Abstract
Galanin-like peptide (GALP) is expressed in the arcuate nucleus and is implicated in the neuroendocrine regulation of metabolism and reproduction. To investigate the physiological significance of GALP, we generated and characterized a strain of mice with a genetically targeted deletion in the GALP gene [GALP knockout (KO) mice]. We report that GALP KO mice have a subtle, but notable, metabolic phenotype that becomes apparent during adaptation to changes in nutrition. GALP KO mice are indistinguishable from wild-type (WT) controls in virtually all aspects of growth, sexual development, body weight, food and water consumption, and motor behaviors, when they are allowed unlimited access to standard rodent chow. However, GALP KO mice have an altered response to changes in diet. 1) Male GALP KO mice consumed less food during refeeding after a fast than WT controls (P < 0.01). 2) GALP KO mice of both sexes gained less weight on a high-fat diet than WT controls (P < 0.01), despite both genotypes having consumed equal amounts of food. We conclude that although GALP signaling may not be essential for the maintenance of energy homeostasis under steady-state nutritional conditions, GALP may play a role in readjusting energy balance under changing nutritional circumstances.
Keywords: reproduction, metabolism, knockout, obesity, body weight
galanin-like peptide (GALP), so named for a 10-amino acid region of sequence identity to galanin (28), is highly conserved between mice, rats, macaques, and humans (4). Despite the common amino acid sequence shared by galanin and GALP, these peptides are derived from separate genes and are thought to have completely different functions (4). Although GALP activates galanin receptors, evidence supports the existence of an as yet uncharacterized receptor specific to GALP (11, 22, 26). In the mammalian species studied so far, expression of GALP in the central nervous system is restricted to the arcuate nucleus of the hypothalamus (Arc), the median eminence, and the posterior pituitary gland (7, 17, 34). The Arc is the nodal point for integration of peripheral and central signals governing energetics, growth, and reproduction (for review see Ref. 36). Lesions of the Arc disrupt energy balance (16), and the loss of GALP neurons may partially explain this phenomenon by virtue of their function in metabolism. 1) The expression of GALP in the Arc is stimulated by leptin, an adipocyte-derived satiety hormone that exerts profound effects on appetite, metabolism, and reproduction (17, 23). 2) The expression of GALP in the Arc is diminished under conditions of negative energy balance, such as dietary restriction and insulin resistance (10, 18, 31). 3) GALP neurons coexpress the signaling form of the leptin receptor Ob-Rb, indicating that leptin interacts directly with these neurons (34). Together, these observations suggest that leptin-sensitive GALP neurons in the Arc are targets for metabolic signals that reflect energy status and, thus, contribute to the process of body weight regulation. This idea is bolstered by studies in mice showing that central (intracerebroventricular) injections of GALP reduce food intake, increase energy expenditure, and decrease body weight (13, 21).
GALP signaling may also serve an important role in the integration of energy balance and reproductive function. The same leptin signal that regulates feeding behavior and body weight appears to act as a metabolic gate for reproduction, allowing reproduction to proceed only if nutritional reserves are adequate for its support (25). A network of perhaps 1,000 neurons that produce gonadotropin-releasing hormone (GnRH) serve as the final common pathway through which the brain regulates gonadotropin secretion and, thus, reproduction (14). Since GnRH neurons themselves do not express the leptin receptor (for review see Ref. 5), leptin's influence on GnRH secretion is thought to be mediated indirectly through neurons that are direct targets for leptin, which include GALP neurons (34). Several lines of evidence indicate that GALP neurons act as a relay station to communicate metabolic status to GnRH neurons, to either engage or disengage reproduction as a function of fuel reserves. 1) Immunohistochemical studies show that GALP neurons project to and appear to make contact with GnRH neurons (34). 2) The administration of GALP directly into the brain induces GnRH-dependent gonadotropin secretion (8, 19, 21, 33). 3) In rodent models of leptin or insulin deficiency, GALP treatment reverses the inhibitory effects of undernutrition on gonadotropin secretion (13, 23, 33). Thus GALP neurons in the Arc may act as sensors of peripheral energy stores, which then transmit information to circuits that control GnRH secretion.
We hypothesized that if GALP plays a physiological role in metabolism or reproduction, then animals with a congenital deficiency in GALP might show specific impairments of metabolic regulation and/or reproductive function. To test this hypothesis, we generated a strain of mice bearing a targeted deletion in the GALP gene [GALP knockout (KO) mice] and analyzed their phenotype, focusing on aspects of metabolism and reproduction to include measures of daily food intake, weight gain, energy expenditure, response to fasting and high-fat diets, sexual development, fertility, gonadal weights, and serum levels of luteinizing hormone (LH). We report that GALP KO mice have altered compensatory responses to changes in diet, despite being reproductively competent.
MATERIALS AND METHODS
Generation of GALP KO strain.
The GALP-coding region is located on chromosome 7 and consists of six exons. Exon 2, which contains the translation start site, was deleted by gene targeting and replaced with a neomycin resistance gene (Neo; Fig. 1A). Correctly targeted clones were identified by neomycin resistance and confirmed by Southern blot analysis (data not shown). Clones were injected into the blastocoele of 3.5-day-old blastocysts, which were then implanted into pseudopregnant C57BL/6J mice. Heterozygous offspring were generated from chimeras; wild-type (WT) and KO littermates were used in phenotyping experiments.
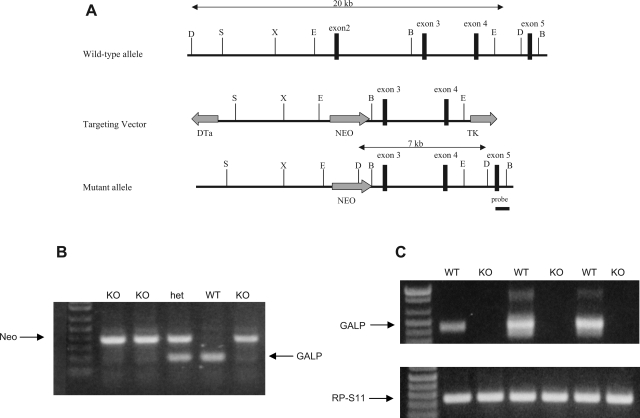
Fig. 1.
A: representational drawing of genomic region surrounding mouse galanin-like peptide gene (GALP): targeting construct, and putative targeted allele. Targeting vector is created to replace exon 2 with a neomycin resistance (Neo) cassette. Vertical bars represent enzymatic cut sites: SpeI (S), XbaI (X), EcoRI (E), and DrdI (D). Diphtheria toxin (DTa) and thymidine kinase (TK) genes flank the targeting construct but are not carried through to the mutant allele. B: representative genotyping gel from genomic DNA. Top bands represent a portion of the Neo cassette (400 bp); bottom bands represent genomic DNA spanning galanin-like peptide (GALP) exon 2 (210 bp). KO, GALP knockout mice; het, heterozygous; WT, wild-type littermates. C: representative RT-PCR gel. Top bands represent cDNA for GALP exons 2-5 (440 bp); bottom bands represent cDNA for riboprotein S11 (RP-S11, 240 bp).
Experimental animals and genotyping.
All animals were housed in the vivarium at Hope College Biology Department or the University of Washington Department of Comparative Medicine and given ad libitum access to water and standard mouse chow. All litters were weaned from the parents at postnatal day 25. The animals were kept on a 12:12-h light-dark cycle. All procedures were approved by the Hope College Animal Care and Use Committee or the University of Washington Institutional Animal Care and Use Committee, in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Genotypes were determined by PCR with a REDExtract-N-Amp kit (Sigma, St. Louis, MO). Primers for genomic GALP DNA spanning exon 2 were 5′-TCCTCAGCTGTGTGCTGTGC-3′ (forward) and 5′-CAGCTCCCCCTGGAT CAGA-3′ (reverse). Primers for the Neo cassette were 5′-CGTCAGAAAGAACACCCAACCAGG-3′ (forward) and 5′- GCCCAGTCATAGCCGAATAGCCTC-3′ (reverse). Tissue samples from the ear or tail were used to isolate DNA for PCR. Mice were then anesthetized by isoflurane, and radio-transmitter tags were injected subcutaneously on the dorsal side of the animal. RT-PCR was performed with a single hypothalamic tissue sample from an adult GALP KO or WT mouse. Before death and tissue collection, mice were injected subcutaneously with 6 nmol of human leptin (Amgen, Thousand Oaks, CA) dissolved in 100 μl of saline to augment GALP mRNA expression. RNA was extracted from hypothalamic tissue using an RNeasy kit (Qiagen, Valencia, CA), and RT-PCR was performed using the Omniscript RT kit (Qiagen, Valencia, CA) with primers [5′-CAGAGATGCAATTAACCCTCACTAAAGGGAGACTGCTCCGTACATCTGGTCC-3′ (forward) and 5′-CCAAGCCTTCTAATAC GACTCACTATAGGGAGAGAAGGAGGAGTGAGGTATGGCTAAC-3′ (reverse)] targeting a region of the GALP mRNA sequence from exon 2 to exon 5. To control for quality among the various cDNA template samples, riboprotein S11 (RP-S11) was also amplified using forward and reverse primers devised by Navarro et al. (27).
Growth, maturation, and body temperature.
After they were weaned, mice were sexed and identified with a unique identification number via radiofrequency transmitters (BMDS, Baltimore, MD) implanted subcutaneously. The transmitters also monitored body temperature at ranges equivalent to that obtained from core body temperature transducers. Body weight and temperature were recorded every week. Observation of vaginal opening and preputial separation in females and males, respectively, was considered the onset of puberty, as previously described by Rich et al. (30).
Motor skills.
Tests of motor coordination were conducted as described by others (19). Motor coordination was assessed at 11 wk of age with a rotarod (Med Associates, St. Albans, VT). Mice were placed in the center of a stationary rotarod cylinder. After a 15-s acclimation period, the rotarod was switched on, and the rotation accelerated through the experimental period from 4 to 40 rpm. Mice were timed until they fell from the rotarod or were removed after 300 s. If mice fell off within the first 15 s, they were subjected a second trial. Each mouse was subjected to two trials separated by 7 days. Data from the final trial are presented.
Refeeding response after fasting.
At 7–8 wk of age, mice were fasted overnight (16 h) or allowed to feed ad libitum. Beginning at 0800 (end of the 16-h period), mice were weighed and then given a known quantity of standard mouse chow. All mice were allowed access to drinking water throughout the experiment. Food was weighed at 60 min. At 1 wk after the initial test, the procedure was repeated, with the treatment groups reversed, so that all animals were both free fed and fasted.
Glucoprivic feeding.
To test the effects of GALP on glucose sensing, mice were injected with either saline or a metabolically inactive form of glucose, 2-deoxy-d-glucose (2-DG, 200 mg/kg sc in saline; Sigma Aldrich, St. Louis, MO), at 9–10 wk of age. This dose of 2-DG is known to elicit food intake without causing other adverse effects in rodents (9). Mice were injected in the morning and returned to their cages with a known quantity of food and free access to water. Food intake was determined from the weight of the food remaining 60 min after 2-DG or saline injection. At 1 wk after the initial test, the procedure was repeated, with the treatment groups reversed, so that all animals received both saline and 2-DG.
Hormonal effects and gonad weights following fasting.
All mice were again fasted as described above, 2 wk after the second half of the refeeding experiment. In the morning after the 16-h fast, fasted and control mice were killed by decapitation, and blood, brain, and gonads were collected (gonads of control females were collected at early proestrus). The testes (males) and uterus plus ovaries (females) were weighed. Sera were collected and used in RIAs for LH and corticosterone. Gonads were weighed immediately after removal. Serum concentrations of LH were measured at Northwestern University (Evanston, IL), with reagents from the National Institutes of Health: anti-rLH-S11 (LH antiserum) and rLH-RP3 (standard). The assay sensitivity was 0.2 ng/ml, and the intra-assay coefficient of variation (CV) was 2.2%. A corticosterone double-antibody RIA kit (catalog no. 07120102, MP Biomedicals, Solon, OH) was used to measure serum corticosterone levels. The intra-assay CV for this assay was <3.7%.
Response to high-fat diet.
Mice were housed singly and fed standard chow or high-fat chow (42% fat; Harlan Teklad, Madison, WI) ad libitum with free access to water for 6 wk. Body weight and food intake were measured twice each week.
Water intake and response to water deprivation.
All mice were housed singly in Nalgene metabolism cages (Fisher Scientific, St. Louis, MO), which allow direct measurement of liquid and fecal waste output from the animals. Water intake was measured over a 24-h period; then access to water was removed from each cage. Mice were weighed at the time of water removal. After 24 h, water was returned and the mice were weighed again. Water intake and body weight were measured for the 24 h following water deprivation. Waste output from each animal was measured throughout the experiment to account for water loss.
In situ hybridization.
Brains from adult male GALP KO or WT mice were collected, immediately frozen on dry ice, and then stored at −80°C. Tissue was sectioned to a thickness of 20 μm, mounted on glass slides (VWR International, So. Plainfield, NY), and stored again at −80°C until further processing. Single-label in situ hybridization for proopiomelanocortin (POMC) mRNA and for neuropeptide Y (NPY) mRNA was performed as previously described (2, 15). Briefly, radiolabeled (33P) antisense POMC or NPY riboprobe was denatured and diluted in hybridization buffer at a concentration of 0.05 pmol/ml along with tRNA (2 mg/ml) and applied to slides (100 μl/slide). After hybridization, slides were treated with RNase (29 μg/ml), washed, and dehydrated as previously reported (7). Slides were then dipped in NTB liquid emulsion (Eastman Kodak, Rochester, NY) and stored at 4°C. Slides were developed 2 days later and then dehydrated with a series of ethanol washes followed by two 10-min washes in Citrisolv (Fisher Scientific, Baltimore, MD). Coverslips were applied with Permaslip mounting medium (Alban Scientific, St. Louis, MO). Slides were coded and randomized to obscure group identification and examined with dark-field microscopy.
Statistical analysis.
Values are expressed as group means ± SE. All growth data were analyzed with repeated-measures ANOVA. Ages of vaginal opening/preputial separation were compared by two-way ANOVA, with sex and genotype as individual variables. Data on water balance and energy regulation during water restriction were analyzed by three-way repeated-measures ANOVA. POMC and NPY mRNA expression data were analyzed by t-test. All other data were analyzed by three-way ANOVA, with genotype, sex, and feeding status as individual variables.
RESULTS
Genotype, general phenotype.
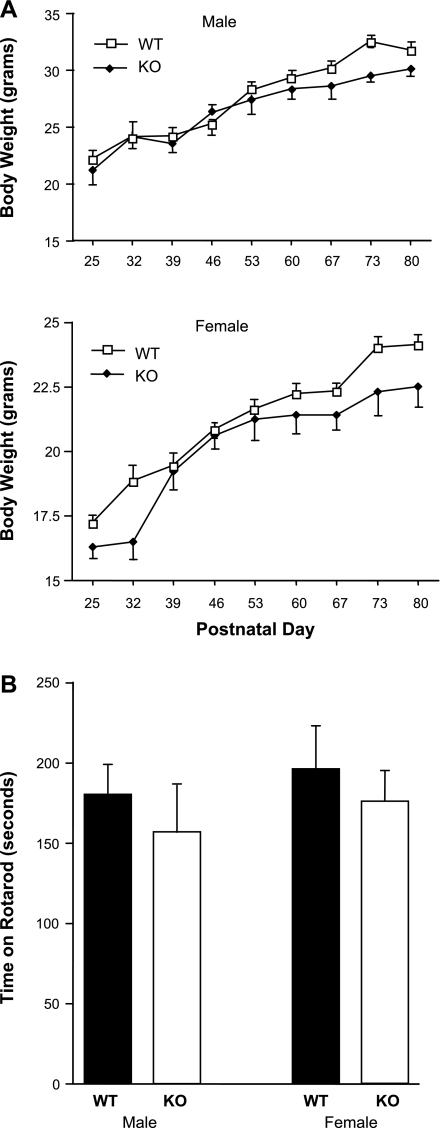
All mice were genotyped at the time of weaning by PCR with primers specific for exon 2 of the GALP gene and for the Neo insert (Fig. 1B). RT-PCR demonstrated consistent expression of RP-S11 mRNA in all animals, whereas only WT animals showed GALP mRNA expression (Fig. 1C). Under general observation, GALP KO mice were indistinguishable from WT littermates in appearance and behavior. Consistent with this observation, KO and WT mice grew at the same rate, with the males of both genotypes gaining weight faster than the females (Fig. 2A). All groups performed similarly in the motor coordination (rotarod) tests (Fig. 2B). Core body temperatures in adult mice were the same regardless of sex or genotype: 38.0 ± 0.16 and 38.2 ± 0.12°C for WT and KO males and 38.8 ± 0.08 and 38.7 ± 0.12°C for WT and KO females (P > 0.05, n = 15–17 per sex or genotype).
Fig. 2.
A: weight gain of ad libitum-fed GALP KO mice and WT littermates (n = 16 per group). Male mice are significantly larger than female mice after postnatal day 25 (P < 0.01). However, there are no significant differences in body weight gain over time between genotypes within either sex. B: nonsignificant trend toward a reduction in motor coordination as determined by time spent on a rotarod apparatus in GALP KO male and female mice compared with WT controls (n = 10–12 per group).
Hormone levels, body temperature, and gonad weights after fasting.
Body weights and core body temperatures were equivalent between genotypes in control and fasted animals (Table 1). All fed groups had similar levels of corticosterone, and all groups, except the KO males, responded to fasting with increased levels of corticosterone (Table 1). Fasting suppressed LH levels in all groups, regardless of genotype, and resulted in a trend for decreased gonad weight in both genotypes; however, the difference was statistically significant only in females (Table 1).
Table 1.
Body weight, body temperature, serum levels of corticosterone and LH and gonad weight in WT and GALP KO mice after an overnight fast
| Body Wt, g | Body Temp, °C | Cort, ng/ml | LH, ng/ml | Gonad Wt, g | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Males | ||||||||||
| WT | ||||||||||
| Control | 36.1±0.46 | 38.4±0.10 | 9.6±1.17 | 0.8±0.14 | 0.3±0.01 | |||||
| Fast | 31.2±0.79* | 37.7±0.19 | 24.1±3.84* | 0.2±0.10* | 0.27±0.01 | |||||
| KO | ||||||||||
| Control | 36.2±0.45 | 38.3±0.10 | 12.9±1.17 | 1.0±0.23 | 0.31±0.01 | |||||
| Fast | 32.0±0.66* | 37.6±0.22 | 14.2±3.39 | 0.2±0.07* | 0.29±0.01 | |||||
| Females | ||||||||||
| WT | ||||||||||
| Control | 25.3±0.66 | 37.7±0.17 | 15.7±3.02 | 0.9±0.2 | 0.2±0.02 | |||||
| Fast | 22.8±0.40* | 37.8±0.39 | 36.2±8.13* | 0.1±0.10* | 0.06±0.02* | |||||
| KO | ||||||||||
| Control | 25.8±0.71 | 37.6±0.15 | 14.8±3.31 | 0.6±0.15 | 0.13±0.02† | |||||
| Fast | 22.1±0.46* | 38.1±0.24 | 30.0±7.76* | 0.3±0.12* | 0.07±0.02* | |||||
Values are means ± SE (n = 5–11 per group). Gonad weight for females includes ovaries + uterus. Cort, corticosterone; LH, luteinizing hormone; WT, wild-type mice; KO, galanin-like peptide knockout mice.
P < 0.05 vs. control group of the same genotype.
P < 0.05 vs. WT group of the same treatment.
Reproductive phenotype.
As an indicator of reproductive maturation (puberty), we observed the postnatal day of vaginal opening in females and preputial separation in males. The time to preputial separation was not significantly different between WT and KO males: 40.2 ± 1.07 and 41.2 ± 1.22 days, respectively (P > 0.05). Similarly, the day of vaginal opening did not differ significantly between WT and KO females: 37.2 ± 1.16 and 37.9 ± 0.85 days, respectively (P > 0.05). Adult gonad weights did not differ significantly between ad libitum-fed WT and KO males, but KO females had significantly smaller ovaries than WT controls (Table 1). Nevertheless, baseline LH levels in adult males and females were similar, regardless of genotype (Table 1), and, on the basis of vaginal cytology, adult KO females appeared to have estrous cycles similar to those of WT females (data not shown). Furthermore, KO females, when placed with a WT male, became pregnant, delivered healthy litters, and raised them to weaning age. Similarly, KO males successfully impregnated WT females.
Refeeding response to fasting and 2-DG treatment.
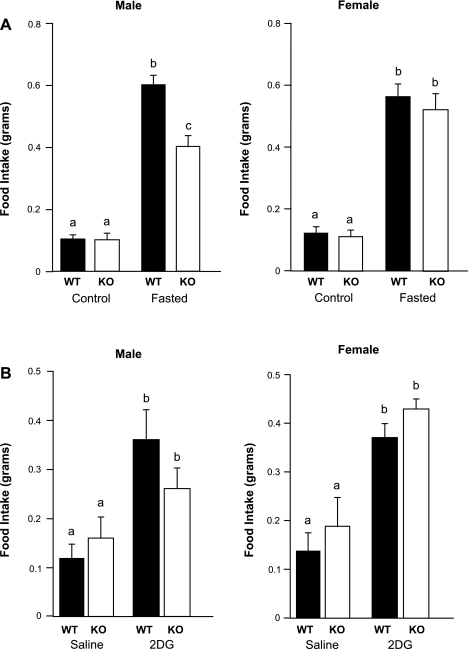
Food intake over 24 h was equivalent for all groups, regardless of sex or genotype (Fig. 3). Food intake over 24 h of refeeding following a 16-h fast was increased in all groups; however, KO males ate significantly less after a fast than WT males or females of either genotype (Fig. 3A). Injection with 2-DG, a glucose analog that inhibits intracellular glycolysis, induced an equivalent feeding response in all groups, regardless of sex or genotype (Fig. 3B).
Fig. 3.
A: significantly attenuated feeding response after a 16-h fast in KO males compared with WT males (P < 0.01). No significant difference was observed in refeeding response to a 16-h fast between KO females and WT females. Values are means ± SE (n = 13–17 per group). B: 2-deoxy-d-glucose (2-DG, 200 mg/kg sc) caused a significant increase in food intake compared with saline in all mice (P < 0.05). There were no significant differences in 2-DG response between genotypes or sexes. Values are means ± SE (n = 13–16 per group). Different letters (a, b, c) indicate significant difference (P < 0.05).
Response to high-fat diet.
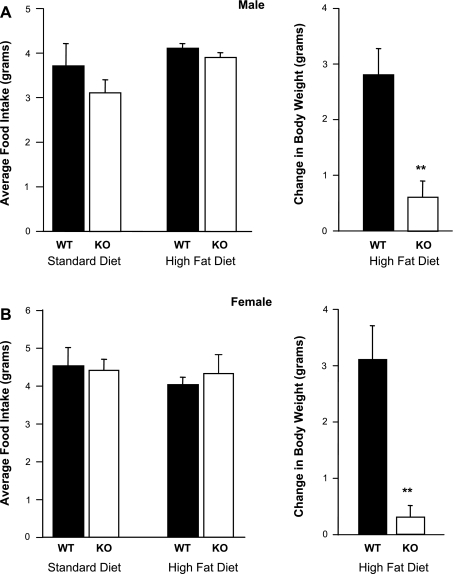
Male and female GALP KO mice gained less weight on a high-fat diet than WT mice (P < 0.001), despite consuming equivalent amounts of food (Fig. 4).
Fig. 4.
Male (A) and female (B) GALP KO mice ate the same amount of high-fat chow but gained less weight than WT littermates. Values are means ± SE (n = 6 per group). **P < 0.05.
Regulation of water intake.
WT and KO mice drank a similar amount of water over 24 h (see supplemental Fig. S1a in the online version of this article). Removal of water for 24 h resulted in an equivalent weight loss in all groups (data not shown). No significant differences were found in the output of feces or urine between genotypes, although females tended to produce more urine than males (see supplemental Figs. S1b and S1c).
POMC and NPY expression.
The number of detectable neurons expressing POMC mRNA was not different between WT and GALP KO mice, under conditions of ad libitum feeding (see supplemental Fig. S2a). Similarly, the number of neurons expressing NPY mRNA was not different between genotypes (see supplemental Fig. S2b).
DISCUSSION
GALP neurons are direct targets for the action of metabolic hormones, including leptin (34), and GALP administered directly into the brain can exert effects on metabolism and reproduction (21; for review see Ref. 6). To determine whether a congenital deficiency in GALP would produce disturbances in the regulation of metabolism and reproduction, we generated and characterized mice with a loss-of-function deletion in the GALP gene (GALP KO mice) and studied their phenotype. We made several predictions about the possible phenotype of GALP KO mice. We posited that a loss of GALP signaling would impair normal homeostatic set points for energy reserves, leading to a phenotype of disturbed feeding behavior and body weight regulation. We also postulated that animals lacking GALP would be incapable of relaying appropriate metabolic information to GnRH neurons and, thus, would show abnormal reproductive function. Contrary to these suppositions, our analyses revealed only a mild phenotype in the GALP KOs; however, animals with a congenital deficiency of GALP showed an altered response to certain metabolic challenges, suggesting that GALP plays a role in the regulation of metabolism.
GALP signaling in the hypothalamus has been implicated in the homeostatic regulation of energy reserves. The administration of GALP into the brain is associated with altered food intake, increased energy expenditure, and reduced body weight (13, 21, 30). Our studies of animals with unlimited access to standard rodent chow revealed no differences between GALP KO and WT mice in their food intake, body weight, or core body temperature. Recent evidence suggests that GALP neurons are influenced by glucose-sensing neurons in the hindbrain (9), so we examined the feeding response to glucoprivia induced by 2-DG treatment in GALP KO mice and WT controls. We found no differences between genotypes, indicating that GALP KO mice are fully capable of sensing glucose deprivation and compensating by increasing glucose intake (e.g., increased feeding). We also examined the response of GALP KO mice to food deprivation by removing access to chow overnight and then allowing the animals to refeed. Fasting produced an equivalent weight loss in GALP KO mice and WT controls. Although both genotypes showed increased feeding after the overnight fast, male GALP KO mice had an attenuated refeeding response, whereas females of both genotypes showed a normal refeeding response. This observation is consistent with earlier studies reporting a sex difference in the response to GALP treatment (1, 30). Sex differences in energy regulation in other mutant genotypes are common. For example, mice that are deficient in both NPY and galanin show sexually differentiated patterns of food intake and body weight (15). Mice that are deficient in either the orexigenic peptide ghrelin or its receptor show a sex-specific phenotype (37, 38); moreover, the effects of ghrelin on appetite can be abolished by treatment with sex steroids (3). Nevertheless, the pattern and intensity of GALP expression are not different between the sexes, nor is GALP gene expression regulated by gonadal steroids (6, 8). Thus the physiological basis for a sex-specific phenotype in the GALP KO animal remains a mystery.
To explore the impact of GALP deficiency on the compensatory response to an altered nutritional plane, we provided groups of KO and WT mice with unlimited access to high-fat chow for several weeks. This manipulation revealed a remarkable effect of GALP deficiency: GALP KO mice, both males and females, gained significantly less weight than their WT counterparts. On average, WT mice gained 2–3 more grams than GALP KO mice over the 6 wk of ad libitum high-fat diet consumption, despite the fact that both genotypes ate similar amounts of food (by weight). This suggests that GALP KO mice have an altered metabolic response to an increase in fat intake. The apparent resistance to weight gain with a high-fat diet may seem counterintuitive, since GALP is generally classified as an endogenous catabolic agent in mice (13, 21; for review see Ref. 12). Notably, similar observations were reported by Wortley et al. (37) in male mice deficient in ghrelin, an anabolic factor known to stimulate feeding and increase weight. While being maintained on a standard chow diet, ghrelin KO mice and their WT littermates show parallel growth and weight gain. In contrast, male ghrelin KO mice fed a high-fat diet gain less weight than WT controls, despite eating the same amount of high-fat chow. Data from this study also suggest that the resistance to diet-induced weight gain in ghrelin KO mice is attributable to an increase in motor activity associated with the high-fat diet (37). On the basis of these observations, we infer that GALP deficiency leads to increased activity and, thus, increased energy use. Although this idea was not directly tested in our experiments, central GALP administration elicits a dramatic effect on motor function in mice. In mice (but not rats), GALP treatment acutely impairs locomotion; however, over several days of chronic GALP treatment, mice become hyperactive (21). In the present study, the rotarod test indicated that coordination, grip, and movement initiation are normal in GALP KO mice, but this does not preclude diet-induced alterations in activity. Further studies are necessary to clarify this aspect of GALP signaling, including studies of GALP or its analogs as therapeutic agents for inducing weight loss.
We postulated that GALP acts as a molecular conduit between peripheral energy stores and the central control of reproduction (4). Thus it was surprising to find that the reproductive system is grossly normal in GALP KO mice. Male and female GALP KO mice showed timely signs of pubertal maturation and normal levels of LH compared with WT controls. This is consistent with studies in rats showing no effect of GALP infusion on timing of puberty (30). Although we observed reduced ovarian weight in ad libitum-fed GALP KO mice compared with WT mice, in all likelihood, this reflects a chance distribution of estrous cycle-induced changes in ovarian size, rather than a true difference between genotypes. We base this inference on our other observations indicating that GALP KO mice show normal estrous cycles, become pregnant within a few days of exposure to an adult male mouse, undergo normal gestation and parturition, and raise normal litters, just as WT females do. Moreover, male GALP KO mice can impregnate adult females. These observations indicate that the reproductive physiology and sexual behaviors are grossly normal in the absence of GALP signaling.
It has been suggested that GALP expression in the posterior pituitary has a role in secretion of vasopressin and oxytocin from the neurohypophysis (for review see Ref. 35). GALP mRNA is expressed in pituicytes, the glia-like cells of the neurohypophysis, where it is upregulated in vivo under conditions of dehydration or salt loading and during lactation (6, 20, 32). Central injections of GALP stimulate vasopressin and oxytocin secretion (29). However, we found that water intake, urine output, weight loss during water restriction, and recovery of fluid intake after water restriction were indistinguishable among genotypes. Furthermore, our observations indicate that congenital deficiency of GALP does not interfere with normal lactation and suckling. Thus the role of GALP signaling in the neurohypophysis remains unclear.
We conclude, on the basis of the analysis of mice bearing null mutations in the GALP gene, that GALP is not indispensable for normal regulation of energy stores, reproduction, motor function, fluid balance, or lactation. However, our results do suggest a modulatory role for GALP signaling in the maintenance of metabolic homeostasis under altered dietary conditions. It is conceivable that GALP may actually have a more prominent role in regulating metabolism and reproduction of WT animals than is revealed by our KO model. Indeed, our findings of significant metabolic abnormalities in GALP KO mice might reflect a highly effective, but incomplete, compensation. It has been previously demonstrated that compensatory processes can correct genetic abnormalities early in development. For example, if NPY/agouti-related protein neurons are selectively ablated by gene targeting early in neonatal development in mice, the animals grow, eat, and thrive normally as adults (24). In contrast, if these same NPY/agouti-related protein neurons are destroyed by gene targeting in the adult, food intake and body weight drop precipitously, and the animals starve (24). These experiments demonstrate that the developing brain is a master of compensation, but the cellular and molecular basis of such compensation remains unknown (and would be worthy of investigation). Our results suggest that compensation by neither NPY nor POMC is directly involved in mitigating the loss of GALP, at least under normal feeding conditions; however, there are numerous other potential candidates. Further research, including the development of an inducible GALP KO strain, is necessary to fully elucidate the role of GALP in the regulation of reproduction and energy homeostasis.
GRANTS
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) through cooperative agreement U54 HD-12629 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research and also by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grants K01 DK-066238 and R01 DK-61517, NICHD Grant R01 HD-027142, and National Science Foundation Grant IBN-110686. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NICHD or NIDDK. We thank the Haward Hughes Medical Institute for support of this research in a grant to the Natural and Applied Sciences Division of Hope College.
Supplementary Material
Acknowledgments
We thank Molly McClain, Rachel Lash, Kathy Lee, Stephanie Krasnow, Jeremy Smith, and Patience Browne for excellent technical assistance. We are grateful to the Hope College Neuroscience capstone students for assistance with various aspects of these experiments and to Brigitte Mann (Northwestern University) for performing RIAs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Castellano JM, Navarro VM, Fernandez-Fernandez R, Roa J, Vigo E, Pineda R, Steiner RA, Aguilar E, Pinilla L, Tena-Sempere M. Effects of galanin-like peptide on luteinizing hormone secretion in the rat: sexually dimorphic responses and enhanced sensitivity at male puberty. Am J Physiol Endocrinol Metab 291: E1281–E1289, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 138: 4489–4492, 1997. [DOI] [PubMed] [Google Scholar]
- 3.Clegg DJ, Brown LM, Zigman JM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 56: 1051–1058, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham MJ Galanin-like peptide as a link between metabolism and reproduction. J Neuroendocrinol 16: 717–723, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham MJ, Clifton DK, Steiner RA. Leptin's actions on the reproductive axis: perspectives and mechanisms. Biol Reprod 60: 216–222, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham MJ, Krasnow SM, Gevers EF, Chen P, Thompson CK, Robinson IC, Smith MS, Clifton DK, Steiner RA. Regulation of galanin-like peptide gene expression by pituitary hormones and their downstream targets. J Neuroendocrinol 16: 10–18, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham MJ, Scarlett JM, Steiner RA. Cloning and distribution of galanin-like peptide mRNA in the hypothalamus and pituitary of the macaque. Endocrinology 143: 755–763, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham MJ, Shahab M, Grove KL, Scarlett JM, Plant TM, Cameron JL, Smith MS, Clifton DK, Steiner RA. Galanin-like peptide as a possible link between metabolism and reproduction in the macaque. J Clin Endocrinol Metab 89: 1760–1766, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Fraley GS Immunolesions of glucoresponsive projections to the arcuate nucleus alter glucoprivic-induced alterations in food intake, luteinizing hormone secretion, and GALP mRNA, but not sex behavior, in adult male rats. Neuroendocrinology 83: 97–105, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Fraley GS, Scarlett JM, Shimada I, Teklemichael DN, Acohido BV, Clifton DK, Steiner RA. Effects of diabetes and insulin on the expression of galanin-like peptide in the hypothalamus of the rat. Diabetes 53: 1237–1242, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Fraley GS, Shimada I, Baumgartner JW, Clifton DK, Steiner RA. Differential patterns of Fos induction in the hypothalamus of the rat following central injections of galanin-like peptide and galanin. Endocrinology 144: 1143–1146, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Gottsch ML, Clifton DK, Steiner RA. Galanin-like peptide as a link in the integration of metabolism and reproduction. Trends Endocrinol Metab 15: 215–221, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Hansen KR, Krasnow SM, Nolan MA, Fraley GS, Baumgartner JW, Clifton DK, Steiner RA. Activation of the sympathetic nervous system by galanin-like peptide—a possible link between leptin and metabolism. Endocrinology 144: 4709–4717, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Herbison AE Physiology of the gonadotropin-releasing hormone neuronal network. Physiol Reprod 1: 1415–1482, 2006. [Google Scholar]
- 15.Hohmann JG, Teklemichael DN, Weinshenker D, Wynick D, Clifton DK, Steiner RA. Obesity and endocrine dysfunction in mice with deletions of both neuropeptide Y and galanin. Mol Cell Biol 24: 2978–2985, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holzwarth-McBride MA, Hurst EM, Knigge KM. Monosodium glutamate induced lesions of the arcuate nucleus. I. Endocrine deficiency and ultrastructure of the median eminence. Anat Rec 186: 185–205, 1976. [DOI] [PubMed] [Google Scholar]
- 17.Jureus A, Cunningham MJ, Li D, Johnson LL, Krasnow SM, Teklemichael DN, Clifton DK, Steiner RA. Distribution and regulation of galanin-like peptide (GALP) in the hypothalamus of the mouse. Endocrinology 142: 5140–5144, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Jureus A, Cunningham MJ, McClain ME, Clifton DK, Steiner RA. Galanin-like peptide (GALP) is a target for regulation by leptin in the hypothalamus of the rat. Endocrinology 141: 2703–2706, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Kauffman AS, Buenzle J, Fraley GS, Rissman EF. Effects of galanin-like peptide (GALP) on locomotion, reproduction, and body weight in female and male mice. Horm Behav 48: 141–151, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Kawasaki M, Saito J, Hashimoto H, Suzuki H, Otsubo H, Fujihara H, Ohnishi H, Nakamura T, Ueta Y. Induction of the galanin-like peptide gene expression in the posterior pituitary gland after acute osmotic stimulus in rats. Neurosci Lett 419: 125–130, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Krasnow SM, Fraley GS, Schuh SM, Baumgartner JW, Clifton DK, Steiner RA. A role for galanin-like peptide in the integration of feeding, body weight regulation, and reproduction in the mouse. Endocrinology 144: 813–822, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Krasnow SM, Hohmann JG, Gragerov A, Clifton DK, Steiner RA. Analysis of the contribution of galanin receptors 1 and 2 to the central actions of galanin-like peptide. Neuroendocrinology 79: 268–277, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Kumano S, Matsumoto H, Takatsu Y, Noguchi J, Kitada C, Ohtaki T. Changes in hypothalamic expression levels of galanin-like peptide in rat and mouse models support that it is a leptin-target peptide. Endocrinology 144: 2634–2643, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310: 683–685, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Magni P, Motta M, Martini L. Leptin: a possible link between food intake, energy expenditure, and reproductive function. Regul Pept 92: 51–56, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Man PS, Lawrence CB. The effects of galanin-like peptide on energy balance, body temperature and brain activity in the mouse and rat are independent of the GALR2/3 receptor. J Neuroendocrinol 20: 128–137, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145: 4565–4574, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Ohtaki T, Kumano S, Ishibashi Y, Ogi K, Matsui H, Harada M, Kitada C, Kurokawa T, Onda H, Fujino M. Isolation and cDNA cloning of a novel galanin-like peptide (GALP) from porcine hypothalamus. J Biol Chem 274: 37041–37045, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Onaka T, Kuramochi M, Saito J, Ueta Y, Yada T. Galanin-like peptide stimulates vasopressin, oxytocin and adrenocorticotropic hormone release in rats. Neuroreport 16: 243–247, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Rich N, Reyes P, Reap L, Goswami R, Fraley GS. Sex differences in the effect of prepubertal GALP infusion on growth, metabolism and LH secretion. Physiol Behav 92: 814–823, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen J, Gundlach AL. Galanin-like peptide mRNA alterations in arcuate nucleus and neural lobe of streptozotocin-diabetic and obese Zucker rats. Further evidence for leptin-dependent and independent regulation. Neuroendocrinology 79: 327–337, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Shen J, Larm JA, Gundlach AL. Galanin-like peptide mRNA in neural lobe of rat pituitary. Increased expression after osmotic stimulation suggests a role for galanin-like peptide in neuron-glial interactions and/or neurosecretion. Neuroendocrinology 73: 2–11, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Stoyanovitch AG, Johnson MA, Clifton DK, Steiner RA, Fraley GS. Galanin-like peptide rescues reproductive function in the diabetic rat. Diabetes 54: 2471–2476, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Takatsu Y, Matsumoto H, Ohtaki T, Kumano S, Kitada C, Onda H, Nishimura O, Fujino M. Distribution of galanin-like peptide in the rat brain. Endocrinology 142: 1626–1634, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Ueta Y, Ozaki Y, Saito J. Novel G-protein-coupled receptor ligands and neurohypophysial hormones. J Neuroendocrinol 16: 378–382, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav 74: 683–701, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW. Absence of ghrelin protects against early-onset obesity. J Clin Invest 115: 3573–3578, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 115: 3564–3572, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.