Abstract
We previously found that statin users had a lower risk of advanced and possibly high-grade prostate cancer compared with nonusers. We hypothesize that statins' effects on cholesterol synthesis may explain those findings because prostate cancer cells exhibit cholesterol dysregulation. Thus, we investigated whether low plasma cholesterol is associated with prostate cancer overall and by stage and grade. Participants were drawn from the 18,018 members of the Health Professionals Follow-up Study who provided blood in 1993-1995. We ascertained 698 incident cases through January 2000. Controls were 698 men who had a PSA test and were matched to cases. Plasma cholesterol was measured enzymatically. Conditional logistic regression was used to estimate multivariable ORs and 95% CIs of total, clinically organ-confined (n=518), advanced (T3b or worse; n=61), low-grade (Gleason < 7; n=386), and high-grade (Gleason ≥ 7, n=247) disease. Low cholesterol (< vs ≥ 25th percentile), was not associated with total (OR = 0.93, 95% CI: 0.72-1.20), organ-confined (OR = 0.87, 95% CI: 0.64-1.18), or low-grade (OR = 1.06, 95% CI: 0.75-1.51) disease. However, men with low cholesterol had a lower risk of high-grade disease (OR = 0.61, 95% CI: 0.39-0.98), especially if organ-confined (OR = 0.54, 95% CI: 0.29-0.99). The association for advanced disease appeared inverse, but number of cases was small (OR = 0.42, 95% CI: 0.13-1.36). Associations remained after excluding cholesterol-lowering drug users. These results coupled with prior statins findings suggest that mechanistic studies on cholesterol metabolism should be pursued to understand a possible target for preventing poorly differentiated prostate cancers.
Keywords: prostate cancer, cholesterol, epidemiology, nested case-control study
Introduction
We previously observed in a large prospective cohort study that men who used statin drugs had half the risk of advanced prostate cancer compared with non-users.1 Risk of advanced prostate cancer and possibly high-grade disease decreased with longer duration of use of statin drugs. In addition, statins were inversely associated with advanced prostate cancer in three subsequent prospective cohort studies2-4 and in one case-control study nested in the Finnish population.5 In addition, a clinic-based case-control study observed an inverse association between statin drugs and total prostate cancer, with the association being stronger for high-grade prostate cancer than for low-grade prostate cancer.6 In contrast, a hospital-based case-control study observed no association between use of statins and prostate cancer overall or by stage.7 Some,8-10 but not all,11 other observational studies that have investigated statin use and prostate cancer have noted inverse associations; however, none of these studies commented on the association by cancer stage or grade. Clinical trials of statins and non-cancer outcomes in which cancers were evaluated as safety or secondary outcomes have not observed associations between statins and total prostate cancer, but these publications did not comment on stage or grade associations.11-13
Given the prospective findings, we hypothesize that one mechanism by which statin drugs influence the risk of advanced and possibly high-grade prostate cancer is via cholesterol-lowering, although other mechanisms are possible. Prostate cancer cells exhibit cholesterol feedback dysregulation and may be particularly susceptible to cholesterol lowering.14 In the 1980s and early 1990s a spate of articles was published investigating the purported link between low serum cholesterol and a higher risk of incident or fatal cancer.15-24 Some of these studies and those more recently published included organ-site specific analyses; variable results have been reported for the association between serum cholesterol and total prostate cancer.21, 23, 25-29 None of these studies evaluated circulating cholesterol and prostate cancer by stage or grade, although those published before the PSA era likely included cases with a stage distribution shifted toward more advanced.
Thus, to begin to address one pathway possibly underlying an inverse association between statins and the development of prostate cancer that has a greater potential to metastasize and cause death, we evaluated the association of circulating plasma cholesterol concentration with prostate cancer overall and by stage and grade in a case-control study nested in the same cohort in which we made the statins and advanced prostate cancer observation previously.1 We hypothesized that men with low plasma cholesterol would have a lower risk of prostate cancer that is of a higher stage or grade at diagnosis than men with higher concentrations, but that there would be no association with low stage and grade prostate cancer.
Material and methods
Study population
Incident prostate cancer cases and controls were identified among members of the Health Professionals Follow-up Study. In 1986, 51,529 U.S. men aged 40-75 years enrolled in this prospective cohort. At baseline and then again every two years the men completed a mailed questionnaire on demographics, lifestyle, and medical history. At baseline and then again every four years they completed a semi-quantitative food frequency questionnaire. The overall response rate to the biennial mailed questionnaires was 94%. Deaths were reported by family members or the postal system in response to the mailed follow-up questionnaires, or were identified through a search of the National Death Index.30 Between 1993 and 1995, 18,018 of the men provided a blood specimen. The blood was collected in tubes containing sodium EDTA and was shipped by overnight courier while chilled. On receipt, the blood was centrifuged, aliquotted into plasma, erythrocytes, and buffy coat and stored in liquid nitrogen freezers. Men who had a diagnosis of any cancer, except nonmelanoma skin cancer, prior to the date of blood draw were excluded from the analysis. This study was approved by the Human Subjects Committee at the Harvard School of Public Health and this analysis was additionally approved by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health.
Prostate cancer cases and controls
For each man who reported a prostate cancer diagnosis on a follow-up questionnaire or when prostate cancer was mentioned on the death certificate we sought medical and pathology records. Study investigators blinded to information from the questionnaires reviewed the records to confirm adenocarcinoma of the prostate and to abstract clinical presentation, stage, and Gleason sum. For the purpose of analysis by stage, we categorized cases based on clinical staging as advanced (regionally invasive or metastatic: ≥ T3b, N1, or M1) or organ-confined (T1b to T2 and N0 and M0) disease; we omitted T3a cases from these categories to maximize the contrast, but included them in the total prostate cancer analysis. For the purpose of analysis by clinical grade we categorized cases as high-grade (Gleason sum ≥ 7) or low-grade (Gleason sum < 7) disease. We excluded incidental microscopic focal tumors (T1a) from the analysis altogether because they are generally indolent and are susceptible to detection bias due to differential rates of surgery for benign prostatic hyperplasia. We identified 698 eligible non-T1a prostate cancer cases between the date of blood draw and January 31, 2000, 96.1% of which were confirmed by medical record review. We included the small proportion of self-reported cases because we found the reporting of a prostate cancer diagnosis to be accurate in this cohort of health professionals.
For each case, we selected one control who was alive and not diagnosed with cancer by the date of the matched case's diagnosis, and who reported on a follow-up questionnaire that he had a PSA test after the date of blood draw. This latter criterion was used to ensure that controls had the opportunity to have an occult prostate cancer diagnosed. The matching criteria were: year of birth (± 1 yr), PSA test prior to blood draw (yes/no), and timing of blood draw – time of day (midnight to before 9 am, 9 am to before noon, noon to before 4 pm, 4 pm to before midnight), season (winter, spring, summer, fall), and year (exact).
Laboratory assays
Case-control pairs were analyzed together in the laboratory of Dr. Steven Clinton at The Ohio State University. Laboratory personnel were blinded to case-control status. Cases diagnosed from the date of blood draw through January 1996 and their matched controls, cases diagnosed from February 1996 through January 1998 and their matched controls, and cases diagnosed from February 1998 through January 2000 and their matched controls were assayed in separate analytical runs. Plasma total cholesterol concentration was measured using an Infinity Total Cholesterol Enzymatic Assay kit (Sigma Diagnostics, St. Louis, MO) for the first and second analytical runs. For the third run, the Infinity kit was not available, so instead we performed an enzymatic assay using reagents from Equal Diagnostics (Exton, PA). Combined over the runs, the mean intra-pair coefficient of variation for cholesterol calculated from blinded quality control samples was 10.9% for the Infinity assay and 9.1% for the Equal Diagnostics assay.
Statistical analysis
We compared mean plasma cholesterol concentration between matched cases and controls using the paired t-test. We used conditional logistic regression to estimate matched odds ratios (OR) and 95% confidence intervals (CI) of total prostate cancer and of advanced, organ-confined, high-grade, and low-grade disease. In another analysis, we restricted the cases to those that were organ-confined. To test for trend, we entered into the model a single ordinal variable with values of 1 to 4 corresponding to the quartile into which an individual fell.
We used indicator variables for quartiles of the plasma cholesterol concentration, with cutpoints based on the distribution among the controls. We used separate quartile cutpoints defined by analytical run because the samples were assayed in three analytical runs and using two assay methods. The shape (normally distributed) and the standard deviation of the cholesterol concentration (1996: 39.8 mg/L; 1998 41.9 mg/L; 2000 38.1 mg/L) measured in the controls was the same in the three analytical batches. However, the mean concentrations in controls differed between the batches run with the Infinity kit (1996: 217.9 mg/L; 1998: 213.9 mg/L) and the batch run using the reagents from Equal Diagnostics (2000: 166.1 mg/L). We could not find any systematic differences between the characteristics of the controls in the third batch versus those in the first and second batches, and thus, we conclude that the variation was due to assay methods differences and that combining the three batches based on their batch-specific ranks would yield correct quartile ranking. Because the mean concentrations differed by kit, we do not report on clinical cutpoints for plasma total cholesterol concentrations in relation to prostate cancer.
Possible confounders considered were those factors previously founded to be associated with total, advanced, or high-grade prostate cancer in this cohort 31: father or brother with prostate cancer, height, vigorous physical activity, diabetes mellitus, vasectomy, cigarette smoking in the past 10 years, intake of energy, red meat, fish, and alcohol, intake of energy-adjusted lycopene, calcium, fructose, and α-linolenic acid, and supplemental vitamin E and selenium.
In a subanalysis, we excluded men (64 cases and 86 controls) who reported ever using cholesterol-lowering drugs on any biennial questionnaire through 1994, broke the matching, and ran logistic regression models. To evaluate whether the associations varied by age at diagnosis (< 64 (25th percentile) versus ≥ 64 years old), family history of prostate cancer through 1994 (yes/no), or body mass index in 1994 (< 25 versus ≥ 25 kg/m2), we ran stratified conditional logistic regression models (age at diagnosis) or ran stratified logistic regression models adjusting for the matching factors age and PSA test prior to blood draw, and the other covariates. To test for statistical interaction, we entered into the appropriate multivariable model the main effect terms for low cholesterol and the covariate (binary) along with a term for their product, the coefficient for which we evaluated using the Wald test. All analyses were conducted using SAS release 9.1 (SAS Institute, Cary, NC). We report two-sided p-values for all hypothesis tests.
Results
The median age at prostate cancer diagnosis was 68.9 ± 7.3 years (range 47.7-84.3 years). Of the 90.5% of cases for whom stage and grade were known, 82.1% were organ confined at diagnosis (T1b-T2b) and the Gleason sum distribution was 2-5: 22.3%, 6: 38.8%, 7: 29.0%, and 8+: 10.0%. The mean time between blood draw for cholesterol analysis and prostate cancer diagnosis was 3.1 ± 1.8 years. Compared with controls, cases were more likely to have a family history of prostate cancer (p = 0.02), consumed slightly more saturated fat (p = 0.11), and consumed fish slightly less frequently (p = 0.05), but otherwise were similar on lifestyle and other dietary factors (Table I). High-grade cases were slightly older (p = 0.08), were more likely to be white (p = 0.11), were taller (p = 0.05), were more likely to use a vitamin E supplement (p = 0.03), and had a higher intake of energy than low-grade cases (p = 0.07) (Table I).
Table I.
Characteristics1 of 698 prostate cancer cases and 698 matched controls nested in the health professionals follow-up study
| Prostate cancer cases | Controls | p3 | p4 | |||
|---|---|---|---|---|---|---|
| Total | High grade2 | Low grade2 | ||||
| No. | 698 | 247 | 386 | 698 | ||
| Age at blood draw (yr) | 65.8 ± 7.4 | 66.1 ± 7.2 | 65.1 ± 7.4 | 65.7 ± 7.4 | Matched | 0.08 |
| White (%) | 94.6 | 93.1 | 96.1 | 94.1 | 0.81 | 0.11 |
| Family history of prostate cancer through 1996 (%) | 21.9 | 21.1 | 22.8 | 17.1 | 0.02 | 0.66 |
| Height in 1986 (in) | 70.1 ± 2.6 | 70.3 ± 2.6 | 69.9 ± 2.7 | 70.1 ± 2.6 | 0.60 | 0.05 |
| Body mass index at age 21 (kg/m2) | 22.8 ± 2.7 | 22.7 ± 2.7 | 22.8 ± 2.7 | 22.9 ± 2.7 | 0.23 | 0.99 |
| Current body mass index (kg/m2) | 25.9 ± 3.6 | 25.9 ± 3.7 | 25.7 ± 3.1 | 25.9 ± 3.5 | 0.76 | 0.58 |
| Vigorous physical activity (MET-hr/wk) | 12.7 ± 23.0 | 11.7 ± 19.6 | 13.6 ± 26.0 | 12.5 ± 23.6 | 0.90 | 0.43 |
| Cigarette smoker in the past 10 years (%) | 16.2 | 15.8 | 16.3 | 17.5 | 0.56 | 0.88 |
| Diabetes mellitus (%) | 5.7 | 6.5 | 4.9 | 5.4 | 0.91 | 0.49 |
| Vasectomy (%) | 25.9 | 28.7 | 25.1 | 26.5 | 0.85 | 0.18 |
| Supplement user | ||||||
| Vitamin E (%) | 38.3 | 42.5 | 33.7 | 35.0 | 0.23 | 0.03 |
| Selenium (%) | 6.9 | 6.1 | 6.5 | 7.6 | 0.69 | 0.80 |
| Calcium (%) | 17.2 | 17.4 | 16.3 | 15.9 | 0.56 | 0.89 |
| Energy-adjusted mean intake | ||||||
| Calcium (mg/day) | 950 ± 395 | 945 ± 375 | 949 ± 400 | 953 ± 426 | 0.89 | 0.72 |
| Lycopene (μg/day) | 7538 ± 7608 | 7342 ± 6358 | 7594 ± 8600 | 7386 ± 4881 | 0.66 | 0.81 |
| Saturated fat (g/day) | 22.1 ± 6.1 | 22.3 ± 6.1 | 22.1 ± 6.0 | 21.6 ± 6.3 | 0.11 | 0.67 |
| α–linolenic acid (g/day) | 1.05 ± 0.27 | 1.05 ± 0.28 | 1.06 ± 0.27 | 1.05 ± 0.28 | 0.91 | 0.49 |
| Energy intake (kcal/day) | 2010 ± 587 | 2087 ± 599 | 1998 ± 574 | 2035 ± 621 | 0.43 | 0.07 |
| Red meat intake (servings/week) | 7.1 ± 4.5 | 7.3 ± 4.6 | 7.2 ± 4.5 | 7.1 ± 4.9 | 0.94 | 0.84 |
| Fish intake (servings/week) | 2.2 ± 1.5 | 2.1 ± 1.3 | 2.2 ± 1.6 | 2.3 ± 1.7 | 0.05 | 0.22 |
| Alcohol intake (g/day) | 11.8 ± 14.6 | 12.8 ± 15.2 | 11.2 ± 14.1 | 11.5 ± 15.0 | 0.68 | 0.16 |
Cases and controls were matched on age, time of day and year of blood draw, and PSA test prior to blood draw. Controls all had a PSA test after blood draw. Unless otherwise indicated, values are from the 1994 follow-up questionnaire. Red meat and fish intake are averages of reports in 1986, 1990, and 1994. Diabetes mellitus includes cases through 1994.
High grade was defined as Gleason sum ≥ 7. Low grade was defined as Gleason sum < 7. Gleason sum was not available for 65 cases.
For the hypothesis test of no difference in means (paired t-test) or proportions (McNemar's test) between total prostate cancer cases and controls. All tests are 2-sided.
For the hypothesis test of no difference in means (t-test) or proportions (Chi-square test) between high- and low-grade cases. All tests are 2-sided.
Among the controls and adjusting for age, compared with men who had higher cholesterol, men who had low cholesterol were leaner (25.5 vs. 26.1 kg/m2, p = 0.03), drank less alcohol (8.6 vs. 12.6 g/day, p = 0.005), were less likely to have smoked in the past 10 years (12.3 vs. 19.1%, p = 0.06), were less likely to have ever had high cholesterol (27.5 vs. 55.1%, p < 0.0001) or triglycerides (18.0 vs. 35.8%, p < 0.0001) although they were similar (all p > 0.4) on intake of energy (2038 vs. 2035 kcal/day), cholesterol (249.0 vs. 251.1 mg/day), total fat (65.5 vs. 66.3 g/day), saturated fat (21.2 vs. 21.7 g/day), and polyunsaturated fat (11.9 vs. 11.8 g/day).
Mean pre-diagnostic plasma cholesterol concentration did not differ between the prostate cancer cases and the controls (Table II). Cases were less likely to have a history of elevated cholesterol (42.8% versus 48.7%; p = 0.03) and were less likely to have ever used cholesterol-lowering medications than controls (9.2% versus 12.3%; p = 0.07) (Table II). There was no association by quartile of plasma cholesterol with total prostate cancer, clinically organ-confined disease, or low-grade disease either before or after multivariable adjustment (Table III). However, risk of high-grade prostate cancer was greater in each of the top three quartiles of total plasma cholesterol than in the bottom quartile (Table III). For advanced disease, men in the top quartile of plasma cholesterol did not have an increased risk when compared to the bottom quartile, although there was a suggestion that men in the middle two quartiles were at increased risk.
Table II.
Mean plasma cholesterol concentration, dietary cholesterol intake, and history of elevated cholesterol in prostate cancer cases and matched controls nested in the health professionals follow-up study
| Prostate cancer cases | Controls | p2 | p3 | |||
|---|---|---|---|---|---|---|
| Cases | High grade1 | Low grade1 | ||||
| No. | 698 | 247 | 386 | 698 | ||
| Plasma cholesterol (mg/dL)4 | 198.5 ± 42.6 | 199.3 ± 44.6 | 196.2 ± 42.1 | 198.8 ± 46.4 | 0.87 | 0.44 |
| Energy-adjusted cholesterol intake (mg/day) | 250.2 ± 98.6 | 253.2 ± 86.7 | 249.7 ± 106.0 | 251.1 ± 111.1 | 0.87 | 0.69 |
| Ever had a serum cholesterol test (%) | 99.0 | 99.1 | 99.0 | 98.7 | 0.78 | 0.73 |
| History of elevated serum cholesterol (%) | 42.8 | 39.3 | 43.0 | 48.7 | 0.03 | 0.35 |
| History of elevated triglycerides (%) | 28.7 | 29.2 | 26.7 | 31.2 | 0.33 | 0.52 |
| Ever use of cholesterol-lowering drugs (%) | 9.2 | 8.9 | 8.8 | 12.3 | 0.07 | 0.98 |
| Use of cholesterol-lowering drugs in 1994 (%) | 7.6 | 6.5 | 7.5 | 9.0 | 0.38 | 0.59 |
High grade was defined as Gleason sum ≥ 7. Low grade was defined as Gleason sum < 7. Gleason sum was not available for 65 cases.
For the hypothesis test of no difference in means (paired t-test) or proportions (McNemar's test) between total prostate cancer cases and controls. All tests are 2-sided.
For the hypothesis test of no difference in means (t-test) or proportions (Chi-square test) between high- and low-grade cases. All tests are 2-sided.
Case-control pairs were run in 3 batches; the same assay method was used for the 1996 and 1998 batches and a different assay method was used for the 2000 batch. Means ± standard deviations in the cases and controls by batch were: 1996: case 218.5 ± 37.8, control 217.9 ± 39.8 mg/L; 1998: case 211.7 ± 38.0, control 213.9 ± 41.9 mg/L; and 2000 case 166.9 ± 32.1, control 166.1 ± 38.1 mg/L.
Table III.
Odds ratio of prostate cancer by plasma cholesterol concentration, 698 matched pairs nested in the health professionals follow-up study
| Prostate cancer | Quartile of plasma cholesterol | p trend | Q1 versus above4 | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| Total | ||||||
| No. cases / controls | 162 / 173 | 173 / 175 | 189 / 176 | 174 / 174 | 162/173 versus 536/525 | |
| OR1 | 1.00 | 1.06 | 1.15 | 1.07 | 0.56 | 0.92 |
| OR2 | 1.00 | 1.04 | 1.15 | 1.05 | 0.63 | 0.93 |
| 95% CI | Reference | 0.76-1.41 | 0.85-1.56 | 0.77-1.43 | 0.72-1.20 | |
| OR3 | 1.00 | 1.02 | 1.15 | 1.05 | 0.60 | 0.93 |
| 95% CI | Reference | 0.74-1.41 | 0.84-1.59 | 0.76-1.45 | 0.72-1.21 | |
|
| ||||||
| Organ-confined | ||||||
| No. cases / controls | 114/128 | 131/134 | 143/130 | 130/126 | 114/128 versus 404/390 | |
| OR1 | 1.00 | 1.10 | 1.24 | 1.16 | 0.33 | 0.86 |
| OR2 | 1.00 | 1.04 | 1.27 | 1.14 | 0.31 | 0.87 |
| 95% CI | Reference | 0.75-1.50 | 0.88-1.82 | 0.79-1.65 | 0.64-1.18 | |
| OR3 | 1.00 | 1.03 | 1.23 | 1.10 | 0.44 | 0.89 |
| 95% CI | Reference | 0.71-1.50 | 0.85-1.79 | 0.75-1.61 | 0.66-1.22 | |
|
| ||||||
| Advanced | ||||||
| No. cases / controls | 14 / 17 | 19 / 18 | 15 / 11 | 13 / 15 | 14/17 versus 47/44 | |
| OR1 | 1.00 | 1.31 | 1.69 | 1.05 | 0.80 | 0.75 |
| OR2 | 1.00 | 3.58 | 3.22 | 1.13 | 0.65 | 0.42 |
| 95% CI | Reference | 0.82-15.7 | 0.64-16.2 | 0.25-5.17 | 0.13-1.36 | |
| OR3 | 1.00 | 1.63 | 2.60 | 1.15 | 0.75 | 0.60 |
| 95% CI | Reference | 0.52-5.05 | 0.70-9.63 | 0.32-4.20 | 0.23-1.61 | |
|
| ||||||
| Low grade | ||||||
| No. cases / controls | 93 / 93 | 97 / 104 | 111 / 91 | 85 / 98 | 93/93 versus 293/293 | |
| OR1 | 1.00 | 0.93 | 1.23 | 0.87 | 0.85 | 1.00 |
| OR2 | 1.00 | 0.86 | 1.19 | 0.83 | 0.75 | 1.06 |
| 95% CI | Reference | 0.56-1.29 | 0.78-1.83 | 0.54-1.27 | 0.75-1.51 | |
| OR3 | 1.00 | 0.89 | 1.18 | 0.81 | 0.66 | 1.05 |
| 95% CI | Reference | 0.58-1.39 | 0.77-1.83 | 0.52-1.27 | 0.73-1.50 | |
|
| ||||||
| High grade | ||||||
| No. cases / controls | 50 / 68 | 63 / 57 | 63 / 61 | 71 / 61 | 50/68 versus 197/179 | |
| OR1 | 1.00 | 1.53 | 1.40 | 1.60 | 0.11 | 0.67 |
| OR2 | 1.00 | 1.79 | 1.49 | 1.68 | 0.13 | 0.61 |
| 95% CI | Reference | 1.00-3.20 | 0.87-2.55 | 0.96-2.96 | 0.39-0.98 | |
| OR3 | 1.00 | 1.38 | 1.48 | 1.47 | 0.17 | 0.69 |
| 95% CI | Reference | 0.80-2.40 | 0.85-2.57 | 0.85-2.54 | 0.44-1.08 | |
Estimated from a conditional logistic regression model.
Estimated from a conditional logistic regression model and adjusted for family history of prostate cancer, height, vigorous physical activity, diabetes mellitus, vasectomy, cigarette smoking in the past 10 years, intake of energy, red meat, fish, and alcohol, intake of energy-adjusted lycopene, calcium, fructose, and α-linolenic acid, and supplemental vitamin E and selenium.
Estimated from a logistic regression model after excluding men who ever used cholesterol-lowering drugs and adjusted for age, PSA test before blood draw, family history of prostate cancer, height, vigorous physical activity, diabetes mellitus, vasectomy, cigarette smoking in the past 10 years, intake of energy, red meat, fish, and alcohol, intake of energy-adjusted lycopene, calcium, fructose, and α-linolenic acid, and supplemental vitamin E and selenium.
Cutpoints differed by analytical batch (see Material and methods for details): 1996 192.8 mg/dL, 1998 185.7 mg/dL, and 2000 140.1 mg/dL).
Because we hypothesized that low plasma cholesterol would be associated with lower risk of prostate cancer, and given the patterns across the quartiles we observed, we evaluated the association between low plasma cholesterol, defined as below the 25th percentile (versus at or above; cutpoints: 1996 192.8 mg/dL, 1998 185.7 mg/dL, and 2000 140.1 mg/dL), and prostate cancer (Table III). Inverse associations for low plasma cholesterol were observed with high-grade disease (OR = 0.61, 95% CI: 0.39-0.98) and possibly with advanced disease (OR = 0.42, 95% CI: 0.13-1.36), but not with prostate cancer overall, organ-confined disease, or low-grade disease. The associations for low cholesterol persisted after excluding men who were diagnosed with prostate cancer within two years of blood draw (data not shown).
To determine whether the inverse association of low cholesterol with high-grade and advanced disease was merely due the use of statins or other cholesterol-lowering drugs by the men who had low cholesterol, we reran the analysis excluding men who had ever used any cholesterol-lowering medications (Table III). The associations with high-grade (OR = 0.69, 95% CI: 0.44-1.08) and advanced disease (OR = 0.60, 95% CI: 0.23-1.61) remained inverse, albeit not statistically significant, among never users and null for the other endpoints. Among men who had ever used cholesterol-lowering drugs, the association for low cholesterol and total prostate cancer was similar to that among never users (data not shown).
This inverse association for low cholesterol was present in men with prostate cancer that was both high grade and organ-confined, even after excluding men who used cholesterol-lowering drugs; there was no association for men who had both low-grade and organ-confined disease (Figure 1).
FIGURE 1.
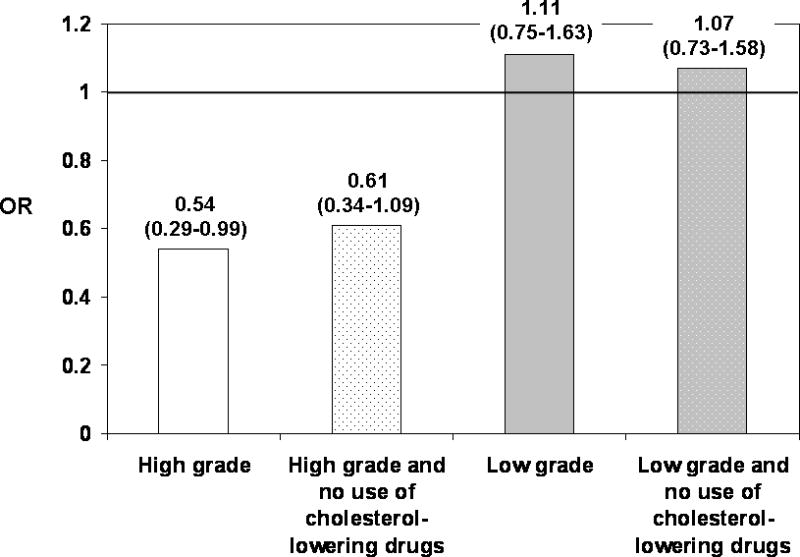
Association of low plasma cholesterol with high and low-grade prostate cancer restricted to men with organ-confined disease. Low plasma cholesterol was defined as below the 25th percentile of the distribution of plasma cholesterol concentration among controls. The analysis included: 167 high-grade organ-confined case-control pairs (excluding ever use of cholesterol-lowering drugs 152 cases, 153 controls) and 333 low-grade organ-confined case-control pairs (excluding ever use of cholesterol-lowering drugs 304 cases, 292 controls). The ORs of high-grade organ-confined disease and low-grade organ-confined disease were estimated from a conditional logistic regression model and adjusted for family history of prostate cancer, height, vigorous physical activity, diabetes mellitus, vasectomy, cigarette smoking in the past 10 years, intake of energy, red meat, fish, and alcohol, intake of energy-adjusted lycopene, calcium, fructose, and α-linolenic acid, and supplemental vitamin E and selenium. The ORs of these endpoints after excluding ever users of cholesterol-lowering drugs were estimated from a logistic regression model and adjusted for age, PSA test before blood draw, and the other covariates listed for the conditional logistic regression model.
Restricting the analysis to cases only, no association was observed for low plasma cholesterol when comparing advanced- with organ-confined disease (OR = 1.01, 95% CI: 0.52-1.99; excluding cholesterol-lowering drug users: OR = 1.08, 95% CI: 0.53-2.13). When comparing high- with low-grade disease, a modest inverse association for low plasma cholesterol (OR = 0.75, 95% CI: 0.50-1.12; excluding cholesterol-lowering drug users: OR = 0.86, 95% CI: 0.57-1.30) was suggested, which was enhanced when restricting the high-grade and low-grade cases to those with organ-confined disease (OR = 0.50, 95% CI: 0.30-0.84; excluding cholesterol-lowering drug users: OR = 0.58, 95% CI: 0.35-0.99).
The null association of low plasma cholesterol with total prostate cancer and the inverse association with high-grade prostate cancer did not differ by age at diagnosis (p interaction = 0.46, 0.91, respectively), family history of prostate cancer (p interaction = 0.44, 0.25), or body mass index (p interaction = 0.80, 0.26).
Discussion
We observed that men whose plasma cholesterol concentration was in the bottom quartile had a lower risk of high-grade prostate cancer and possibly advanced disease, but not prostate cancer overall or prostate cancer that was clinically organ-confined or low-grade. This association for high-grade disease was more pronounced when restricting to cancers that were confined within the prostate. The inverse associations for high-grade and advanced disease were not fully explained by use of cholesterol-lowering drugs. Our results indirectly suggest that cholesterol metabolism may be a target for preventing the development of poorly differentiated prostate cancers and that one mechanism by which statin drugs may influence the development of such prostate cancers by reducing circulating cholesterol concentrations.
Few prospective studies have investigated the association between blood cholesterol and prostate cancer. Some studies reported no association between cholesterol and prostate cancer incidence24, 26, 27, 32 or mortality.28 Other studies have reported that the risk of prostate cancer decreased with increasing cholesterol and/or that risk of prostate cancer was elevated in men with low cholesterol overall21, 25 or in a subgroup.28 Low cholesterol was typically defined as the bottom quartile or quintile of the distribution for the given population. Aside from the large study by Tulinius et al.,27 these findings were based on fewer than 100 prostate cancer cases. The findings from our study may not be comparable to those published in the past because our study was conducted in the era of routine screening for elevated PSA in the U.S. and during a time when serum cholesterol concentrations have been declining.33 Further, compared with the early studies reporting on cholesterol and death from prostate cancer and that had relatively short follow-up, we likely minimized bias due to reverse causation by studying cases that had an aggressive phenotype (i.e., early high-grade disease).
How circulating cholesterol level or cholesterol-lowering might influence risk of advanced or high-grade prostate cancer could be related to many of the functions cholesterol has in cells and a developing cancer. Cholesterol is a critical structural component of cellular membranes that serves to modulate fluidity and the interactions among many proteins, including those involved in cell survival signaling such as Akt34-36. Other cholesterol-dependent pathways include sonic hedgehog signal transduction pathway, which is active in metastatic prostate cancer37. Whether the difference in circulating cholesterol concentration that we observed between the bottom quartile and the top three quartiles or that would occur with use of statins (17-35% decline38) would influence cholesterol-dependent pathways and thus risk of prostate cancer with a poorer prognosis is unclear. A pathway that is unlikely to explain our findings is androgen production from plasma cholesterol: circulating cholesterol concentration is far greater than steroid hormone concentrations and because a recent study found no difference in androgen concentrations between statin users and non-users in a large cross-sectional study.39
The strengths of this study include its prospective design, large size, and rich covariate information. Also, the men in this cohort, who were on average 66 years old at the time of blood draw in 1993-1994, had a mean plasma cholesterol concentration (216 ± 41.0 mg/dL in the controls assayed using the Infinity assay kit) that was comparable to that in the same aged men over the same time interval nationally (216 and 214 in men 50-59 and 60-74 years old).33 We defined low cholesterol as below the bottom quartile of the cholesterol distribution among the controls. Based on the two case-control batches for which cholesterol was measured by the Infinity kit (1996: 192.8 mg/dL, 1998: 185.7 mg/dL) the bottom quartile was below the clinical cutpoint (200 mg/dL) for borderline high total cholesterol.40
We restricted controls to men who had had a PSA test after blood collection for cholesterol evaluation; by doing so, we likely limited observation bias that might have resulted if opportunity for disease detection was differential by factors that influence cholesterol. Because of widespread PSA screening in this cohort there were few advanced cases at diagnosis in which to evaluate the cholesterol association with precision and it precluded some subgroup analyses. Although we did not independently confirm clinical staging or Gleason grading, we do not believe that major error occurred when classifying cases into extremes of disease characteristics. In addition, misclassification of grade could not explain our results because errors in grading would have tended to attenuate associations rather than to accentuate associations.
Rather than relying on self-reported serum cholesterol or an estimate of dietary cholesterol intake, we measured plasma total cholesterol concentration in blood samples that were drawn months to years prior to prostate cancer diagnosis. Although we cannot rule out reverse causation, we do not expect that extant but not yet detected prostate cancers at the time blood was collected would have influenced circulating cholesterol concentration given that PSA-detected cancers tend to be early stage and small volume disease. Circulating cholesterol is an integrated measure of endogenously produced cholesterol and intake of cholesterol from the diet. We measured plasma total cholesterol, rather than separately cholesterol carried as part of low (LDL) versus high density lipoproteins (HDL). It is possible to speculate that lipoprotein profiles may better capture the amount of cholesterol produced by the liver and available to prostate cells. However, cholesterol is produced endogenously by many cells in various tissues, including by the prostate. We do not understand the dynamics of circulating cholesterol and prostate cell cholesterol synthesis as contributing to the intracellular cholesterol necessary for structural components or signaling, and how this may change during prostate carcinogenesis.
We used two total cholesterol assay methods for this study because the kit we used for the first two analytical batches was not available at the time we ran the third analytical batch. Although the two assay methods yielded notably different means, the distributions (shape and spread) were comparable and thus the correct ranking of individuals with respect to cholesterol concentration was maintained. For this reason, we did not report the OR of prostate cancer for cutpoints used clinically for treatment recommendations. Nevertheless, the association between low plasma total cholesterol (bottom quartile versus at or above) and high-grade prostate cancer was similar when restricting the analysis to case-control pairs that were assayed in the first two analytical batches for which we used the same assay (156 pairs; multivariable OR 0.58, 95% CI 0.32-1.05) and the third batch only (91 pairs; multivariable OR = 0.67, 95% CI: 0.28-1.63) as when we included all 3 batches as given as the primary results from this study (698 pairs; OR = 0.61, 95% CI: 0.39-0.98).
In summary, our prospective findings suggest that low cholesterol may decrease the risk of high-grade prostate cancer. The results of the present study coupled with ours and others' findings for statins, suggest a line of mechanistic studies on cholesterol metabolism that should be pursued as a possible target for preventing clinically important prostate cancers.
Acknowledgments
We thank the research staff of the Health Professionals Follow-up study for their continued help in conducting the study.
Grant sponsor: The Health Professionals Follow-up Study is supported by Public Health Service research grants CA55075 and CA72036 (National Cancer Institute) and HL35464 (National Heart, Lung, and Blood Institute) from the National Institutes of Health, Department of Health and Human Services (Harvard). This analysis was also supported by Public Health Service research grant P50 CA58236 from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (Hopkins). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Abbreviations
- PSA
prostate specific antigen
- CI
confidence interval
- OR
odds ratio
- EDTA
ethylenediaminetetraacetic acid
- LDL
low-density lipoprotein
- HDL
high-density lipoprotein
Footnotes
Novelty of paper: This is the first study to specifically examine the association of plasma cholesterol and prostate cancer by stage and grade at diagnosis as a possible mechanism underlying the emerging consistent inverse association between statins, a class of cholesterol-lowering drugs, and advanced and high-grade prostate cancer.
Impact of paper: The present study coupled with the prior findings for statins, suggest a line of mechanistic studies on cholesterol metabolism that should be pursued for understanding a possible target for the prevention of poorly differentiated prostate cancers. The clinical import of our two studies is the suggestion that cholesterol metabolism may be a possible target for preventing the development of advanced and poorly differentiated prostate cancers.
References
- 1.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213–7. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 3.Flick ED, Habel LA, Chan KA, Van Den Eeden SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry CP, Jr, Sternfeld B, Jacobsen SJ, et al. Statin use and risk of prostate cancer in the California Men's Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2218–25. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 4.Friedman GD, Flick ED, Udaltsova N, Chan Pharm DJ, Quesenberry CP, Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361 859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 5.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–32. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 6.Shannon J, Tewoderos S, Garzotto M, Beer TM, Derenick R, Palma A, Farris PE. Statins and prostate cancer risk: A case-control study. Am J Epidemiol. 2005;162:318–25. doi: 10.1093/aje/kwi203. [DOI] [PubMed] [Google Scholar]
- 7.Coogan PF, Rosenberg L, Strom BL. Statin use and the risk of 10 cancers. Epidemiology. 2007;18:213–9. doi: 10.1097/01.ede.0000254694.03027.a1. [DOI] [PubMed] [Google Scholar]
- 8.Singal R, Khurana V, Caldito G, Fort C. Statins and prostate cancer risk: a large case-control study in veterans; 2005 American Society of Clinical Oncology Annual Meeting; 2005. [Google Scholar]
- 9.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–94. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, Gaziano JM. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100:134–9. doi: 10.1093/jnci/djm286. [DOI] [PubMed] [Google Scholar]
- 11.Browning DR, Martin RM. Statins and risk of cancer: A systematic review and metaanalysis. Int J Cancer. 2007;120:833–43. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 12.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 13.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 14.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 15.Rose G, Shipley MJ. Plasma lipids and mortality: a source of error. Lancet. 1980;1:523–6. doi: 10.1016/s0140-6736(80)92775-0. [DOI] [PubMed] [Google Scholar]
- 16.Williams RR, Sorlie PD, Feinleib M, McNamara PM, Kannel WB, Dawber TR. Cancer incidence by levels of cholesterol. JAMA. 1981;245:247–52. [PubMed] [Google Scholar]
- 17.Garcia-Palmieri MR, Sorlie PD, Costas R, Jr, Havlik RJ. An apparent inverse relationship between serum cholesterol and cancer mortality in Puerto Rico. Am J Epidemiol. 1981;114:29–40. doi: 10.1093/oxfordjournals.aje.a113171. [DOI] [PubMed] [Google Scholar]
- 18.Keys A, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Karvonen MJ, Menotti A, Nedeljkovic S, Punsar S, Toshima H. Serum cholesterol and cancer mortality in the Seven Countries Study. Am J Epidemiol. 1985;121:870–83. doi: 10.1093/oxfordjournals.aje.a114057. [DOI] [PubMed] [Google Scholar]
- 19.Schatzkin A, Hoover RN, Taylor PR, Ziegler RG, Carter CL, Larson DB, Licitra LM. Serum cholesterol and cancer in the NHANES I epidemiologic followup study. National Health and Nutrition Examination Survey. Lancet. 1987;2:298–301. doi: 10.1016/s0140-6736(87)90890-7. [DOI] [PubMed] [Google Scholar]
- 20.Sherwin RW, Wentworth DN, Cutler JA, Hulley SB, Kuller LH, Stamler J. Serum cholesterol levels and cancer mortality in 361,662 men screened for the Multiple Risk Factor Intervention Trial. JAMA. 1987;257:943–8. [PubMed] [Google Scholar]
- 21.Knekt P, Reunanen A, Aromaa A, Heliovaara M, Hakulinen T, Hakama M. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. J Clin Epidemiol. 1988;41:519–30. doi: 10.1016/0895-4356(88)90056-x. [DOI] [PubMed] [Google Scholar]
- 22.Neaton JD, Blackburn H, Jacobs D, Kuller L, Lee DJ, Sherwin R, Shih J, Stamler J, Wentworth D. Serum cholesterol level and mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med. 1992;152:1490–500. [PubMed] [Google Scholar]
- 23.Schuit AJ, Van Dijk CE, Dekker JM, Schouten EG, Kok FJ. Inverse association between serum total cholesterol and cancer mortality in Dutch civil servants. Am J Epidemiol. 1993;137:966–76. doi: 10.1093/oxfordjournals.aje.a116769. [DOI] [PubMed] [Google Scholar]
- 24.Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse, and physical activity. Cancer Epidemiol Biomarkers Prev. 1995;4:807–11. [PubMed] [Google Scholar]
- 25.Morris DL, Borhani NO, Fitzsimons E, Hardy RJ, Hawkins CM, Kraus JF, Labarthe DR, Mastbaum L, Payne GH. Serum cholesterol and cancer in the Hypertension Detection and Follow-up Program. Cancer. 1983;52:1754–9. doi: 10.1002/1097-0142(19831101)52:9<1754::aid-cncr2820520933>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Schatzkin A, Hoover R, Taylor P, Ziegler R, Carter C, Albanes D, Larson D, Licitra L. Site-specific analysis of total serum cholesterol and incidence cancer in the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Cancer Res. 1988;48:452–8. [PubMed] [Google Scholar]
- 27.Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6:863–73. [PubMed] [Google Scholar]
- 28.Eichholzer M, Stahelin HB, Gutzwiller F, Ludin E, Bernasconi F. Association of low plasma cholesterol with mortality for cancer at various sites in men: 17-y follow-up of the prospective Basel study. Am J Clin Nutr. 2000;71:569–74. doi: 10.1093/ajcn/71.2.569. [DOI] [PubMed] [Google Scholar]
- 29.Wuermli L, Joerger M, Henz S, Schmid HP, Riesen WF, Thomas G, Krek W, Cerny T, Gillessen S. Hypertriglyceridemia as a possible risk factor for prostate cancer. Prostate Cancer Prostatic Dis. 2005;8:316–20. doi: 10.1038/sj.pcan.4500834. [DOI] [PubMed] [Google Scholar]
- 30.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the National Death Index. Am J Epidemiol. 1984;119:837–9. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 31.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the Health Professionals Follow-up Study. Int J Cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson MM, Garland C, Barrett-Connor E, Khaw KT, Friedlander NJ, Wingard DL. Heart disease risk factors, diabetes, and prostatic cancer in an adult community. Am J Epidemiol. 1989;129:511–7. doi: 10.1093/oxfordjournals.aje.a115162. [DOI] [PubMed] [Google Scholar]
- 33.Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, Grundy SM, Johnson CL. Trends in serum lipids and lipoproteins of adults, 1960-2002. JAMA. 2005;294:1773–81. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 34.Hager MH, Solomon KR, Freeman MR. The role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab Care. 2006;9:379–85. doi: 10.1097/01.mco.0000232896.66791.62. [DOI] [PubMed] [Google Scholar]
- 35.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168:1107–18. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–12. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 38.Edwards JE, Moore RA. Statins in hypercholesterolaemia: a dose-specific meta-analysis of lipid changes in randomised, double blind trials. BMC Fam Pract. 2003;4:18. doi: 10.1186/1471-2296-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hall SA, Page ST, Travison TG, Montgomery RB, Link CL, McKinlay JB. Do statins affect androgen levels in men? Results from the Boston area community health survey. Cancer Epidemiol Biomarkers Prev. 2007;16:1587–94. doi: 10.1158/1055-9965.EPI-07-0306. [DOI] [PubMed] [Google Scholar]
- 40.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) National Health, Lung, and Blood Institute, National Institutes of Health; 2002. [Google Scholar]


