Abstract
Rationale
The mGluR5 antagonist MPEP has effects that suggest potential as a pharmacotherapy for cocaine addiction. MPEP can attenuate self-administration of cocaine in animals; however, studies usually involved only acute treatment with MPEP and a single dose of self-administered cocaine. Cocaine addicts use varied amounts of cocaine over long periods of time, and an effective pharmacotherapy would almost certainly require more chronic treatment.
Objectives
The present study: 1) compared the effects of repeated treatment with MPEP or the NMDA receptor antagonist dizocilpine on the reinforcing effects of a range of doses of cocaine and 2) determined the pharmacological specificity of the effects of the drugs in attenuating cocaine self-administration compared to food-reinforced behavior. An effective pharmacotherapy should selectively reduce cocaine self-administration.
Methods
Groups of monkeys responded under a fixed-ratio schedule of i.v. cocaine self-administration or food-pellet delivery. The effects of daily treatment with MPEP and dizocilpine were determined under both the schedule of i.v. cocaine injection and food delivery.
Results
Treatment with MPEP and dizocilpine significantly reduced cocaine self-administration, producing rightward and downward shifts in the ascending limb of the cocaine dose-response function. MPEP and dizocilpine selectively and significantly attenuated self-administration of a low reinforcing dose of cocaine compared to food without evidence of tolerance.
Conclusions
Both MPEP and dizocilpine functioned as partially surmountable antagonists of the reinforcing effects of cocaine. The similar effects of the two drugs raises the possibility that MPEP attenuated the reinforcing effects of cocaine, at least in part, via mGluR5-mediated inhibition of NMDA receptor activity.
Keywords: Glutamate, Metabotropic glutamate receptors, Ionotropic glutamate receptors, Cocaine self-administration, Food self-administration, Pharmacotherapy, Squirrel monkey (Saimiri sciureus)
A substantial body of literature supports the importance of glutamate receptor mechanisms in the behavioral effects of cocaine (Kalivas 2004). Increasingly, research has focused on the role of metabotropic glutamate receptors (mGluRs), in comparison to ionotropic glutamate receptors (i.e., NMDA, AMPA and kainate receptors), in the abuse-related effects of cocaine (Kenny & Markou 2004). The mGluRs are G protein-coupled receptors that have been classified into three main groups (groups I – III) encompassing eight receptor subtypes (mGluR 1 – 8) based on sequence homology, signal transduction pathways, and pharmacology (Conn & Pin 1997; Kenny & Markou 2004). The mGluR5 subtype has received considerable attention due its high expression levels in limbic and forebrain regions that are believed to serve as important neuroanatomical substrates underlying cocaine addiction (Spooren et al. 2001; Muly et al. 2003; Kenny & Markou 2004).
Behavioral studies have shown that mice lacking the mGluR5 gene fail to acquire cocaine self-administration (Chiamulera et al. 2001). However, responding for food under a similar schedule of reinforcement was unaffected in these same mice, demonstrating a potentially selective regulation of cocaine self-administration by mGluR5 receptors (Chiamulera et al. 2001). Consistent with the findings in knockout mice, the mGluR5 receptor antagonist MPEP [2-methyl-6-(phenylethynyl)-pyridine] attenuated cocaine, but not food, self-administration in wild-type mice (Chiamulera et al. 2001). Since this initial study, several additional studies in rodents have provided concordant results. For example, MPEP has been shown to attenuate cocaine self-administration in rats under both fixed ratio and progressive ratio schedules and under short and long access conditions (Tessari et al. 2004; Kenny et al. 2003, 2005; Paterson & Markou 2005). The cocaine-blocking effects of MPEP extend to nonhuman primates as well. Lee et al. (2005) showed that MPEP attenuated cocaine self-administration under a second-order schedule of i.v. drug injection in squirrel monkeys. MPEP also attenuated drug seeking and blocked the discriminative stimulus effects of cocaine at doses of MPEP that did not markedly impair motor behavior.
In previous studies of the effects of MPEP on cocaine self-administration, the ability of MPEP to modulate self-administration of a single dose of cocaine (e.g., peak of the cocaine dose-response function) was evaluated. It is almost certain, though, that cocaine abusers self-administer a wider array of cocaine doses. Moreover, evaluating the effects of a pretreatment drug on a single dose of cocaine can lead to ambiguous conclusions. That is, depending on where that specific dose lies in the full dose-response function, decreases in self-administration could reflect either enhancement or attenuation of cocaine’s reinforcing effects (cf. Mello & Negus 1996). One purpose of the present study was to extend the findings of earlier studies by assessing the effects of MPEP on a wider range of doses of self-administered cocaine. This latter approach was intended to provide a more definitive characterization of the effects of MPEP on cocaine reinforcement.
Earlier studies with MPEP also typically utilized single-day tests with each MPEP dose. With this approach, it is difficult to determine whether decreases in self-administration are due to reductions in cocaine’s reinforcing effects or to non-specific disruptions in motor behavior. If MPEP reduces the reinforcing effects of cocaine, changes in cocaine self-administration with repeated MPEP administration should parallel those observed across days of saline substitution. In addition, given that cocaine addiction is a chronic disorder, it is likely that any treatment medication will be administered over an extended period of time. Studies involving repeated treatment with mGluR5 antagonists have shown that tolerance can develop rapidly to some of the effects of these drugs (e.g., antinociceptive effects, Sevostianova & Danysz 2006, but see Nordquist et al. 2007). With these considerations in mind, the present study also evaluated the effects of MPEP across several days of repeated treatment (minimum of five days). Finally, the effects of MPEP were evaluated in a second group of monkeys trained to self-administer food under an identical schedule of reinforcement as the cocaine group to determine whether reduction in self-administration by MPEP was specific to cocaine reinforcement.
In the present studies, the effects of MPEP were compared to those of the reference NMDA receptor antagonist dizocilpine. Although MPEP exhibits high selectivity at mGluR5 receptors in vitro and in vivo (Gasparini et al. 1999; Thomas et al. 2001), MPEP also has been reported to either directly or indirectly inhibit NMDA receptor activity (Lea & Faden 2006). MPEP has been shown to decrease NMDA-mediated neuronal toxicity (O’Leary et al. 2000; Movsesyan et al. 2001; Lea et al. 2005) and to indirectly modulate NMDA receptor function (Homayoun et al. 2004). The latter findings raise the possibility that the cocaine-attenuating effects of MPEP may be mediated, at least in part, by NMDA receptor mechanisms.
Materials and Methods
Subjects and surgical procedure
Eleven experimentally naïve, adult male squirrel monkeys (Saimiri sciureus), weighing 800 to 1100 g, were studied in daily experimental sessions (Monday to Friday). Between sessions, monkeys lived in individual home cages where they had unlimited access to water. Monkeys in the cocaine self-administration study also had unrestricted access to food (Teklad Monkey Diet, supplemented with fresh fruit), whereas monkeys in the food self-administration study were maintained at approximately 90% of their free-feeding body weight by adjusting their caloric intake in the home cage. All monkeys were maintained in accordance with the guidelines of the Committee on Animals of the Harvard Medical School and the “Guide for Care and Use of Laboratory Animals” of the Institute of Laboratory Animal Resources, National Research Council, Department of Health, Education and Welfare Publication No. (NIH) 85-23, revised 1996. Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Before beginning the present study, monkeys in the cocaine self-administration study were prepared with a chronic indwelling venous catheter (polyvinyl chloride, inside diameter: 0.015 mm; outside diameter: 0.035 mm) using the surgical procedures described by Platt et al. (2005). Under isoflurane anesthesia and aseptic conditions, one end of a catheter was passed to the level of the right atrium by way of a femoral or jugular vein. The distal end of the catheter was passed subcutaneously and exited in the mid-scapular region. Catheters were flushed daily with saline and were sealed with stainless steel obturators when not in use. Monkeys wore nylon-mesh jackets (Lomir Biomedical; Toronto, Canada) at all times to protect the catheter.
Apparatus
Monkeys were seated in primate chairs equipped with a response lever and stimulus lights mounted on the front panel (Med Associates, Inc.; Georgia, VT). Each press of the lever with a minimum downward force of approximately 0.25 N was recorded as a response. In cocaine self-administration experiments, catheters were connected to a syringe pump (Med Associates, Inc.; Georgia, VT) located outside of the chamber. Each operation of the pump lasted 1 s and delivered 0.18 ml of drug solution into the catheter. In food self-administration experiments, 190 mg sucrose pellets (Bio-Serv Precision pellets, Formula 0069, Bio-Serv; Frenchtown, NJ) were delivered to a tray that was accessible through an opening in the front panel of the chair. Experimental sessions were conducted in ventilated, sound-attenuating chambers, which were equipped with white noise to mask external sounds.
Cocaine- and food-maintained behavior
Monkeys (n = 6) were trained to respond under a fixed-ratio (FR) schedule of reinforcement. In the presence of a white light, completion of every 10th response (FR 10) resulted in a 1-sec change in illumination from white to red light and an i.v. injection of cocaine. Between injections, all lights were off briefly and responses had no scheduled consequences. Daily sessions ended after 60 min elapsed or 100 injections were delivered, whichever occurred first. A cocaine dose-response function initially was established in each monkey. The effects of a range of doses of cocaine (0.003–0.1 mg/kg/injection) as well as saline were tested in irregular order. These data were used to identify the dose of cocaine that maintained maximum levels of self-administration for each monkey (0.01 or 0.03 mg/kg/injection), which was then used as a standard training dose.
A separate group of monkeys (n = 5) was trained to respond under a FR 10 schedule of food reinforcement. This schedule was procedurally identical to the schedule of cocaine self-administration described above except that completion of every FR 10 produced a food pellet instead of an injection of cocaine. Daily sessions ended after 60 min elapsed or 100 food pellets were delivered, whichever occurred first.
To habituate subjects in both groups to the injection procedures as well as to establish baseline rates of responding, monkeys received i.m. injections of saline before each experimental session. Injections were made in a thigh or calf muscle of either leg. Drug testing began once stable cocaine or food self-administration (i.e., no upward or downward trend in injections/session or pellets/session over a period of at least three consecutive days) was observed under the baseline conditions.
Modulation of the cocaine dose-response function by MPEP and dizocilpine
Initially, for subjects trained to self-administer cocaine, each monkey’s cocaine dose-response function was re-determined following pretreatment with MPEP, dizocilpine and their corresponding vehicles. Intramuscular injections of the drugs or their vehicles were administered immediately before the start of the session. The doses and pretreatment times of MPEP and dizocilpine were selected based on a previous study with these drugs in squirrel monkeys (Lee et al. 2005). Each dose of MPEP or dizocilpine (including vehicle) was studied for a minimum of 5 consecutive cocaine self-administration sessions and until self-administration performance was stable (i.e., no upward or downward trend in injections/session over a period of at least three consecutive days). Doses of MPEP and dizocilpine were studied in irregular order in individual monkeys, and all doses of one drug were studied before moving on to test the other drug. Between tests with different doses of MPEP or dizocilpine, self-administration of the training dose of cocaine (0.01 or 0.03 mg/kg/injection depending on the monkey) was determined for 3–10 days.
Effects of MPEP and dizocilpine on cocaine- versus food-maintained behavior
Finally, the effects of pretreatment with a range of doses of MPEP (0.1 – 1.8 mg/kg) and dizocilpine (0.001 – 0.03 mg/kg) were determined on self-administration of two cocaine doses (0.01 and 0.03 mg/kg/injection) that maintained the highest levels of cocaine intake. Each pretreatment dose of MPEP or dizocilpine was tested for at least five consecutive sessions and until stable self-administration was observed. Doses were administered to individual monkeys in irregular order with the restriction that all doses of one drug were evaluated before proceeding to testing with the other drug. Baseline responding following saline pretreatment was re-determined between tests with different doses to insure that intake and patterns of self-administration had not changed over the course of the study. Each experiment was conducted in at least four monkeys out of a total of six.
As with the cocaine-trained monkeys, monkeys trained to self-administer food pellets were pretreated with an i.m. injection of either MPEP or dizocilpine immediately before the start of a food self-administration session. A range of doses of MPEP (0.1 – 1.8 mg/kg), dizocilpine (0.001 – 0.1 mg/kg) or their vehicles were studied. Each dose was tested for at least five consecutive sessions and until stable food self-administration was observed. Between testing with a dose of a particular drug, baseline responding (i.e., following saline injection) was re-determined. Studies were conducted in at least four monkeys out of a total of five.
Analysis of drug effects
Data are expressed as the mean (± SEM) number of reinforcers (cocaine injections or food pellets) obtained over the last three self-administration sessions under baseline conditions (i.e., following saline pretreatment) or after pre-session administration of MPEP, dizocilpine or their vehicles. In the initial study using a full range of doses of cocaine, statistical significance of treatment effects was assessed with separate two-way repeated measures ANOVAs followed by Bonferroni t-tests, in which the number of injections delivered during cocaine self-administration sessions following pretreatment with different doses of MPEP or dizocilpine was compared to the number of injections delivered after vehicle administration. In addition, the dose of cocaine following pretreatment with vehicle, MPEP or dizocilpine which resulted in delivery of either 25% or 50% of the maximum injections/session for cocaine alone (ED25 and ED50, respectively) was estimated for individual monkeys by linear regression analysis in cases where the ascending portion of the log dose-response function was defined by three or more data points or by linear interpolation in cases where the log dose-response function was defined best by two points. The mean (± SEM) ED25 and ED50 value for each drug was determined by averaging ED25 or ED50 values for individual monkeys.
To compare the effects of MPEP and dizocilpine on cocaine and food self-administration, the number of reinforcers delivered following pretreatment with each dose of a particular drug was converted to a percentage of control (i.e., number of reinforcers delivered during baseline self-administration sessions). Statistical significance of treatment effects was assessed with separate one-way repeated measures ANOVAs followed by Bonferroni t-tests, in which the number of reinforcers delivered during cocaine or food self-administration sessions following pretreatment with different doses of MPEP or dizocilpine were compared to the number of reinforcers delivered after vehicle administration. In addition, the dose of MPEP or dizocilpine needed to decrease the maximum number of reinforcers obtained by 50% (A50) was determined for individual monkeys by linear regression analysis in cases where the descending portion of the log dose-response function was defined by three or more data points or by linear interpolation in cases where the log dose-response function was defined best by two points. The mean (± SEM) A50 value for each drug was determined by averaging A50 values for individual monkeys. Finally, separate one-way repeated measures ANOVAs followed by Bonferroni t-tests compared reinforcers/session (injections/session or pellets/session) across the last five days of treatment with an effective dose of MPEP or dizocilpine. For cocaine (0.01 mg/kg/injection), the effects of repeated 0.3 mg/kg MPEP and 0.01 mg/kg dizocilpine were analyzed; for cocaine (0.03 mg/kg/injection), the effects of repeated 1 mg/kg MPEP and 0.01 mg/kg dizocilpine were analyzed; and for food self-administration, the effects of repeated 1.8 mg/kg MPEP and 0.1 mg/kg dizocilpine were analyzed.
Drugs
(−)-Cocaine hydrochloride (Sigma RBI, St. Louis, MO), MPEP hydrochloride [2-methyl-6-(phenylethynyl)-pyridine] and dizocilpine maleate (also, MK-801; Tocris Cookson, Ellisville, MO) were dissolved in small amounts of ethanol and 0.1N HCl as required and diluted to the desired concentrations with sterile water or 0.9% saline solution. Intramuscular injection volumes were 0.1 ml/kg of body weight.
Results
Cocaine- and food-maintained behavior
Intravenous injections of cocaine maintained consistent self-administration in all six monkeys under the FR schedule of i.v. drug injection. For individual monkeys, the dose that maintained maximal injections/session was 0.01 or 0.03 mg/kg/injection. Under baseline conditions (i.e., after saline injection), the mean (± SEM) number of self-administered injections was 70 ± 9.2 injections/session for the group of six monkeys. During sessions in which vehicle was substituted for cocaine, responding decreased markedly and monkeys obtained an average of 4.3 ± 1.7 injections/session. For the group of monkeys trained under the FR schedule of food delivery, performances were comparable to those observed for the cocaine self-administration group. Under baseline conditions, the mean (± SEM) number of food pellet deliveries was 75 ± 7.7 pellets/session.
Modulation of the cocaine dose-response function by MPEP and dizocilpine
Pretreatment with MPEP (0.3 or 1 mg/kg) for five or more consecutive sessions resulted in dose-dependent rightward and downward shifts in the ascending limb of the cocaine dose-response function with little change in the effects of the highest dose of cocaine (Figure 1). Two-way repeated measures ANOVA identified significant main effects of cocaine dose [F(3,9) = 34.54, p<0.001], MPEP dose [F(2,6) = 7.41, p<0.05], and a significant cocaine X MPEP interaction [F(6,18) = 12.29, p<0.001]. Subsequent post-hoc analyses showed that pretreatment with 0.3 mg/kg of MPEP significantly reduced self-administration of 0.01 mg/kg/injection cocaine compared to vehicle pretreatment (Bonferroni t-tests, p<0.05) and that this effect of MPEP could be surmounted partially by increasing the dose of self-administered cocaine to 0.03 mg/kg/injection or greater. Additional analysis of ED25 and ED50 values revealed that 0.3 mg/kg of MPEP produced a 3 to 4-fold rightward shift in the ascending limb of the cocaine dose-response function (ED25: 0.004 ± 0 for cocaine alone, 0.014 ± 0.006 for cocaine after treatment with MPEP; ED50: 0.005 ± 0 for cocaine alone, 0.016 ± 0.005 for cocaine after treatment with MPEP). When the dose of MPEP was increased to 1 mg/kg, self-administration of both 0.01 and 0.03 mg/kg/injection of cocaine was decreased significantly compared to vehicle pretreatment (Bonferroni t-tests, p<0.05). Increasing the dose of self-administered cocaine to 0.1 mg/kg/injection resulted in numbers of injections/session that did not differ from that observed after vehicle pretreatment. ED25 analysis showed that this higher MPEP dose also shifted the ascending limb of the cocaine dose-response function 4-fold to the right (ED25: 0.004 ± 0 for cocaine alone, 0.015 ± 0.003 for cocaine after treatment with MPEP). An ED50 value could not be calculated because the number of injections/session did not reach that of 50% of cocaine alone.
Figure 1.
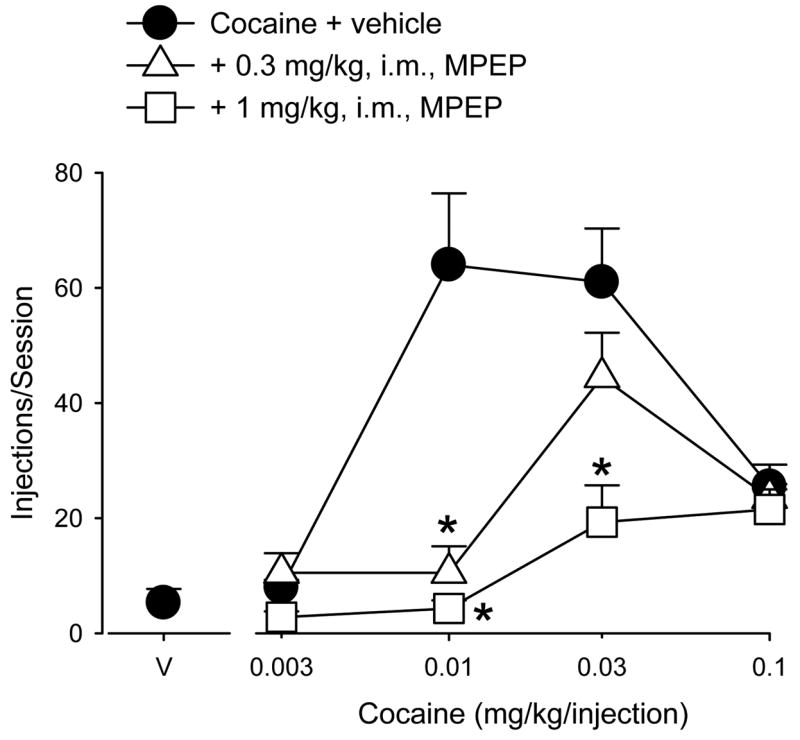
Attenuation of cocaine self-administration by MPEP pretreatment for ≥ 5 consecutive sessions. Data are means (± SEM) for the last three sessions at each condition in each of four monkeys. The cocaine dose response function following vehicle pretreatment is the average dose-response function for only those monkeys that had received MPEP pretreatment. Asterisks indicate significant reductions in cocaine self-administration after MPEP pretreatment compared to vehicle pretreatment.
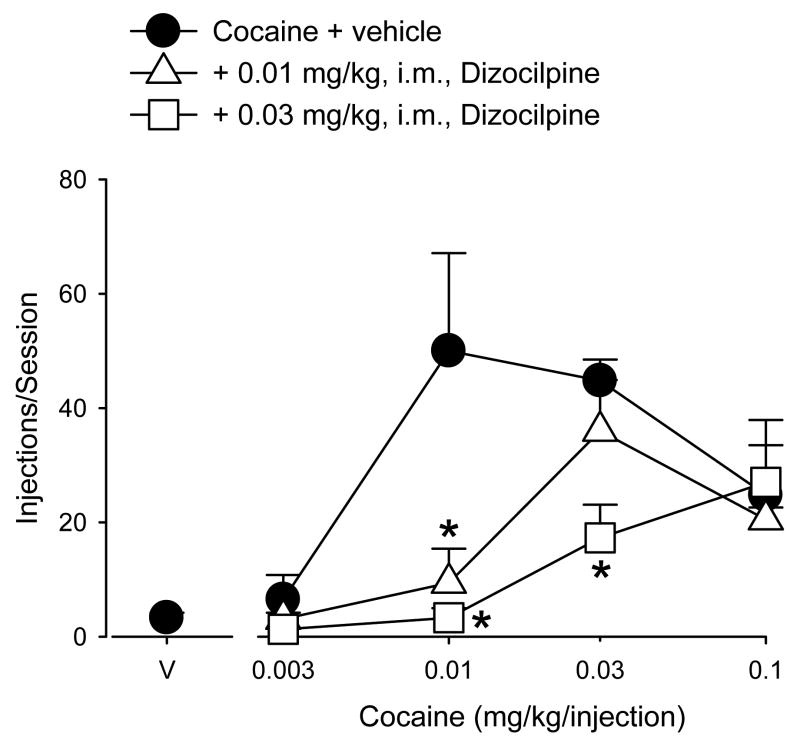
Similar rightward and downward shifts in the ascending limb of the cocaine dose-response function were observed following administration of dizocilpine (Figure 2). Two-way repeated measures ANOVA identified significant main effects of cocaine dose [F(3,9) = 8.12, p<0.05], dizocilpine dose [F(2,6) = 25.67, p<0.05], and a significant cocaine X dizocilpine interaction [F(6,18) = 3.36, p<0.05]. Post-hoc analyses showed that pretreatment with 0.01 mg/kg of dizocilpine significantly reduced self-administration of 0.01 mg/kg/injection cocaine compared to vehicle pretreatment (Bonferroni t-tests, p<0.05), an effect that was surmounted by increasing the dose of self-administered cocaine to 0.03 mg/kg/injection or higher. Additional analysis of ED25 and ED50 values revealed that 0.01 mg/kg of dizocilpine produced a 4-fold rightward shift in the ascending limb of the cocaine dose-response function (ED25: 0.004 ± 0 for cocaine alone, 0.015 ± 0.003 for cocaine after treatment with dizocilpine; ED50: 0.007 ± 0.001 for cocaine alone, 0.029 ± 0.012 for cocaine after treatment with dizocilpine). When the dose of dizocilpine was increased further to 0.03 mg/kg, self-administration of both 0.01 and 0.03 mg/kg/injection of cocaine was significantly reduced compared to vehicle pretreatment (Bonferroni t-tests, p<0.05). Increasing the dose of self-administered cocaine to 0.1 mg/kg/injection resulted in numbers of injections/session that did not differ from that observed after vehicle pretreatment. ED25 analysis showed that this higher dizocilpine dose shifted the ascending limb of the cocaine dose-response function 6-fold to the right (ED25: 0.004 ± 0 for cocaine alone, 0.023 ± 0.006 for cocaine after treatment with dizocilpine). ED50 values could not be calculated for 0.03 mg/kg of dizocilpine because the number of injections/session did not reach that of 50% of cocaine alone.
Figure 2.
Attenuation of cocaine self-administration by dizocilpine pretreatment for ≥ 5 consecutive sessions. Data are means (± SEM) for the last three sessions at each condition in each of four monkeys. The cocaine dose response function following vehicle pretreatment is the average dose-response function for only those monkeys that had received dizocilpine pretreatment. Asterisks indicate significant reductions in cocaine self-administration after dizocilpine pretreatment compared to vehicle pretreatment.
Effects of MPEP and dizocilpine on cocaine- versus food-maintained behavior
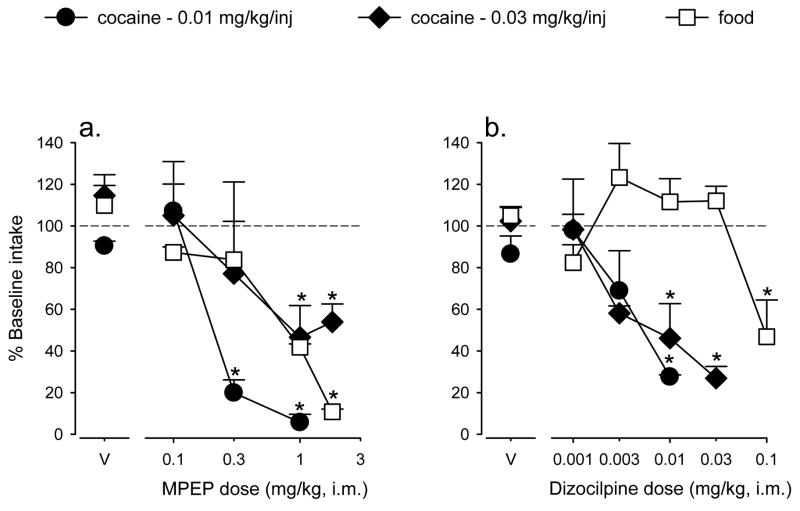
Daily pretreatment with MPEP produced dose-related decreases in the average injections/session maintained by 0.01 mg/kg/injection cocaine (Figure 3, left; filled circles). The two highest doses of MPEP (0.3 and 1 mg/kg) significantly reduced the average injections/session to 6–20% of the values maintained by cocaine self-administration under baseline conditions [F(3,6) = 43.55, p<0.001; Bonferroni t-test p<0.05]. When the dose of self-administered cocaine was increased to 0.03 mg/kg/injection, higher doses of MPEP (1 and 1.8 mg/kg) were needed to significantly reduce the number of self-administered injections/session [Figure 3, left, filled diamonds; F(4,12) = 6.70, p<0.05; Bonferroni t-test p<0.05]. Maximum reduction of intake maintained by the higher dose of cocaine by MPEP was only to 47% of intake under baseline conditions. MPEP induced a similar dose-dependent reduction in pellets/session in monkeys trained under the fixed-ratio schedule of food delivery (Figure 3, left, open squares), with the highest dose of MPEP (1.8 mg/kg) significantly reducing intake to approximately 11% of the intake under baseline conditions [F(4,12) = 5.67, p<0.05; Bonferroni t-test p<0.05]. A comparison of A50 values showed that a lower dose of MPEP was needed to reduce self-administration of 0.01 mg/kg/injection of cocaine (A50 = 0.25 ± 0.06 mg/kg) compared to the dose required to reduce self-administration of either 0.03 mg/kg/injection cocaine (A50 = 1.75 ± 0.07 mg/kg) or of food pellets (A50 = 0.65 ± 0.21 mg/kg).
Figure 3.
Effects of daily pretreatment with MPEP (a, left), dizocilpine (b, right) or their vehicles (points over “V”) on reinforcers/session under the fixed-ratio schedule of i.v. cocaine injections or food pellet deliveries. For each monkey, intake (injections/session or pellets/session) was converted to a percent of baseline intake (i.e., intake after saline injection). Data are means (± SEM) based on data from groups of 4 monkeys. Asterisks indicate significant reductions in intake after MPEP or dizocilpine pretreatment compared to vehicle pretreatment.
Like MPEP, daily pretreatment with dizocilpine produced dose-dependent reductions in the average injections/session maintained by 0.01 mg/kg/injection cocaine (Figure 3, right, filled circles). Dizocilpine, at a dose of 0.01 mg/kg, significantly reduced the average number of cocaine injections/session to 28% of the value maintained under baseline conditions [F(3,9) = 6.04, p<0.05; Bonferroni t-test p<0.05]. When the dose of cocaine was increased to 0.03 mg/kg/injection, the number of self-administered injections/session were also reduced significantly to 27–46% of baseline values by 0.01 and 0.03 mg/kg dizocilpine [Figure 3, right, filled diamonds; F(4,12) = 10.16, p<0.001; Bonferroni t-test p<0.05]. Higher doses of dizocilpine were needed to attenuate food-maintained behavior (Figure 3, right, open squares). Only 0.1 mg/kg dizocilpine significantly reduced the number of pellets/session below the level observed under baseline conditions [F(4,12) = 7.55, p<0.05; Bonferroni t-test p<0.05). Based on A50 values, lower doses of dizocilpine were needed to reduce self-administration of 0.01 mg/kg/injection of cocaine (A50 = 0.004 ± 0.001 mg/kg) and 0.03 mg/kg/injection of cocaine (A50 = 0.014 ± 0.004 mg/kg) compared to the dose required to reduce self-administration of food pellets (A50 = 0.079 ± 0.008 mg/kg).
Time course analysis
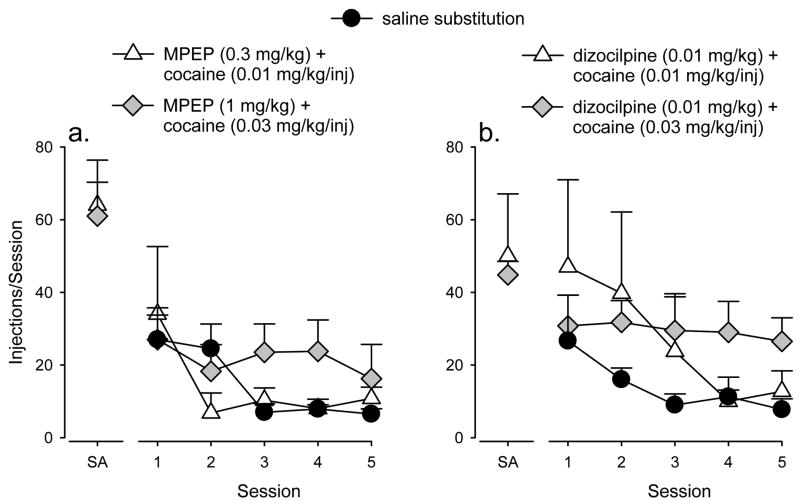
If the reductions in cocaine self-administration induced by pretreatment with MPEP and dizocilpine are due to reductions in the reinforcing effects of cocaine, then the changes in cocaine injections across consecutive drug pretreatment sessions would be expected to be similar to the changes in injections/session across consecutive sessions of saline substitution. Figure 4 depicts the number of injections/session across the first 5 sessions of saline substitution (filled circles). Saline data were obtained during the course of determining the cocaine dose-response function. Visual inspection of the figure shows that i.v. self-administration during consecutive saline substitution sessions was highest on day 1 and then declined to a low, stable level by day 3. When the effects of MPEP pretreatment on self-administration of cocaine were compared to the effects of saline substitution, the effects of MPEP on the low dose of cocaine (0.01 mg/kg/injection; white triangles) appeared to parallel the effects of saline substitution. In contrast, for the higher dose of self-administered cocaine (0.03 mg/kg/injection; gray diamonds), the effect observed on day 5 of MPEP treatment was essentially the same as on day 1 of treatment.
Figure 4.
Number of injections across the first 5 sessions of treatment with the minimally effective dose of MPEP (a, left) or dizocilpine (b, right) in combination with cocaine and compared with number of injections across the first 5 sessions of saline availability (filled circles). Points are means (± SEM) based on data from groups of 4 monkeys. Points above “SA” represent the mean injections/session delivered during cocaine self-administration in the absence of drug pretreatment.
A similar time course was observed with daily dizocilpine administration (Figure 4, right). With repeated dizocilpine treatment, the number of injections maintained by the low cocaine dose (white triangles) was initially high and then declined to low levels by day 4. This pattern generally follows the pattern observed when saline was substituted for self-administered cocaine (filled circles). For the higher dose of cocaine (gray diamonds), however, the number of injections obtained remained relatively stable with no marked increases or decreases across five days of repeated dizocilpine treatment.
Daily administration of MPEP or dizocilpine also permitted assessment of whether tolerance developed to the suppressant effects of these drugs on self-administration. In no case did the effects of MPEP or dizocilpine diminish across days. Separate one-way repeated measures ANOVAs showed that the effects of MPEP and dizocilpine on self-administration of the two cocaine doses did not decrease across days [Figure 4; MPEP (0.3 mg/kg)/cocaine (0.01 mg/kg/injection): F(4,12) = 1.81, p = 0.192, MPEP (1.0 mg/kg)/cocaine (0.03 mg/kg/injection): F(4,12) = 0.88, p = 0.503, dizocilpine (0.01 mg/kg)/cocaine (0.01 mg/kg/injection): F(4,12) = 1.93, p = 0.171, dizocilpine (0.01 mg/kg)/cocaine (0.03 mg/kg/injection): F(4,12) = 0.11, p = 0.977]. Similarly, tolerance to the effects of MPEP and dizocilpine on food self-administration was not observed (data not shown), as the effect observed on day 1 of treatment with either MPEP (1.8 mg/kg) or dizocilpine (0.1 mg/kg_ did not differ significantly from the effect observed on day 5 of treatment [MPEP: F(4,12) = 0.73, p = 0.590; dizocilpine: F(4,12) = 1.40, p = 0.292].
Discussion
In the present study, daily pretreatment with MPEP attenuated cocaine self-administration by squirrel monkeys responding under a FR schedule of i.v. drug injection. MPEP pretreatment produced dose-dependent, rightward and downward shifts in the ascending limb of the cocaine dose-response function which could be at least partially surmounted by increasing the dose of self-administered cocaine. This result extends earlier work in both rodents and monkeys indicating that MPEP can reduce self-administration of a single dose of cocaine under a variety of schedules of reinforcement (Chiamulera et al. 2001; Tessari et al. 2004; Kenny et al. 2005; Lee et al. 2005; Paterson & Markou 2005). Together, these findings support the idea that MPEP can act as a functional antagonist of the reinforcing effects of cocaine.
Although MPEP displays high selectivity for MGluR5 receptors (Gasparini et al. 1999; Thomas et al. 2001), MPEP also has been reported to either directly or indirectly inhibit NMDA receptor activity (Lea & Faden 2006). To explore the potential role of NMDA receptor mechanisms in the effects of MPEP, we compared the effects of MPEP with those of the NMDA receptor antagonist dizocilpine. Dizocilpine produced dose-dependent, rightward and downward shifts in the ascending limb of the cocaine dose-response function that were comparable to those produced by MPEP. Like MPEP, the effects of dizocilpine also could be surmounted by increasing the dose of self-administered cocaine. Moreover, daily treatment with both MPEP and dizocilpine induced changes in the number of injections/session, at least for 0.01 mg/kg/injection cocaine, that paralleled the changes in injections/session across days of saline substitution. This observation suggests that the attenuation of cocaine self-administration by MPEP and dizocilpine is due to a reduction in reinforcing effects as opposed to a nonspecific suppression of behavior.
Under some conditions, repeated exposure to self-administered cocaine can lead to sensitization of the behavioral effects of cocaine, including its reinforcing effects (Ben-Shahar et al. 2005; Liu et al. 2005; but see Mutschler et al. 2001). Behavioral sensitization has been considered an important mechanism facilitating drug-taking behavior and relapse, and glutamate receptor mechanisms have been implicated in the sensitization process (for review see Cornish & Kalivas 2001). In a direct comparison of the role of NMDA and mGluR5 receptor mechanisms in psychostimulant-induced locomotor sensitization, Yap and colleagues (2005) found that the development of behavioral sensitization to amphetamine could be prevented by the co-administration of dizocilpine, but not MPEP. These results implicate NMDA, but not mGluR5, receptor mechanisms in psychostimulant-induced behavioral sensitization. Furthermore, these findings also demonstrate a dissociation between sensitization and the reinforcing effects of cocaine and, given the similar effects of MPEP and dizocilpine in the present study, suggest that sensitization played little role in the self-administration behavior of the monkeys.
The similarity in the effects of MPEP and dizocilpine raises the possibility that the effects of MPEP are mediated at least in part via attenuation of NMDA receptor activity. In an earlier study of the effects of these two glutamatergic ligands in the behavioral effects of cocaine in squirrel monkeys, Lee et al. (2005) also found that both MPEP and dizocilpine could attenuate the reinforcing effects of a maximally reinforcing dose of cocaine. However, MPEP, but not dizocilpine, also blocked cocaine-induced reinstatement of drug seeking as well as the discriminative stimulus effects of cocaine (Lee et al. 2005). These results raise the possibility that inhibition of NMDA receptor activity may play a more significant role in attenuation of the reinforcing effects of cocaine compared to other effects of cocaine related to its abuse. The idea that NMDA receptor mechanisms may be particularly important for the reinforcing effects of cocaine is underscored by the observation that dizocilpine can block the acquisition of cocaine self-administration in rats. Schenk et al. (1993) found that upon discontinuation of daily dizocilpine treatment, rats acquired cocaine self-administration in much the same way as cocaine-naïve rats (e.g., exhibited the same acquisition time course as naïve rats).
Our finding that dizocilpine can attenuate cocaine self-administration, and hence the reinforcing effects of cocaine in monkeys, differs from the majority of self-administration studies conducted in rodents. For example, in rats self-administering cocaine under a FR schedule, dizocilpine has been shown to decrease the number of cocaine injections earned (Shoaib et al. 1995; Pierce et al. 1997; Hyytia et al. 1999; Allen et al. 2005). This change in responding for cocaine is similar to that which occurs when the dose of self-administered cocaine is increased, leading to the interpretation that the reinforcing effects of cocaine are enhanced rather than attenuated. This interpretation is supported by the observation that pretreatment with dizocilpine resulted in increased numbers of injections and breakpoints in rats self-administering cocaine under a progressive-ratio schedule (Ranaldi et al. 1996; Hyytia et al. 1999; Allen et al. 2005). The reasons underlying the different effects of dizocilpine in the present study compared to studies in rats are unclear. An obvious possibility would be the existence of underlying differences in the NMDA receptor system of the two species. However, in vitro studies have identified relatively few differences in the regional distribution of functional NMDA receptors in primates compared to non-primate species (Hof et al. 1996; Huntley et al. 1997; Meoni et al. 1998; Gonzalez-Albo & DeFelipe 2000). Moreover, these differences appear to involve brain regions not traditionally associated with processes involved in drug reinforcement (e.g., claustrum, Meoni et al. 1998) Alternatively, the different effects of dizocilpine could reflect procedural differences among the various studies, in particular the doses of dizocilpine tested. It has been suggested, for example, that dizocilpine enhances the reinforcing effects of cocaine in rats only over a relatively narrow range of doses and that doses of dizocilpine below this range have no effect on cocaine self-administration (Ranaldi et al. 1996; Pierce et al. 1997); whereas doses above this range tend to attenuate cocaine self-administration (Shoaib et al. 1995; Ranaldi et al. 1996). Finally, there is the possibility that dizocilpine acts uniquely in the rat to enhance rather than attenuate cocaine reinforcement. Other NMDA receptor antagonists, including dextromethorphan and memantine, have been shown to reduce cocaine self-administration in rats under both FR and progressive-ratio schedules, consistent with an attenuation of the reinforcing effects of cocaine (Pulvirenti et al. 1997; Hyytia et al. 1999).
Both MPEP and dizocilpine attenuated cocaine self-administration raising the possibility that these or related drugs may have some utility as cocaine pharmacotherapies. An important consideration in the development of a cocaine pharmacotherapy is the pharmacological specificity of the proposed medication. One method used to evaluate potential nonspecific effects is to compare the doses of a drug needed to reduce behavior maintained by cocaine self-administration relative to the dose needed to reduce behavior maintained by a non-drug reinforcer. If a drug reduces behavior maintained by cocaine self-administration to a greater extent or more potently than it reduces behavior maintained by the non-drug reinforcer, then the drug may have sufficiently selective effects on cocaine self-administration to be considered as a potential pharmacotherapy (Mello & Negus 1996). In the present study, compared to its effects on food-reinforced behavior, MPEP selectively reduced self-administration of 0.01 mg/kg/injection cocaine. When the dose of cocaine was increased to 0.03 mg/kg/injection, higher doses of MPEP were needed to decrease cocaine self-administration, and these same doses of MPEP also attenuated food self-administration suggesting that the cocaine-specific effects of MPEP are limited to relatively low doses of cocaine. The observation that MPEP can selectively modulate the reinforcing effects of a comparatively low dose of cocaine is consistent with reports from studies in rodents responding under a FR schedule of cocaine administration (Chiamulera et al. 2001; Kenny et al. 2003; Tessari et al. 2004). The finding that a higher dose of MPEP reduced both cocaine self-administration and food-reinforced behavior may reflect impairment of operant responding. It is noteworthy, however, that these same doses did not decrease food-reinforced responding in the context of a cocaine discrimination procedure nor did they markedly alter observable behavior in squirrel monkeys (Lee et al. 2005). Alternatively, MPEP may have a generalized effect on the process of reinforcement, reducing reinforcing effectiveness of different types of drug and non-drug reinforcers. This latter viewpoint is supported by the finding that a similar dose of MPEP reduced breakpoints in rats self-administering cocaine, nicotine or food under a progressive-ratio schedule (Paterson & Markou 2005).
Consistent with data from rodent studies (Shoaib et al. 1995; Pierce et al. 1997), dizocilpine also selectively modulated the reinforcing effects of cocaine compared to food. In this case, similar doses of dizocilpine reduced self-administration of both 0.01 and the 0.03 mg/kg/injection of cocaine. At least a 6-fold higher dose of dizocilpine was needed to decrease food-reinforced responding. The reduction in food self-administration was likely due to motor impairing effects of dizocilpine, as the monkeys exhibited ataxia and increased muscle resistance post-session.
Given the chronic nature of cocaine addiction, an effective treatment regimen likely would entail repeated dosing. It was of interest, then, to determine the degree to which the effects of MPEP and dizocilpine were sustained across days. In contrast to other procedures (e.g., antinociception, Sevostianova & Danysz 2006), MPEP and dizocilpine’s ability to reduce cocaine self-administration did not wane across a minimum of five consecutive sessions. Although a cocaine pharmacotherapy almost certainly would be administered longer than five days, these results suggest that the likelihood of tolerance developing to the cocaine-attenuating effects of the two drugs may be low.
In summary, MPEP antagonized the reinforcing effects of cocaine in squirrel monkeys in a partially surmountable manner. MPEP also selectively reduced self-administration of a low, but not high, reinforcing dose of cocaine compared to food self-administration, without evidence of tolerance over several days of treatment. The results with MPEP were similar to those obtained with the NMDA receptor antagonist dizocilpine. These findings raise the possibility that the ability of MPEP to attenuate the reinforcing effects of cocaine may be due, at least in part, to inhibition of NMDA receptor activity either directly or indirectly subsequent to mGluR5 receptor blockade.
Acknowledgments
We thank L. Teixeira and S. Langer for technical assistance.
This research was supported by DA17700, DA11054 and RR00168.
References
- Allen RM, Carelli RM, Dykstra LA, Suchey TL, Everett CV. Effects of the competitive N-methyl-D-aspartate receptor antagonist, LY235959 [(−)-6-phosphonomethyl-deca-hydroisoquinoline-3-carboxylic acid], on responding for cocaine under both fixed and progressive ratio schedules of reinforcement. J Pharmacol Exp Ther. 2005;315:449–457. doi: 10.1124/jpet.105.086355. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to i.v. cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Cocaine sensitization and craving: Differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis. 2001;20:43–54. doi: 10.1300/J069v20n03_05. [DOI] [PubMed] [Google Scholar]
- Gasparini F, Lingenhöhl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, Biollaz M, Allgeier H, Heckendorn R, Urwyler S, Varney MA, Johnson EC, Hess SD, Rao SP, Sacaan AI, Santori EM, Veliçelebi G, Kuhn R. 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Albo MC, DeFelipe J. Colocalization of glutamate ionotropic receptor subunits in the human temporal neocortex. Cereb Cortex. 2000;10:621–631. doi: 10.1093/cercor/10.6.621. [DOI] [PubMed] [Google Scholar]
- Hof PR, Vissavajjhala P, Rosenthal RE, Fiskum G, Morrison JH. Distribution of glutamate receptor subunit proteins GluR2(4), GluR5/6/7 and NMDAR1 in the canine and primate cerebral cortex: A comparative immunohistochemical analysis. Brain Res. 1996;723:77–89. doi: 10.1016/0006-8993(96)00218-1. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional interaction between NMDA and mGlu5 receptors: Effects on working memory, instrumental learning, motor behaviors, and dopamine release. Neuropsychopharmacology. 2004;29:1259–1269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Huntley GW, Vickers JC, Morrison JH. Quantitative localization of NMDAR1 receptor subunit immunoreactivity in inferotemporal and prefrontal association cortices of monkey and human. Brain Res. 1997;749:245–262. doi: 10.1016/S0006-8993(96)00847-5. [DOI] [PubMed] [Google Scholar]
- Hyytia P, Backstrom P, Liljequist S. Site-specific NMDA receptor antagonists produce differential effects on cocaine self-administration in rats. Eur J Pharmacol. 1999;378:9–16. doi: 10.1016/s0014-2999(99)00446-x. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. The ups and downs of addiction: Role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology. 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann NY Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Lea PM, Faden AI. Metabotropic glutamate receptor subtype 5 antagonists MPEP and MTEP. CNS Drug Reviews. 2006;12:149–166. doi: 10.1111/j.1527-3458.2006.00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea PM, Movsesyan VA, Faden AI. Neuroprotective activity of the mGluR5 antagonists MPEP and MTEP against acute excitotoxicity differs and does not reflect actions at mGluR5 receptors. Br J Pharmacol. 2005;145:527–534. doi: 10.1038/sj.bjp.0706219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: Comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DCS, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: Effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Meoni P, Mugnaini M, Bunnemann BH, Trist DG, Bowery NG. [3H]MK-801 binding and the mRNA for the NMDAR1 subunit of the NMDA receptor are differentially distributed in human and rat forebrain. Mol Brain Res. 1998;54:13–23. doi: 10.1016/s0169-328x(97)00289-1. [DOI] [PubMed] [Google Scholar]
- Movsesyan VA, O’Leary DM, Fan L, Bao W, Mullins PGM, Knoblach SM, Faden AI. mGluR5 antagonists 2-methyl-6-(phenylethynyl)-pyridine and (E)-2-methyl-6-(2-phenylethyenyl)-pyridine reduce traumatic neuronal injury in vitro and in vivo by antagonizing N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2001;296:41–47. [PubMed] [Google Scholar]
- Muly EC, Maddox M, Smith Y. Distribution of mGluR1alpha and mGluR5 immunolabeling in primate prefrontal cortex. J Comp Neurol. 2003;467:521–535. doi: 10.1002/cne.10937. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Covington HE, 3rd, Miczek KA. Repeated self-administered cocaine “binges” in rats: Effects on cocaine intake and withdrawal. Psychopharmacology. 2001;154:292–300. doi: 10.1007/s002130000646. [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Durkin S, Jaeschke G, Spooren W. Stress-induced hypothermia: Effects of acute and repeated dosing of MPEP. Eur J Pharmacol. 2007;568:199–202. doi: 10.1016/j.ejphar.2007.04.034. [DOI] [PubMed] [Google Scholar]
- O’Leary DM, Movsesyan V, Vicini S, Faden AI. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol. 2000;131:1429–1437. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Meil WM, Kalivas PW. The NMDA antagonist, dizocilpine, enhances cocaine reinforcement without influencing mesoaccumbens dopamine transmission. Psychopharmacology. 1997;133:188–195. doi: 10.1007/s002130050390. [DOI] [PubMed] [Google Scholar]
- Platt DM, Carey GJ, Spealman RD. Intravenous self-administration techniques in monkeys. In: Enna S, Williams M, Ferkany J, Kenakin T, Porsolt R, Sullivam J, editors. Current Protocols in Neuroscience. Wiley; New York: 2005. pp. 9.21.1–9.21.15. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Balducci C, Koob GF. Dextromethorphan reduces intravenous cocaine self-administration in the rat. Eur J Pharmacol. 1997;321:279–283. doi: 10.1016/s0014-2999(96)00970-3. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, French E, Roberts DCS. Systemic pretreatment with MK-801 (dizocilpine) increases breaking points for self-administration of cocaine on a progressive-ratio schedule in rats. Psychopharmacology. 1996;128:83–88. doi: 10.1007/s002130050113. [DOI] [PubMed] [Google Scholar]
- Schenk S, Valadez A, Worley CM, McNamara C. Blockade of the acquisition of cocaine self-administration by the NMDA antagonist MK-801 (dizocilpine) Behav Pharmacol. 1993;4:652–659. [PubMed] [Google Scholar]
- Sevostianova N, Danysz W. Analgesic effects of mGlu1 and mGlu5 receptor antagonists in the rat formalin test. Neuropharmacology. 2006;51:623–630. doi: 10.1016/j.neuropharm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Shippenberg TS, Goldberg SR, Schindler CW. Behavioral studies with the glycine partial agonist (+)-HA966 on cocaine-induced locomotor activity and reinforcement. Behav Pharmacol. 1995;6:568–576. [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, Salt TE, Kuhn R. Novel allosteric antagonists shed light on mGlu(5) receptors and CNS disorders. Trends Pharmacol Sci. 2001;22:331–337. doi: 10.1016/s0165-6147(00)01694-1. [DOI] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Thomas LS, Jane DE, Gasparini F, Croucher MJ. Glutamate release inhibiting properties of the novel mGlu(5) receptor antagonist 2-methyl-6-(phenylethynyl)-pyrridine (MPEP): Complimentary in vitro and in vivo evidence. Neuropharmacology. 2001;41:523–527. doi: 10.1016/s0028-3908(01)00091-0. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Covington HE, 3rd, Gale MC, Datta R, Miczek KA. Behavioral sensitization due to social defeat stress in mice: Antagonism at mGluR5 and NMDA receptors. Psychopharmacology. 2005;179:230–239. doi: 10.1007/s00213-004-2023-3. [DOI] [PubMed] [Google Scholar]