absence of hepatic metabolism of pancreatic polypeptide in the dog. Am J Physiol Endocrinol Metab 245: E171–E177, 1983. [DOI] [PubMed] [Google Scholar]
- 20.Heptulla RA, Tamborlane WV, Ma TY, Rife F, Sherwin RS. Oral glucose augments the counterregulatory hormone response during insulin-induced hypoglycemia in humans. J Clin Endocrinol Metab 86: 645–648, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Hevener AL, Bergman RN, Donovan CM. Novel glucosensor for hypoglycemic detection localized to the portal vein. Diabetes 46: 1521–1525, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Hevener AL, Bergman RN, Donovan CM. Portal vein afferents are critical for the sympathoadrenal response to hypoglycemia. Diabetes 49: 8–12, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Hevener AL, Bergman RN, Donovan CM. Hypoglycemic detection does not occur in the hepatic artery or liver: findings consistent with a portal vein glucosensor locus. Diabetes 50: 399–403, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Ionut V, Hucking K, Liberty IF, Bergman RN. Synergistic effect of portal glucose and glucagon-like peptide-1 to lower systemic glucose and stimulate counter-regulatory hormones. Diabetologia 48: 967–975, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Jones KL, O'Donovan D, Russo A, Meyer JH, Stevens JE, Lei Y, Keogh J, Tonkin A, Horowitz M. Effects of drink volume and glucose load on gastric emptying and postprandial blood pressure in healthy older subjects. Am J Physiol Gastrointest Liver Physiol 289: G240–G248, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Levin BE Metabolic sensing neurons and the control of energy homeostasis. Phys Behav 89: 486–489, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Matveyenko AV, Donovan CM. Metabolic sensors mediate hypoglycemic detection at the portal vein. Diabetes 55: 1276–1282, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Matveyenko AV, Bohland MA, Saberi M, Donovan CM. Portal vein hypoglycemia is essential for full induction of hypoglycemia-associated autonomic failure with slow-onset hypoglycemia. Am J Physiol Endocrinol Metab 293: E857–E864, 2007. [DOI] [PubMed] [Google Scholar]
- 29.McCrimmon RJ, Song Z, Cheng H, McNay CE, Weikart-Yeckel C, Fan X, RouthVH, Sherwin RS. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia induced hormonal counterregulation. J Clin Invest 116: 1723–1730, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore MC, Cardin S, Edgerton DS, Farmer B, Neal DW, Lautz M, Cherrington AD. Unlike mice, dogs exhibit effective glucoregulation during low-dose portal and peripheral glucose infusion. Am J Physiol Endocrinol Metab 286: E226–E233, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Mundinger TO, Mei Q, Figlewicz DP, Lernmark Å, Taborsky GJ. Impaired glucagon response to sympathetic nerve stimulation in the BB diabetic rat: effect of early sympathetic islet neuropathy. Am J Physiol Endocrinol Metab 285: E1047–E1054, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, Hüfner M, Schmiegel WH. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab 87: 1239–1246, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Routh VH, Song Z, Liu X. The role of glucosensing neurons in the detection of hypoglycemia. Diabetes Tech Ther 6: 413–421, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Saberi M, Bohland M, Donovan CM. The locus for hypoglycemic detection shifts with the rate of fall in glycemia: the role of portal-superior mesenteric vein glucose sensing. Diabetes 57: 1380–1386, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Smith D, Pernet A, Reid H, Bingham E, Rosenthal JM, Macdonald IA, Umpleby AM, Amiel SA. The role of hepatic portal glucose sensing in modulating responses to hypoglycaemia in man. Diabetologia 45: 1416–1424, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Thorens B, Larsen PJ. Gut-derived signaling molecules and vagal afferents in the control of glucose and energy homeostasis. Curr Op Clin Nut Met Care 7: 471–478, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Wide L, Porath J. Radioimmunoassay of proteins with the use of sephadex-coupled antibodies. Biochim Biophys Acta 130: 257–260, 1966. [Google Scholar]
- 38.Zangeneh F, Basu R, Shah P, Arora P, Camilleri M, Rizza RA. Enteral infusion of glucose at rates approximating EGP enhances glucose disposal but does not cause hypoglycemia. Am J Physiol Endocrinol Metab 285: E280–E286, 2003. [DOI] [PubMed] [Google Scholar]