Abstract
Accumulated evidence over the last several years indicates that insulin regulates multiple steps in the overall translocation of GLUT4 vesicles to the fat/muscle cell surface, including formation of an intracellular storage pool of GLUT4 vesicles, its movement to the proximity of the cell surface, and the subsequent docking/fusion with the plasma membrane. Insulin-stimulated formation of phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3; and in some cases, of its catabolite PtdIns(3,4)P2] plays a pivotal role in this process. PtdIns(3,4,5)P3 is synthesized by the activated wortmannin-sensitive class IA phosphoinositide (PI) 3-kinase and controls the rate-limiting cell surface terminal stages of the GLUT4 journey. However, recent research is consistent with the conclusion that signals by each of the remaining five PIs, i.e., PtdIns(3)P, PtdIns(4)P, PtdIns(5)P, PtdIns(3,5)P2, and PtdIns(4,5)P2, may act in concert with that of PtdIns(3,4,5)P3 in integrating the insulin receptor-issued signals with GLUT4 surface translocation and glucose transport activation. This review summarizes the experimental evidence supporting the complementary function of these PIs in insulin responsiveness of fat and muscle cells, with particular reference to mechanistic insights and functional significance in the regulation of overall GLUT4 vesicle dynamics.
Keywords: glucose transporter 4, glucose transport, fat and muscle cells, phosphatidylinositol monophosphates, phosphatidylinositol bisphosphates
insulin stimulation of glucose transport in muscle and fat tissues is essential for maintenance of glucose homeostasis. The principal glucose transporter that mediates this uptake is the integral membrane protein glucose transporter 4 (GLUT4), a facilitative glucose transporter that, upon insulin receptor activation, translocates to the fat/muscle cell surface (recently reviewed in Refs. 13, 19, 37, 47, 50, 73, and 74). The dominant role of GLUT4 in maintaining normal whole body glucose homeostasis has been illustrated by a multitude of genetically engineered mouse models with modulated GLUT4 expression levels (41). Although the molecular and cellular mechanism underlying insulin-induced GLUT4 translocation from the intracellular storage sites to the plasma membrane is under intensive investigation, our knowledge is still incomplete and subject to debate. According to currently accepted models, GLUT4, through both clathrin-assisted and clathrin-independent processes, is internalized from the cell surface into endosomes from where it exits into a postendosomal pool of GLUT4 storage vesicles (GSVs). Under basal conditions, GLUT4 is sequestered in this GSV intracellular pool by a mechanism of dynamic recycling/recapturing and/or tethering to retention receptors (13, 19, 37, 47, 50, 74). Newly synthesized GLUT4 is also sorted to this compartment directly from the trans-Golgi network (TGN). In response to insulin, GSVs undergo enhanced cytoskeleton-dependent trafficking toward the cell surface, where the plasma membrane fusion is markedly increased. Whereas the GSV movement and docking/fusion now appear to be the major regulated events (19, 37, 74), experimental evidence is provided to indicate that insulin also influences additional steps of the complex GLUT4 trafficking itinerary, such as the de novo GSV formation (76), GLUT4 interaction with retention receptors (50), GLUT4 interendosomal trafficking (15), remodeling of the cortical filamentous actin (F-actin) (13, 73), GLUT4 unmasking, and activation at the cell surface (17, 26). These events are crucial for building up a reservoir pool of GSVs with correct properties for movement, surface insertion, or glucose uptake as well as for creating a proper microenvironment extrinsic to GSVs, which is a prerequisite for optimal insulin responsiveness. In fulfilling this mission, the activated insulin receptor triggers signaling cascades that are both dependent on, and independent of, class IA phosphoinositide 3-kinase (PI3K), with more than 60 protein and lipid intermediate players implicated thus far in orchestrating the overall process (19, 37, 47, 50, 70, 73, 74). Among those, a central role is attributed to phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3], one of the seven members of the PI group (Fig. 1). PtdIns(3,4,5)P3 is generated from PtdIns(4,5)P2 at the cell surface by the action of the wortmannin-sensitive class IA PI3K that is activated via the insulin receptor substrate signaling pathway. Inositol polyphosphate phosphatases SHIP and SKIP1, Src homology 2-containing inositol 5-phosphatase, and skeletal muscle- and kidney-enriched inositol phosphatase respectively rapidly convert PtdIns(3,4,5)P3 to PtdIns(3,4)P2 whose signaling functions may not completely overlap those of PtdIns(3,4,5)P3 (10). Besides PtdIns(3,4,5)P3 and PtdIns(3,4)P2, mammalian cells exhibit five more PI species that are produced by reversible phosphorylation of the inositol hydroxyls at positions 3, 4, and/or 5 (Fig. 1). As PtdIns(3,4,5)P3 and PtdIns(3,4)P2, they are low-abundance membrane-anchored lipid signals that are now implicated in nearly all aspects of cell physiology, including membrane trafficking and actin cytoskeleton organization (12, 39, 64, 69). Recent work has revealed unsuspected roles of these other PIs in insulin-regulated GLUT4 dynamics acting in parallel with PtdIns(3,4,5)P3 signals and contributing to the fine tuning of the overall insulin response. Although molecular details related to their function in GLUT4 translocation remain to be revealed in many cases, the wortmannin-independent elevation observed with some of these PIs further supports the concept that insulin stimulates signaling cascade(s) that acts in parallel with PI3K activation and PtdIns(3,4,5)P3 generation. This review focuses on our current knowledge about the function of PtdIns(3)P, PtdIns(4)P, PtdIns(5)P, PtdIns(3,5)P2, and PtdIns(4,5)P2 in insulin-regulated GLUT4 translocation in adipose and muscle cells. The role of PtdIns(3,4,5)P3/PtdIns(3,4)P2 and that of their metabolizing enzymes in insulin responsiveness has been reviewed recently (47, 53, 70, 72).
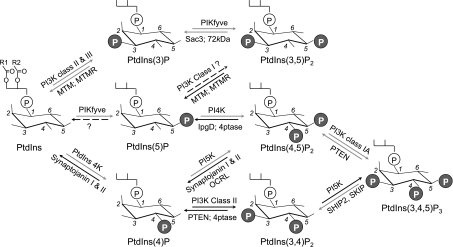
Fig. 1.
The 7 phosphoinositides (PIs) and their metabolism. The PI molecule includes inositol 1-phosphate (a hydrophilic portion exposed to the cytosol) bound via the phosphate group to 1-R1, 2-R2 diacylglycerol (a hydrophobic membrane-anchored portion). Depicted are the major pathways for synthesis and turnover confirmed both in vitro and in vivo (solid arrows). The routes implicated in insulin-regulated glucose transporter 4 (GLUT4) vesicle translocation discussed herein are in gray arrows. R1/R2, fatty acid; P, phosphate; PI3K, PI4K, and PI5K, PI 3-kinase, PI 4-kinase, and PI 5-kinase, respectively; PtdIns 4K, phosphatidylinositol 4-kinase; MTM, myotubularin; MTMR, myotubularin related; IpgD, entry-mediating invasin phosphatase; OCRL, phosphatase implicated in the oculocerebrorenal syndrome of Lowe; PtdIns(3)P, phosphatidylinositol 3-phosphate; PtdIns(3,5)P2, phosphatidylinositol 3,5-bisphosphate; PtdIns(5)P, phosphatidylinositol 5-phosphate; PtdIns(4,5)P2, phosphatidylinositol 4,5-bisphosphate; PTEN, phosphatase and tensin homolog deleted on chromosome 10; PIKfyve, phosphoinositide kinase for position five containing the FYVE domain; SHIP2, Src homology 2 containing inositol 5-phosphatase; SKIP, skeletal muscle- and kidney-enriched inositol phosphatase; PtdIns(3,4,5)P3, phosphatidylinositol 3,4,5-trisphosphate; PtdIns(4)P, phosphatidylinositol 4-phosphate; PtdIns(3,4)P2, phosphatidylinositol 3,4-bisphosphate.
PtdIns(3)P
Adipose and muscle cells display a constitutive, although small, pool of PtdIns(3)P (1–3% of total PIs; Refs. 33, 43, and 54) that is maintained through a balance between PI3Ks and inositol polyphosphate 3-phosphatase activities. The role of PtdIns(3)P in the insulin-signaling cascade has been suggested in early studies by the finding of a rapid wortmannin-independent increase of the PI3K class II C2α (PI3K-C2α) in vitro activity in response to insulin stimulation of adipose and muscle cells (4). However, insulin-dependent changes in [32P]PtdIns(3)P-accumulated cellular levels have not been registered by HPLC inositol head group separation of lipids extracted from 32Pi-labeled primary or cultured adipocytes (33, 43). More recent HPLC analyses in L6 cells and 3T3-L1 adipocytes, metabolically labeled with [3H]myoinositol, document a transient [3H]PtdIns(3)P increase in response to acute insulin (38). Although the PtdIns(3)P could be generated by class II and class III PI3Ks, the insulin-dependent PtdIns(3)P rise is attributed to wortmannin-resistant PI3K-C2α (14). Using a PtdIns(3)P-binding Fab1p/YOTB/Vac1p/EEA1 (FYVE) domain as an intracellular reporter, PtdIns(3)P generation is suggested to occur at the plasma membrane lipid raft subdomain, where the PI3K-C2α enzyme translocates in response to insulin (14, 38). Carrier-mediated delivery of exogenous PtdIns(3)P into primary or cultured adipocytes and L6 myoblasts or myotubes has been shown by several laboratories to promote wortmannin-resistant movements of GLUT4 to the plasma membrane in the absence of insulin (26, 29, 38, 68). Insulin-independent cell surface gain of GLUT4 is also observed by an enzymatic increase in plasma membrane PtdIns(3)P levels through ectopic expression of the 72-kDa inositol polyphosphate 5-phosphatase in cultured adipocytes (34). However, despite the cell surface GLUT4 accumulation upon plasma membrane PtdIns(3)P increases, in neither case is the glucose transport stimulated (34, 38, 68). This failure has been attributed to the inability of delivered PtdIns(3)P to support GLUT4 membrane fusion (38, 68), a process that requires activation of class IA PI3K-catalyzed PtdIns(3,4,5)P3 production (19, 37, 74). However, exogenously delivered PtdIns(3,4,5)P3 is also insufficient to stimulate glucose transport, which could result partly from a requirement for an additional unmasking step of GLUT4 following plasma membrane fusion (26). Although PtdIns(3)P is suggested to fulfill such a function (26), it is worth noting that the combined carrier-based delivery of PtdIns(3)P and PtdIns(3,4,5)P3 into 3T3-L1 adipocytes also does not suffice to activate glucose transport (68). These observations indicate either that the concentration/localization of the delivered lipids is inadequate to trigger glucose uptake or that PtdIns(3)P/PtdIns(3,4,5)P3-independent regulatory signals are required in parallel. Alternatively or additionally, the exogenous lipids may induce unintended changes in other endogenous PI or protein players, which could also explain why the combined PtdIns(3)P and PtdIns(3,4,5)P3 delivery fails to activate glucose transport. Nonetheless, the expected perturbation in PtdIns(3)P levels by either ectopic expression of the PI 3-phosphatase myotubularin or depletion of PI3K-C2α reduces the insulin-activated glucose uptake (7, 14), consistent with the requirement for PtdIns(3)P in this process. Furthermore, whereas PtdIns(3)P signals are apparently required at a step of GLUT4 movement, inputs at the cell surface, in addition to GLUT4 unmasking (26), are also possible (Fig. 2). This is suggested by recent findings in adipocytes demonstrating that PtdIns(3)P is permissive for GLUT4 fusion with the plasma membrane under knockout of Munc18c (29), a protein that masks syntaxin 4 at the cell surface and thus disables fusion (73). Likewise, GLUT4 plasma membrane fusion does occur in the experimental system of enzymatically increased surface PtdIns(3)P upon ectopic expression of the 72-kDa 5-phosphatase in 3T3-L1 adipocytes (34).
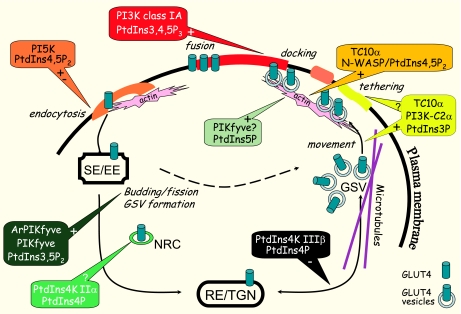
Fig. 2.
Schematic model for the sites of PI's action in insulin-regulated multistep process of GLUT4 translocation in adipose and muscle cells. GLUT4 endocytosis requires PtdIns(4,5)P2, whose insulin-dependent regulation is uncertain. A subpopulation of GLUT4 exits sorting endosomes/early endosomes (SE/EE) and via recycling endosomes/trans-Golgi network (RE/TGN) enters into GLUT4 storage vesicles (GSVs) in an insulin-regulated process that requires robust endosomal PtdIns(3,5)P2 synthesis by PIKfyve and its associated regulator ArPIKfyve. A subpopulation of small GLUT4 vesicle intermediates that may also bud off from SE/EE contain PtdIns4K IIα and remain nonresponsive to insulin (NRC). Insulin-dependent movement of GSV requires PI3K-C2α-catalyzed PtdIns(3)P synthesis that may also affect events at the cell surface. GLUT4 movement/tethering requires an insulin-regulated filamentous actin remodeling regulated by robust change in PtdIns(5)P and/or PtdIns(4,5)P2. Signals mediated by insulin-activated class IA PI3K and PtdIns(3,4,5)P3 synthesis regulate GLUT4 docking/fusion. The PtdIns(3,4,5)P3 (red), PtdIns(3)P (yellow), and PtdIns(4,5)P2 (orange) basal or insulin-regulated localizations at plasma membrane subdomains are confirmed by specific reporters containing selective pleckstrin homology domains [for PtdIns(3,4,5)P3 and PtdIns(4,5)P2 binding] or the FYVE finger domain [for PtdIns(3)P binding]. Compartments/microdomains of basal or altered PtdIns(3,5)P2, PtdIns(5)P, and PtdIns(4)P in response to insulin have not been verified by specific bioreporters. The individual steps in the overall GLUT4 translocation are in italics. See text for more details. N-WASP, neural Wiskott-Aldrich syndrome protein.
Activation of PI3K-C2α, and thus PtdIns(3)P production, is proposed to occur via the Rho-like GTPase TC10 (14). TC10 resides constitutively in lipid raft microdomains of the plasma membrane, where it is activated by insulin in a manner independent of class IA PI3K (73). TC10α, but not TC10β, is required in insulin-induced activation of glucose transport in cultured adipocytes (6), but whether such TC10 isoform specificity operates in the PI3K-C2α activation remains to be seen. In response to insulin, activated TC10 mobilizes the exocyst, an evolutionarily conserved vesicle-tethering complex that is crucial for targeting GSVs to the plasma membrane in 3T3-L1 adipocytes (25). Whether PtdIns(3)P is engaged in this process is unknown. It has become increasingly clear that GLUT4 translocation in response to insulin is associated with dynamic actin remodeling mediated, in part, via activated TC10 (13, 31, 73). A plausible link between PtdIns(3)P and submembranous actin remodeling has been suggested on the basis of data with actin-depolymerizing agents (34). Further work is needed to clarify specific downstream molecular effectors of the wortmannin-resistant TC10/PI3K-C2α/PtdIns(3)P pathway signaling to GLUT4 in response to insulin as well as PtdIns(3)P's role in the GSV cell surface events.
PtdIns(3,5)P2
PtdIns(3,5)P2 is widespread in mammalian cells but present only in minute quantities (20). In 3T3-L1 adipocytes, a cell type where PtdIns(3,5)P2 has been studied exclusively in the context of insulin action, this PI constitutes <0.2% of total PIs (54). Data from an in vitro [32P]ATP-membrane labeling assay combined with HPLC inositol head group separation of extracted lipids indicate marked insulin-dependent increases in PtdIns(3,5)P2 on intracellular membranes and less so on plasma membranes fractionated from 3T3-L1 adipocytes (23). In cell-based assays, however, insulin stimulation for over a period of 1 to 30 min fails to significantly upregulate [32P]PtdIns(3,5)P2 accumulation in labeled 3T3-L1 adipocytes as measured by HPLC analyses of total cellular PIs (54). Noteworthy, similar analyses in this cell type document a profound and unprecedented hyperosmotic rise in [32P]PtdIns(3,5)P2-accumulated levels (54). Therefore, it is highly likely that increased PtdIns(3,5)P2 production in response to insulin engages only a distinct subset of membranes and could not be registered by HPLC-based measurements of total 32P-labeled PIs. Although PtdIns(3,5)P2-binding molecules as well as PtdIns(3,5)P2-specific antibodies have been described (44, 51), we found both to be unsuitable as reporters for quantitative imaging or morphological definition of the PtdIns(3,5)P2-rich membranes in various mammalian cell types, including 3T3-L1 adipocytes (Ikonomov O and Shisheva A, unpublished observations). As a result, the exact intracellular sites of localized PtdIns(3,5)P2 generation in response to insulin or hyperosmolarity in 3T3-L1 adipocytes are elusive. Based on information about the localization and functionality of PIKfyve (phosphoinositide kinase for position five containing the FYVE domain), the sole enzyme producing PtdIns(3,5)P2 (comprehensively reviewed recently in Ref. 63), the membranes of the dynamic endosomal system are candidate sites. In mammalian cells, PIKfyve localizes at the endosomal PtdIns(3)P-substrate pool in a wortmannin-sensitive manner (57). The targeted PtdIns(3)P pool is therefore most likely produced by the wortmannin-sensitive class III and perhaps class IA PI3Ks, but this is yet to be experimentally verified. PIKfyve has been initially isolated from a mouse adipocyte library through a screen for transcripts that, like GLUT4, are expressed in a fat/muscle-specific/enriched manner (67). Subsequent studies with the endogenous enzyme revealed presence of the protein and activity to various degrees in all mammalian cell types tested thus far, including PC12, human embryonic kidney (HEK 293), Chinese hamster ovary (CHO), COS, 3T3-L1, and others (Refs. 60 and 63 and Sbrissa D and Shisheva A, unpublished observations). Importantly, a transition of 3T3-L1 cells from a fibroblastic to adipocytic phenotype is associated with considerable upregulation of PIKfyve protein, implying a key role for the two PIKfyve lipid products, PtdIns(3,5)P2 and PtdIns(5)P (see below), in 3T3-L1 adipocyte-specific functions (21). However, stimulation of 3T3-L1 adipocytes with insulin or with other external cell stimuli, such as EGF, PDGF, or osmolarity, does not affect the lipid kinase activity to a significant degree as assayed in vitro with PIKfyve preparations immunopurified from cell lysates (55, 56, 58). An alternative regulatory mechanism may involve intracellular redistribution of PIKfyve without changes in the intrinsic activity. Biochemical observations in 3T3-L1 adipocytes for a recruitment of cytosolic PIKfyve to intracellular microsomes in an insulin-dependent manner are consistent with such a mechanism (66). Because the PIKfyve molecule harbors a FYVE domain that binds PtdIns(3)P (57), regulated formation of PtdIns(3)P-enriched endosomal platforms could serve as plausible targets for the enzyme. However, other recruitment mechanisms, operating separately or in conjunction, could not be excluded. In this regard, it is interesting that the membrane PIKfyve fractionated from insulin-treated cells displays an electrophoretic mobility shift (66). The latter is phosphatase sensitive, indicative of an insulin-regulated phosphorylation step in the PIKfyve membrane recruitment mechanism, but more work is needed to clarify the affected residues and kinase responsible (66). On the basis of data with phospho-specific PIKfyve antibodies directed to a peptide in a sequence context of phospho-S318, Berwick et al. (1) proposed that PIKfyve is phosphorylated by Akt (also known as PKB) in response to insulin, which activates its enzymatic activity. However, the specificity of the S318 site in PIKfyve for phosphorylation by Akt, as claimed previously (1), has not been confirmed recently (62).
Several lines of experimentation implicate localized production of PtdIns(3,5)P2 as a positive regulator of insulin responsiveness in 3T3-L1 adipocytes. Thus, expression of dominant-negative kinase-deficient PIKfyve mutants, some of which are selectively deficient for PtdIns(3,5)P2 synthesis, inhibit insulin-induced gain of surface GLUT4 in a background of no noticeable aberrations in adipocyte morphology (21). Likewise, depletion of PIKfyve by siRNAs inhibits the cell surface accumulation of ectopically expressed GLUT4 and endogenous insulin-responsive aminopeptidase, or the activation of glucose transport by insulin, concomitant with a reduction of the intracellular PtdIns(3,5)P2 pool (23). Conversely, insulin-dependent gain in surface GLUT4 and glucose uptake is observed under ablation of the inositol polyphosphate 5-phosphatase Sac3 that associates with PIKfyve and dephosphorylates PtdIns(3,5)P2 (59, 65). However, PtdIns(3,5)P2, delivered by cytoplasmic microinjections, does not promote GLUT4 translocation in 3T3-L1 adipocytes, which correlates with the inability of PIKfyveWT expression or Sac3 depletion to activate GLUT4 translocation or glucose transport in the absence of insulin stimulation (21, 61, 65). These data imply that the step sensitive to PtdIns(3,5)P2 signals most likely precedes the GSV movement to the cell surface (Fig. 2). Recent findings from in vitro reconstitution assays suggest that vesicle budding/fission (or maturation) and fusion events in the endosomal network correlate with PtdIns(3,5)P2 synthesis and turnover, respectively (22, 59). Consistent with PtdIns(3,5)P2's key role in early endosome plasticity are data on the performance of membrane trafficking pathways in several mammalian cell types. Thus, disruption of PtdIns(3,5)P2 synthesis by either knockdown of PIKfyve or expression of dominant-negative kinase-deficient PIKfyveK1831E selectively inhibits the course of endocytic cargo transport out of early endosomes to the TGN or other postendosomal compartments in HeLa or HEK 293 cells (24, 52). However, both constitutive and regulated membrane recycling to the cell surface, as exemplified by the insulin-induced GLUT1 recycling in 3T3-L1 adipocytes or the constitutive transferrin receptor (TfR) recycling in HEK-293 cells, are intact (23, 24). Therefore, it is likely that insulin accelerates exit of GLUT4 from early endosomes en route to GSVs through a mechanism that requires a robust PIKfyve-catalyzed PtdIns(3,5)P2 production. It is conceivable that such a step is critical for the endosomal GLUT4 to reenter and replenish the rapidly depleting GSV pool during insulin challenge. Observations that both GSV formation and interendosomal traffic are activated by insulin support this idea (15, 76). More work is needed to directly implicate PtdIns(3,5)P2 function in the insulin-regulated step of GLUT4 vesicle budding/fission from (or maturation of) early endosomes en route from GSVs.
An adapter protein that physically associates with PIKfyve to activate its enzymatic activity is associated regulator of PIKfyve (ArPIKfyve) (60). That ArPIKfyve also functions as an upstream activator of PIKfyve-catalyzed PtdIns(3,5)P2 production in 3T3-L1 adipocytes is supported by the observations for reduced [32P]PtdIns(3,5)P2 intracellular accumulation upon ArPIKfyve depletion (54). Of significant importance is the finding that, under the conditions of ArPIKfyve depletion, insulin-stimulated glucose uptake in 3T3-L1 adipocytes is reduced (23). Combined loss of PIKfyve and ArPIKfyve proteins has a greater inhibition on insulin-stimulated glucose uptake, correlating with a greater reduction in the intracellular PtdIns(3,5)P2 pool (23). However, the association between ArPIKfyve and PIKfyve appears not to be an insulin-regulated step (23), and thus the upstream mechanism(s) underlying the ArPIKfyve/PIKfyve-controlled PtdIns(3,5)P2 increase in response to insulin requires further investigation. In another study, insulin-activated Akt is suggested to be an upstream activator of PIKfyve-catalyzed PtdIns(3,5)P2 rise, but this, unlike in our studies (21, 23), inhibits GLUT4 translocation (1). However, as has recently become apparent, the PIKfyveS318A mutant, used solely to draw such a conclusion, has been inaccurately characterized as a specific substrate for Akt-dependent phosphorylation. In fact, PIKfyveS318A and PIKfyveWT are equally phosphorylated by Akt in vitro (62). This notable inconsistency requires careful reevaluation of the notion that PIKfyve is a downstream Akt effector in insulin signaling to GLUT4.
Several candidate downstream protein effectors of PtdIns(3,5)P2 have recently been found to operate in other trafficking systems in both yeast and mammalian cells (63). It remains to be seen whether these or other yet-to-be-identified targets of PtdIns(3,5)P2 fit into the insulin-signaling network leading to GLUT4 translocation and glucose transport. Intriguingly, evidence is provided for a potential link between PIKfyve lipid products and Akt. Thus, Akt exhibits reduced and increased phosphorylation by insulin under PIKfyve/ArPIKfyve depletion and PIKfyveWT expression, respectively, in cultured adipocytes (21, 23). In other cell types, Akt is found phosphorylated in a PtdIns(5)P-dependent manner (Ref. 49; also see below), but how these two are mechanistically coupled is unknown.
PtdIns(5)P
PtdIns(5)P is barely detectable in many cell types, but it constitutes a substantial subfraction of the total PIs in resting 3T3-L1 adipocytes (58, 61). It exceeds by approximately fivefold the basal PtdIns(3)P in these cells and, by at least one order of magnitude, the PtdIns(5)P levels measured in other cell types (58, 61). Importantly, insulin action in 3T3-L1 adipocytes and transformed (T) CHO cells expressing the human insulin receptor induces a robust and transient increase in PtdIns(5)P mass levels (61). The PtdIns(5)P rise appears to be independent of class IA PI3K activation and signaling because it fully resists cell treatment with wortmannin (61). The intracellular place and enzyme(s) responsible for the PtdIns(5)P increase are uncertain. On the basis of observations that PIKfyve produces PtdIns(5)P both in vivo and in vitro and that ectopically expressed PIKfyveWT mimics the effect of exogenously delivered PtdIns(5)P on F-actin breakdown in CHO-T cells, PIKfyve is suggested as the candidate kinase (61). Moreover, recruitment of a cytosolic subfraction of PIKfyve to intracellular membranes is observed in 3T3-L1 adipocytes in response to insulin, which may mechanistically link the PtdIns(5)P increase with PIKfyve (66). However, as mentioned above, the in vitro-measured PIKfyve activity is not significantly altered by acute insulin, and given the complex enzymology of PtdIns(5)P metabolism (64), the identity of the enzyme subjected to insulin regulation is inconclusive. Whatever the enzyme, it is highly significant that PtdIns(5)P, exogenously delivered into 3T3-L1 adipocytes by cytoplasmic microinjections, increases cell surface abundance of ectopically expressed GLUT4 in the absence of insulin (61). Conversely, agents that sequester PtdIns(5)P, such as the 3xPHD (plant homeodomain) derived from different PHD-containing proteins, disable insulin-triggered cell surface gain of ectopically expressed GLUT4. Kinetic considerations are consistent with a PtdIns(5)P-dependent acceleration of GLUT4 exocytosis rather than with an inhibition of endocytosis (61). Noteworthy, as in the case of PtdIns(3)P, carrier-based delivery of PtdIns(5)P is unable to significantly activate glucose transport in 3T3-L1 adipocytes in the absence of insulin (Sbrissa D and Shisheva A, unpublished observations). Because PtdIns(5)P induces a wortmannin-resistant breakdown of actin stress fibers in CHO-T cells, it is conceivable that its robust rise is required in the class IA PI3K-independent remodeling of actin filaments shown to be critical for optimal efficiency of GLUT4 movements in response to insulin (13, 73). Experimental support for PtdIns(5)P functioning in F-actin reorganization is also coming from studies in HeLa and NIH-3T3 cells, in which PtdIns(5)P has been elevated by expression of IpgD (entry-mediating invasin phosphatase), a bacterial inositol polyphosphate 4-phosphatase (45). Further work is required to support the predicted insulin-regulated link of PtdIns(5)P with complex F-actin remodeling in 3T3-L1 adipocytes and determine molecular effector(s) downstream of PtdIns(5)P in the context of GLUT4. Evidence from other cell systems indicates that the IpgD-dependent PtdIns(5)P rise activates Akt, presumably via inhibiting PtdIns(3,4,5)P3 dephosphorylation (5) and/or via activating class IA PI3K (49), but mechanistic details as to how this happens are thus far unavailable.
Whereas the downstream effector(s) as well as the enzyme(s) responsible for the insulin-induced PtdIns(5)P rise in adipocyte context are yet to be determined, of significant interest are data in mice deficient for PI4Kβ, the enzyme that clears PtdIns(5)P by converting it to PtdIns(4,5)P2 (Fig. 1). The PI4Kβ−/− mice display increased insulin sensitivity and reduced adipocity, with enhanced Akt activation by insulin in skeletal muscle and liver (36). Although the PI profile in the knockout mice is yet to be seen, inhibition of PI4Ks could potentially represent an approach in treatment of obesity and insulin resistance.
PtdIns(4)P
Unlike the first three PIs discussed above, PtdIns(4)P is highly abundant in mammalian cells, including adipose and muscle cells (33, 38, 43, 54, 58). Early studies have demonstrated that GLUT4-containing vesicles isolated from rat fat and skeletal muscle cells display abundant PtdIns(4)P-synthesizing activity, which is not regulated by insulin (11, 35). PtdIns 4K type IIα, a ubiquitous integral membrane protein (64), has recently been assigned to account for virtually all PtdIns 4K activity found in GLUT4 vesicles in 3T3-L1 adipocytes (75). However, GSVs are not the place of this enzyme's residence. Rather, PtdIns 4K type IIα partitions exclusively to a subpopulation of GLUT4 vesicles that do not translocate in response to insulin and are positive for cellugyrin (75). Cellugyrin is a homolog of neuronal synaptogyrin that is involved in the biogenesis of vesicle carriers shuttling between sorting/early endosomes and the TGN. Cellugyrin-positive GLUT4 vesicles are suggested to represent intracellular transport intermediates that bud from endosomes en route to GSV via the TGN (75), but it remains to be seen whether PtdIns(4)P contributes to this process. In another study, expression of a different type of PtdIns(4)P-generating activity, PtdIns 4K type IIIβ, or its activator, neuronal calcium sensor 1, in 3T3-L1 adipocytes inhibits GLUT4 translocation in response to insulin under preserved proximal insulin signaling via both class IA PI3K-dependent and -independent pathways (42). Notably, surface translocations of GLUT1 and manose 6-phosphate receptor by insulin are intact, implying that yet-to-be identified events en route to, or at the pool of GSVs, but not at the endosomal recycling compartment, are sensitive to high PtdIns(4)P (42). How PtdIns(4)P produced by PtdIns 4K type IIIβ negatively affects insulin responsiveness remains to be clarified. Whether increased PtdIns(4)P hydrolysis results in gain of insulin responsiveness has not yet been examined. It should be noted, however, that exogenously delivered PtdIns(4)P, by either microinjection or carriers, does not affect the cell surface accumulation of ectopically expressed or endogenous GLUT4 in 3T3-L1 adipocytes and L6 myoblasts, nor does it alter the basal glucose transport (38, 61).
PtdIns(4,5)P2
The abundant PtdIns(4,5)P2 that is synthesized primarily by PI5Ks has been known for quite some time to affect almost every step of neurotransmitter endocytosis and regulated secretion, including vesicle budding, fission, motility, and fusion (12, 39, 69). Given the ability of PtdIns(4,5)P2 to recruit multiple components of the endocytic machinery at the plasma membrane and to regulate the activity of a variety of proteins associated with reorganization of F-actin, it is indeed hard to envision proper GLUT4 translocation by insulin without correct PtdIns(4,5)P2 dynamics. However, direct experimental evidence to implicate localized PtdIns(4,5)P2 production in insulin responsiveness of GLUT4 translocation in muscle and adipose cells has emerged only recently. In addition to serving as a precursor for insulin-induced PtdIns(3,4,5)P3 synthesis by class IA PI3K at the cell surface (10), PtdIns(4,5)P2 may intersect the elaborate GLUT4 vesicle trafficking at several additional levels, including endocytosis, motility, cell surface insertion, and possibly activation. The link between PtdIns(4,5)P2 and GLUT4 trafficking is likely through PtdIns(4,5)P2's capacity to induce cortical actin remodeling that is crucial in insulin responsiveness in both adipose and muscle cells (13, 71, 73). Concordantly, deconvolution microscopy analysis in cultured adipocytes documents that PtdIns(4,5)P2 is unevenly distributed on the cell surface and that the PtdIns(4,5)P2-enriched regions [called PtdIns(4,5)P2-rich membrane patches] associate exclusively with regions of dense F-actin (18). However, several observations in primary and cultured adipocytes suggest that PtdIns(4,5)P2 may function independently of F-actin. Thus, GLUT4 endocytosis in rat adipocytes is found to be independent of F-actin (46). Concordantly, a blockade in the function of PtdIns(4,5)P2, but not that of the cortical F-actin, completely arrests clathrin-assisted endocytosis in 3T3-L1 adipocytes (18). Likewise, the localization of PtdIns(4,5)P2 to the plasma membrane or formation of aberrant structures seen under increased PtdIns(4,5)P2 production is largely independent of cortical F-actin (30).
Two studies have addressed the role of PtdIns(4,5)P2 in endocytosis of TfR and GLUT4 by directly modulating the local presence of PtdIns(4,5)P2 at the plasma membrane in 3T3-L1 adipocytes. Sequestration of PtdIns(4,5)P2 with the pleckstrin homology domain of PLCδ1, a powerful PtdIns(4,5)P2 binding module, markedly inhibits TfR endocytosis (18), consistent with deregulation of the PtdIns(4,5)P2-binding proteins of the clathrin coat and membrane fission such as AP2, epsin, and dynamin (10, 12). Intriguingly, expression of PI5Ks to increase adipocytic PtdIns(4,5)P2 production also results in inhibition of TfR and GLUT4 endocytosis, concomitant with derangement of F-actin (30). Whereas the similar outcome under these two seemingly opposite effects on the local presence of functional PtdIns(4,5)P2 may appear unexpected, it should be taken into consideration that, following clathrin coat assembly and membrane fission, a subsequent PtdIns(4,5)P2 hydrolysis disassembles the coat and is required very early in the endocytosis process (10, 12). Potential block in uncoating of AP2, whose α-subunit binds PtdIns(4,5)P2 with high activity, has been suggested to mechanistically link the reduced GLUT4 endocytosis with increased plasma membrane PtdIns(4,5)P2 (30).
Unlike GLUT4 endocytosis, GLUT4 translocation to the cell surface is facilitated by actin filaments that may serve both as linear tracks for directing GSV movements and as anchors in the cell surface insertion (48, 71, 73). The nonconventional motor myosin 1c (Myo1c) that induces plasma membrane ruffling at locations marked by PtdIns(4,5)P2-rich membrane patches and resides, at least in part, in GSVs may provide such a link (2, 3, 18). Upon insulin, GLUT4 vesicles translocate to the regions of Myo1c-driven cell surface ruffling, where they may even undergo plasma membrane fusion if Myo1c is expressed at high levels (3). Notably, both events are independent of PI3K activation and PtdIns(3,4,5)P3 generation (2, 3). Consistent with the critical role of Myo1c in insulin responsiveness, depletion of the endogenous protein inhibits insulin-stimulated glucose uptake (2). Furthermore, Myo1c was recently found to associate with the exocyst complex in 3T3-L1 adipocytes, consistent with a mutual cooperation of the two complexes in the insulin-dependent surface anchoring of GLUT4 vesicles (8). Whereas a parallel PtdIns(4,5)P2-regulated remodeling of actin cytoskeleton is likely to be coupled with the Myo1c/exocyst-driven events at the cell surface, direct data are thus far unavailable. A plausible link may be provided by the members of the Wiskott-Aldrich syndrome protein (WASP) family that are recruited to PtdIns(4,5)P2 and activated to promote formation of a branching network of actin filaments (64, 69). An association of the ubiquitously expressed isoform neural (N)-WASP with TC10 is found to control a PI3K-independent F-actin assembly and GLUT4 responsiveness to insulin in 3T3-L1 adipocytes (28, 32). Thus, insulin-activated TC10 emanates PtdIns(3,4,5)P3-independent signals to at least two candidate PI downstream effectors that function in GLUT4 motility and cell surface anchoring, i.e., PtdIns(3)P and N-WASP/PtdIns(4,5)P2. How these signals are coordinated in space and time remains to be seen, as does the expected diversity in the TC10-controlled effectors in muscle vs. adipose cells (27).
More direct work to implicate PtdIns(4,5)P2-dependent F-actin remodeling in insulin-regulated GLUT4 dynamics has been carried out in insulin-resistant cell models. Sustained insulin exposure of 3T3-L1 adipocytes or L6 myotubes, a treatment that renders cells insulin resistant, is associated with reduction of cortical F-actin and a concomitant loss of plasma membrane PtdIns(4,5)P2 (9, 40). The restoration of plasma membrane PtdIns(4,5)P2 levels by exogenous carrier-linked delivery restores cortical F-actin and alleviates insulin responsiveness of GLUT4 translocation and glucose transport (9, 40). However, in normal L6 muscle or 3T3-L1 adipose cells, exogenous PtdIns(4,5)P2, delivered by cytoplasmic microinjections or carriers, is ineffective in triggering GLUT4 dynamics in the absence of insulin (26, 61, 68). It should also be noted that statistically significant insulin-dependent changes of the PtdIns(4,5)P2 total levels or surface distribution in cultured adipocytes have not been observed by HPLC analyses or deconvolution microscopy with a PtdIns(4,5)2 reporter, respectively (18, 61). Since such alterations are clearly inevitable (64), a more sensitive technology for monitoring PtdIns(4,5)P2 dynamic and spatially discrete fluctuations is apparently required. Experimental evidence was recently provided to suggest that PtdIns(4,5)P2 may also stimulate GLUT4 transport activity in 3T3-L1 adipocytes, which proceeds in an F-actin-dependent manner (16). p38 MAPKs have previously been implicated in enhancing the intrinsic glucose transport activity of GLUT4 at the plasma membrane (17), but how PtdIns(4,5)P2 and F-actin fit into this activation paradigm remains to be addressed.
Concluding Remarks and Perspectives
Although the class IA PI3K product PtdIns(3,4,5)P3 is undoubtedly a critical intermediate player in insulin action to GLUT4, the available data in both muscle and adipose cells are consistent with the notion that the other five PI species also play a pivotal role in the overall insulin responsiveness of GLUT4 translocation, relaying signals complementary to those of PtdIns(3,4,5)P3. These other PIs most likely affect distinct and, probably, not overlapping segments in the insulin-regulated GLUT4 translocation process, and their relative contributions and detailed mechanistic information should be addressed further. A recurring theme in their function appears to be the wortmannin insensitivity, highlighting the concept for concerted activation of one or more PI3K-independent pathways for optimal insulin responsiveness. Mechanistic insights about the spatial and temporal insulin-dependent regulation of the enzymes responsible for the synthesis and turnover of these PIs would definitely be a priority for future work. The development of more specific tools to monitor PIs will provide critical insights for the localized PI presence and insulin-regulated dynamics at the levels of both GSVs and the extrinsic compartments, including endosomes, TGN, plasma membrane, and actin cytoskeleton.
GRANTS
The work described from my laboratory was funded by the American Diabetes Association, Juvenile Diabetes Foundation International, and National Institute of Diabetes and Digestive and Kidney Diseases (DK-58058).
Acknowledgments
I express gratitude to laboratory members Drs. Ogi Ikonomov and Diego Sbrissa for their excellent work and stimulating discussions, to Dr. Kostya Kandror for insightful comments, to the late Violeta Shisheva for many years of support, and to Linda McCraw for excellent secretarial assistance in preparation of this review article.
REFERENCES
- 1.Berwick DC, Dell GC, Welsh GI, Heesom KJ, Hers I, Fletcher LM, Cook FT, Tavaré JM. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J Cell Sci 117: 5985–5993, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bose A, Guilherme A, Robida SI, Nicoloro SM, Zhou QL, Jiang ZY, Pomerleau DP, Czech MP. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420: 821–824, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bose A, Robida S, Furcinitti PS, Chawla A, Fogarty K, Corvera S, Czech MP. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol Cell Biol 24: 5447–5458, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown RA, Domin J, Acaro A, Waterfield MD, Shepherd PR. Insulin activates the alpha isoform of class II phosphoinositide 3-kinases. J Biol Chem 274: 14529–14532, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Carricaburu V, Lamia KA, Lo E, Lavereaux L, Payrastre B, Cantley LC, Rameh LE. The phosphatidylinositol (PI)-5-phosphate 4-kinase type II enzyme controls insulin signaling by regulating PI-3,4,5-trisphosphate degradation. Proc Natl Acad Sci USA 100: 9867–9872, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Chiang SH, Saltiel AR. TC10α is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology 148: 27–33, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chaussade C, Pirola L, Bonnafous S, Blondeau F, Brenz-Verca S, Tronchère H, Portis F, Rusconi S, Payrastre B, Laporte J, Van Obberghen E. Expression of myotubularin by an adenoviral vector demonstrates its function as a phosphatidylinositol 3-phosphate [PtdIns(3)P] phosphatase in muscle cell lines: involvement of PtdIns(3)P in insulin-stimulated glucose transport. Mol Endocrinol 17: 2448–2460, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell 3: 391–404, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Chen G, Raman P, Bhonagiri P, Strawbridge AB, Pattar GR, Elmendorf JS. Protective effect of phosphatidylinositol 4,5-bisphosphate against cortical filamentous actin loss and insulin resistance induced by sustained exposure of 3T3-L1 adipocytes to insulin. J Biol Chem 279: 39705–39709, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czech MP Dynamics of phosphoinositides in membrane retrieval and insertion. Annu Rev Physiol 65: 791–815, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Del Vecchio RL, Pilch PF. Phosphatidylinositol 4-kinase is a component of glucose transporter (GLUT4)-containing vesicles. J Biol Chem 266: 13278–13283, 1991. [PubMed] [Google Scholar]
- 12.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Dugani CB, Klip A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep 6: 1137–1142, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falasca M, Hughes WE, Dominguez V, Sala G, Fostira F, Fang MQ, Cazzolli R, Shepherd PR, James DE, Maffucci T. The role of phosphoinositide 3-kinase C2α in insulin signaling. J Biol Chem 282: 28226–28236, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Foster LJ, Li D, Randhawa VK, Klip A. Insulin accelerates inter-endosomal GLUT4 traffic via phosphatidylinositol 3-kinase and protein kinase B. J Biol Chem 276: 44212–44221, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Funaki M, DiFransico L, Janmey PA. PI 4,5-P2 stimulates glucose transport activity of GLUT4 in the plasma membrane of 3T3-L1 adipocytes. Biochim Biophys Acta 1763: 889–899, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furtado LM, Poon V, Klip A. GLUT4 activation: thoughts on possible mechanisms. Acta Physiol Scand 178: 287–296, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Lifshitz L, Patki-Kamath V, Ruft R, Fogarty K, Czech MP. Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes. Mol Cell Biol 24: 9102–9123, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Ikonomov OC, Sbrissa D, Shisheva A. Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J Biol Chem 276: 26141–26147, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Ikonomov OC, Sbrissa D, Mlak K, Shisheva A. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology 143: 4742–4754, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Ikonomov OC, Sbrissa D, Shisheva A. Localized PtdIns 3,5-P2 synthesis to regulate early endosome dynamics and fusion. Am J Physiol Cell Physiol 291: C393–C404, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Ikonomov OC, Sbrissa D, Dondapati R, Shisheva A. ArPIKfyve-PIKfyve interaction and role in insulin-regulated GLUT4 translocation and glucose transport in 3T3-L1 adipocytes. Exp Cell Res 313: 2404–2416, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikonomov OC, Sbrissa D, Fligger J, Foti M, Carpentier JL, Shisheva A. PIKfyve controls fluid-phase endocytosis but not recycling/degradation of endocytosed receptors or sorting of procathepsin D by regulating multivesicular body morphogenesis. Mol Biol Cell 14: 4581–4591, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue M, Chiang SH, Chang L, Chen XW, Saltiel AR. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol Biol Cell 17: 2303–2311, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishiki M, Randhawa VK, Poon V, JeBailey L, Klip A. Insulin regulates the membrane arrival, fusion, and C-terminal unmasking of glucose transporter-4 via distinct phosphoinositides. J Biol Chem 280: 28792–28802, 2005. [DOI] [PubMed] [Google Scholar]
- 27.JeBailey L, Rudich A, Huang X, Di Ciano-Oliveira C, Kapus A, Klip A. Skeletal muscle cells and adipocytes differ in their reliance on TC10 and Rac for insulin-induced actin remodeling. Mol Endocrinol 18: 359–372, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Jiang ZY, Chawla A, Bose A, Way M, Czech MP. A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter recycling. J Biol Chem 277: 509–515, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Kanda H, Tamori Y, Shinoda H, Yoshikawa M, Sakaue M, Udagawa J, Otani H, Tashiro F, Miyazaki JI, Kasuga M. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest 115: 291–301, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanzaki M, Furukawa M, Raab W, Pessin JE. Phosphatidylinositol 4,5-bisphosphate regulates adipocyte actin dynamics and GLUT4 vesicle recycling. J Biol Chem 279: 30622–30633, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Kanzaki M, Watson RT, Hou JC, Stamnes M, Saltiel AR, Pessin JE. Small GTP-binding protein TC10 differentially regulates two distinct populations of filamentous actin in 3T3L1 adipocytes. Mol Biol Cell 13: 2334–2346, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanzaki M, Watson RT, Khan AH, Pessin JE. Insulin stimulates actin comet tails on intracellular GLUT4-containing compartments in differentiated 3T3L1 adipocytes. J Biol Chem 276: 49331–49336, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Kelly KL, Ruderman NB. Insulin-stimulated phosphatidylinositol 3-kinase. J Biol Chem 268: 4391–4398, 1993. [PubMed] [Google Scholar]
- 34.Kong AM, Horan KA, Sriratana A, Bailey CG, Collyer LJ, Nandurkar HH, Shisheva A, Layton MJ, Rasko JE, Rowe T, Mitchell CA. Phosphatidylinositol 3-phosphate [PtdIns3P] is generated at the plasma membrane by an inositol polyphosphate 5-phosphatase: endogenous PtdIns3P can promote GLUT4 translocation to the plasma membrane. Mol Cell Biol 26: 6065–6081, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristiansen S, Ramlal T, Klip A. Phosphatidylinositol 4-kinase, but not phosphatidylinositol 3-kinase, is present in GLUT4-containing vesicles isolated from rat skeletal muscle. Biochem J 335: 351–356, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamia KA, Peroni OD, Kim YB, Rameh LE, Kahn BB, Cantley LC. Increased insulin sensitivity and reduced adiposity in phosphatidylinositol 5-phosphate 4-kinase β−/− mice. Mol Cell Biol 24: 5080–5087, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larance M, Ramm G, James DE. The GLUT4 code. Mol Endocrinol 22: 226–233, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maffucci T, Brancaccio A, Piccolo E, Stein RC, Falasca M. Insulin induces phosphatidinylinositol-3-phosphate formation through TC10 activation. EMBO J 22: 4178–4189, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin TF PI(4,5)P(2) regulation of surface membrane traffic. Curr Opin Cell Biol 13: 493–499, 2001. [DOI] [PubMed] [Google Scholar]
- 40.McCarthy AM, Spisak KO, Brozinick JT, Elmendorf JS. Loss of cortical actin filaments in insulin-resistant skeletal muscle cells impairs GLUT4 vesicle trafficking and glucose transport. Am J Physiol Cell Physiol 291: C860–C868, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minokoshi Y, Kahn CR, Kahn BB. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin action and glucose homeostasis. J Biol Chem 278: 33609–33612, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Mora S, Durham PL, Smith JR, Russo AF, Jeromin A, Pessin JE. NCS-1 inhibits insulin-stimulated GLUT4 translocation in 3T3L1 adipocytes through a phosphatidylinositol 4-kinase-dependent pathway. J Biol Chem 277: 27494–27500, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Navé BT, Siddle K, Shepherd PR. Phorbol esters stimulate phosphatidylinositol 3,4,5-trisphosphate production in 3T3-L1 adipocytes: implications for stimulation of glucose transport. Biochem J 318: 203–205, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicot AS, Fares H, Payrastre B, Chisholm AD, Labouesse M, Laporte J. The phosphoinositide kinase PIKfyve/Fab1p regulates terminal lysosome maturation in Caenorhabditis elegans. Mol Biol Cell 17: 3052–3074, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niebuhr K, Giuriato S, Pedron T, Philpott DJ, Gaits F, Sable J, Sheetz MP, Parsot C, Sansonetti PJ, Payrastre B. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S. flexneri effector IpgD reorganizes host cell morphology. EMBO J 21: 5069–5078, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omata W, Shibata H, Li L, Takata K, Kojima I. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem J 346: 321–328, 2000. [PMC free article] [PubMed] [Google Scholar]
- 47.Patel N, Huang C, Klip A. Cellular location of insulin-triggered signals and implications for glucose uptake. Pflügers Arch 451: 499–510, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Patki V, Buxton J, Chawla A, Lifshitz L, Fogarty K, Carrington W, Tuft R, Corvera S. Insulin action on GLUT4 traffic visualized in single 3T3-L1 adipocytes by using ultra-fast microscopy. Mol Biol Cell 12: 129–141, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pendaries C, Tronchère JM, Arbibe L, Mounier J, Gozani O, Cantley L, Fry MJ, Gaits-Iacovoni F, Sansonetti PJ, Payrastre B. PtdIns(5)P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J 25: 1024–1034, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pilch PF The mass action hypothesis: formation of Glut4 storage vesicles, a tissue-specific, regulated exocytic compartment. Acta Physiol (Oxf) 192: 89–101, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Rusten TE, Rodahl LNW, Pattni K, Englund C, Samakovlis C, Dove S, Brech A, Stenmark H. Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol Biol Cell 17: 3989–4001, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rutherford AC, Traer C, Wassmer T, Pattni K, Bujny MV, Carlton JG, Stenmark H, Cullen PJ. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci 119: 3944–3957, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaoka T, Wada T, Tsuneki H. Lipid phosphatases as a possible therapeutic target in cases of type 2 diabetes and obesity. Pharmacol Ther 112: 799–809, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Sbrissa D, Shisheva A. Acquisition of unprecedented phosphatidylinositol 3,5-bisphosphate rise in hyperosmotically stressed 3T3-L1 adipocytes, mediated by ArPIKfyve-PIKfyve pathway. J Biol Chem 280: 7883–7889, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Sbrissa D, Ikonomov OC, Shisheva A. PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. Effect of insulin. J Biol Chem 274: 21589–21597, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Sbrissa D, Ikonomov O, Shisheva A. Selective insulin-induced activation of class IA phosphoinositide 3-kinase in PIKfyve immune complexes from 3T3-L1 adipocytes. Mol Cell Endocrinol 181: 35–46, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Sbrissa D, Ikonomov O, Shisheva A. PtdIns 3-P-binding domains in PIKfyve: binding specificity and consequences to the protein endomembrane localization. J Biol Chem 277: 6073–6079, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Sbrissa D, Ikonomov OC, Deeb R, Shisheva A. Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J Biol Chem 277: 47276–47284, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex. J Biol Chem 282: 23878–23891, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Sbrissa D, Ikonomov OC, Strakova J, Dondapati R, Mlak K, Deeb R, Silver R, Shisheva A. A mammalian ortholog of Saccharomyces cerevisiae Vac14 that associates with and up-regulates PIKfyve phosphoinositide 5-kinase activity. Mol Cell Biol 24: 10437–10447, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sbrissa D, Ikonomov OC, Strakova J, Shisheva A. Role for a novel signaling intermediate, phosphatidylinositol 5-phosphate, in insulin-regulated F-actin stress fiber breakdown and GLUT4 translocation. Endocrinology 145: 4853–4865, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Tavaré JM, Lang F. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res 100: 686–692, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Shisheva A PIKfyve: partners, significance, debates and paradoxes. Cell Biol Int 32: 591–604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shisheva A Regulating GLUT4 vesicle dynamics by phosphoinositide kinases and phosphoinositide phosphatases. Front Biosci 8: s945–s967, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Shisheva A, Ikonomov O, Sbrissa D. Novel Sac3 phosphatase associates with PtdIns 3,5-P2-synthesizing PIKfyve and functions as a negative regulator of insulin responsiveness in 3T3-L1 adipocytes. In: Xth International Symposium on Insulin Receptors and Insulin Action. Stockholm, Sweden: May 2–6, 2007, p. 46.
- 66.Shisheva A, Rusin B, Ikonomov OC, DeMarco C, Sbrissa D. Localization and insulin-regulated relocation of 5′-phosphoinositide kinase PIKfyve in 3T3-L1 adipocytes. J Biol Chem 276: 11859–11869, 2001. [DOI] [PubMed] [Google Scholar]
- 67.Shisheva A, Sbrissa D, Ikonomov O. Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinositide kinase in insulin-sensitive cells. Mol Cell Biol 19: 623–634, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sweeney G, Garg RR, Ceddia RB, Li D, Ishiki M, Somwar R, Foster LJ, Neilsen PO, Prestwich GD, Rudich A, Klip A. Intracellular delivery of phosphatidylinositol (3,4,5)-trisphosphate causes incorporation of glucose transporter 4 into the plasma membrane of muscle and fat cells without increasing glucose uptake. J Biol Chem 279: 32233–32242, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Takenawa T, Itoh T. Phosphoinositides, key molecules for regulation of actin cytoskeletal organization and membrane traffic from the plasma membrane. Biochim Biophys Acta 1533: 190–206, 2001. [DOI] [PubMed] [Google Scholar]
- 70.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signaling pathways: insights into insulin action. Mol Cell Biol 7: 85–96, 2006. [DOI] [PubMed] [Google Scholar]
- 71.Tong P, Khayat ZA, Huang C, Patel N, Ueyama A, Klip A. Insulin-induced cortical actin remodeling promotes GLUT4 insertion at muscle cell membrane ruffles. J Clin Invest 108: 371–381, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vinciguerra M, Foti M. PTEN and SHIP2 phosphoinositide phosphatases as negative regulators of insulin signaling. Arch Physiol Biochem 112: 89–104, 2006. [DOI] [PubMed] [Google Scholar]
- 73.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev 25: 177–204, 2004. [DOI] [PubMed] [Google Scholar]
- 74.Watson RT, Pessin JE. GLUT4 translocation: the last 200 nanometers. Cell Signal 19: 2209–2217, 2007. [DOI] [PubMed] [Google Scholar]
- 75.Xu Z, Huang G, Kandror KV. Phosphatidylinositol 4-kinase type IIalpha is targeted specifically to cellugyrin-positive glucose transporter 4 vesicles. Mol Endocrinol 20: 2890–2897, 2006. [DOI] [PubMed] [Google Scholar]
- 76.Xu Z, Kandror KV. Translocation of small preformed vesicles is responsible for the insulin activation of glucose transport in adipose cells. Evidence from the in vitro reconstitution assay. J Biol Chem 277: 47972–47975, 2002. [DOI] [PubMed] [Google Scholar]